Abstract
Purpose
The aim of this study was to evaluate the effect of dietary nitrate on secretory function of pancreatic islet and oxidative stress status in streptozotocin (STZ) induced type 1 diabetes in absence or presence of nitric oxide synthase inhibitor (L-NAME).
Methods
Fifty adult male sprague-dawly rats were divided into 5 groups: controls (C), diabetes (D), diabetes+nitrate (DN), diabetes +L-NAME (D + Ln), and diabetes+nitrate+L-NAME (DN + Ln) for 45 days. The concentrations of sodium nitrate and L-NAME were respectively 80 mg/L in drinking water and 5 mg/kg intraperitoneally. Body weight gain, plasma levels of glucose and insulin, islet insulin secretion and content, lipid peroxidation and antioxidant status in the pancreas of rats were determined.
Results
Compared to control group, the body weight gain and plasma insulin level were significantly decreased and plasma glucose and pancreatic NO and MDA concentrations and antioxidant enzymes activities were significantly increased in the STZ diabetic rats. In the diabetic rats, nitrate alone significantly reduced plasma glucose and increased pancreatic SOD and GPx activity. Reduced plasma glucose, pancreatic MDA and NO concentrations and increased plasma insulin level and pancreatic islet insulin secretion were observed in D + Ln and DN + Ln groups. Antioxidant enzymes activities were increased in diabetic rats which received combination of nitrate and L-NAME.
Conclusions
Our results showed that nitrate without effect on pancreatic islet insulin content and secretion decreased the blood glucose and slightly moderate oxidative stress and its effects in the presence of L-NAME on glucose hemostasis and pancreatic insulin secretion higher than those of nitrate alone.
Keywords: Nitrate, Nitric oxide synthase inhibitor, Oxidative stress, Insulin secretion
Introduction
Inorganic nitrate and nitrite are the nutritional elements that have recently been taken into consideration. The general population can be exposed to nitrate and nitrite through ingestion of food and drinking water. These inorganic compounds are in the human diet as plant-based foods and food additive compounds. It is generally believed that these compounds are unpleasant residues in the food chain and useless products of nitric oxide (NO) oxidation [1, 2]. There is a lot of confusion about the effects of nitrates and nitrites on human health. During the past decade, some studies have shown therapeutic and nutritional effects of nitrate and nitrite in many diseases such as cardiovascular disease, obesity, sickle cell anemia, gastric ulcer, systemic and pulmonary hypertension, metabolic syndrome, and even type 2 diabetes [1, 3–5]. The role of nitrate/nitrite in some biological pathways was considered after discovering the non-enzymatic pathway of NO production from nitrate in the diet that acts as physiological alternative sources of NO in healthy tissues [6]. Nitric oxide is a simple and highly bioactive molecule with complex function in the body. NO acts as a signaling molecule that regulates many biological functions and has an essential role in the pathogenesis of many disorders including diabetes. It has been shown that nitric oxide synthesis occurs by two pathways, dependent and independent L-arginine-nitric oxide synthase (NOS) pathway. There are three isoforms of NOS including endothelial NOS (or eNOS), neuronal NOS (or nNOS) and inducible NOS (or iNOS) that can be expressed in many tissues and cell types [1]. All three isoforms of NOS (iNOS, eNOS and nNOS) are expressed in pancreatic β-cells [7–9]. Effects of NO on insulin secretion are different, so that both the stimulatory [10] and inhibitory [11] effects of nitric oxide on insulin secretion were reported. In contrast, Jones et al. [12] have shown that nitric oxide has no effect on insulin secretion from the rat pancreatic islets. This controversy contributed to different concentrations of NO or different isoforms of the enzyme NO synthase (NOS) involved in its production. It has been shown that in the physiological state, eNOS-derived NO increases the pancreatic insulin secretion, while in the pathological state, higher NO concentrations derived by iNOS decrease te insulin secretion [13, 14].
In comparison to other cells, low levels of antioxidant enzymes in the pancreatic beta cells cause high sensitivity of these cells to oxidative stress. It is well known that increased ROS production plays a key role in the pathogenesis of pancreatic beta cells disorder and diabetes development [15, 16]. To the best of our knowledge, there is no study on the effects of inorganic nitrate on oxidative status of pancreatic tissues. Therefore, the purpose of this study was to investigate the effect of this component on pancreatic oxidative stress markers.
Although there have been few reports about the beneficial effects of dietary nitrite/nitrate on the pancreatic islet insulin secretion in type 2 diabetes mellitus, it is unclear whether these components are beneficial in streptozotocin (STZ) induced type 1 diabetes that shows islet beta cell dysfunction, increased oxidative stress, and pancreatic NO production induced by iNOS. It is not known whether inhibition of nitric oxide synthase (NOS) by N-G-Nitro-L-Arginine Methyl Ether (L-NAME) and reduction of NO production in the pancreas alter the effects of dietary nitrate on insulin secretion and lipid peroxidation and antioxidant enzymes activities in STZ induced diabetic rats.
Therefore, the aims of this study were to evaluate the effects of dietary nitrate supplementation on glucose homeostasis, oxidative stress status and secretory function of pancreatic islets in STZ- induced diabetic rats in absence or presence of L-NAME, a nonspecific NOS inhibitor.
Materials and methods
Chemicals
All reagent-grade chemicals were purchased from Sigma (St. Louis, MO, USA) or Merck (Darmstadt, Germany). Rat insulin kit (Mercodia, Uppsala, Sweden) and commercial glucose assay kit (Pars Azmoon Co, Ira) were respectively used for determination of the plasma levels of insulin and glucose. Antioxidant enzymes assay kits were purchased from ZellBio (ZellBio GmbH, Ulm, Germany). Phosphate-buffered saline (PBS), fetal bovine serum (FBS) and other culture materials were obtained from Gibco (Waltham, MA, USA).
Animals and study design
Adult sprague-Dawley male rats (230–260 g) were purchased from laboratory animal center of the Research Institute of Shiraz University of Medical Sciences (Shiraz, Iran) and kept in controlled conditions of light (12 h–12 h light–dark cycles), temperature (24 ± 2 °C) and relative humidity (23 ± 5%) with free access to food and water. The rats were randomized into 5 groups, including controls (C), diabetes (D), diabetes + nitrate (DN), diabetes +L-NAME (D + LN) and diabetes + nitrate + L-NAME (DN + LN). In the present study, the concentrations of sodium nitrate and L-NAME were respectively, 80 mg/L in drinking water [17] and 5 mg/kg intraperitoneally [18].
Induction of diabetes mellitus in adult male rats was performed by intraperitoneal injection of streptozotocin (STZ, 65 mg/kg). After 72 h of STZ injection, diabetes was confirmed by the blood glucose levels higher than 250 mg/dL. The experiment lasted for 45 days. During the experiment, the body weights of all animals were measured every week. At the end of the experiments, the animals were anaesthetized with intra-peritoneal injection of pentobarbital (120 mg/kg), and the pancreas was removed to evaluate the islet insulin secretion and oxidative stress parameters and antioxidant enzymes activities.
Animal handling and surgical procedures were carried out in accordance with the standard principles of laboratory animal care to reduce the animal suffering, and the study was approved by the local ethics committee of Shiraz University of Medical Sciences (Approval No: IR.SUMS.REC.1395.S1119)
Intraperitoneal glucose tolerance test (IPGTT)
After 40 days, intraperitoneal glucose tolerance test (IPGTT) was performed in all experimental animals. For IPGTT, after overnight (10–12 h) fasting and under general anaesthesia, blood samples were collected by cutting the tail tip at time zero before IP injection of glucose, and at 15, 30, 60, 90, and 120 min after glucose injection (2 g/kg body weight). The glucose oxidase method (Pars Azmoon Co., Tehran, Iran) and ultrasensitive rat insulin enzyme-linked immunosorbent assay method (ELISA, Mercodia, Uppsala, Sweden) were used for assessment of the plasma levels of glucose and insulin concentrations, respectively. Both insulin and glucose measurements were repeated three times. Intra- and inter-assay coefficients of variation for insulin measurement were 4.8 and 8.2% and for glucose assay was 3.6 and 6.9%, respectively.
The following formula [19] was used for calculation of homeostasis model assessment of insulin resistance (HOMA-IR):
HOMA IR = Glucose (mmol/l) × Insulin (U/ml) /22.5.
Islet isolation, insulin secretion and content
Islet isolation was performed based on the method described in a previous study [20]. Briefly, after anesthesia (60 mg/kg pentobarbital I.P.), the pancreas of the rats was exposed; the pancreatic main duct to the intestine was clamped and then 10 ml ice-cold Hank’s Balanced Salt Solution (HBSS) containing 0.5 mg/ml of collagenase P was injected through the bile duct. The inflated pancreas was removed and digested by collagenase at 37 °C for 17 min. The islets were washed four times with cold HBSS, passed through a sterile 50 μm mesh cell strainer, and hand-picked under a stereomicroscope.
For insulin secretion, batches of eight islets were placed in each well of 12-well plate and cultured in 1 ml RPMI-1640 media supplemented with 5 mmol/l glucose, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin for 60 min in 37 °C CO2 incubator. After that, the incubation medium was collected and used for insulin measurement. Insulin secretion was evaluated in the basal concentration of glucose of 5 mM.
As described in a previous study, the total pancreatic insulin content was measured with acid- ethanol extraction (0.18 M HCl in 70% ethanol) [21]. The released insulin, total intracellular insulin content, and the insulin released from the islets were assayed by the ELISA method. Measurements were repeated three times.
Measurement of oxidative stress markers and nitric oxide metabolites (NOx) in the pancreas
On day 45, after deep anesthesia, the animals were killed and immediately their pancreases were removed. The pancreases were weighted, washed with cold saline, sliced, and homogenized in cold sodium phosphate buffer (pH 7.4) containing 1 mM EDTA. After centrifuging, the pancreatic tissue was homogenated at 4000 rpm for 15 min at 4 °C; then, their supernatants were separated and used to assay lipid peroxidation, enzyme activities, Glutathione (GSH) and protein determination. Superoxide dismutase (SOD) activity was measured by SOD Assay Kit (ZellBio GmbH, Ulm, Germany) according to the manufacturer’s instructions using colorimetrically method at 450 nm. Glutathione Peroxidase (GPx) activity was assayed by GPx Assay kit (ZellBio GmbH, Ulm, Germany) according to the manufacturer’s instructions. GPx activity was measured according to coupled enzyme assay in which GPx reduces cumene hydroperoxide while oxidizing GSH to oxidised glutathione (GSSG). Then, GSSG was converted to the GSH with consumption of NADPH and its oxidation to NADP+ by glutathione reductase (GR). The decrease of NADPH was proportional to GPx activity measured by colorimetric method at OD 340 nm. The glutathione (GSH) concentration was determined by glutathione colorimetric assay kit (ZellBio GmbH, Ulm, Germany). In this assay, DTNB (5,5′-dithio-bis-[2-nitrobenzoic acid]) reacts with reduced glutathione to form a yellow product that absorbs at 412 nm. The optical density, measured at 412 nm, is directly proportional to glutathione concentration in the sample. MDA generated during lipid peroxidation was determined by TBARS method [20]. The pancreatic level of total NOx was assayed by the Griess method. The Bradford method [22] was used for assessment of protein content of the supernatant using bovine serum albumin (BSA) as standard. Measurement of oxidative stress markers and nitric oxide metabolites were repeated twice.
Statistical analysis
GraphPad Prism software, version 8.0 (Graphpad Software, La Jolla, CA, USA) was used for statistical analysis. For multiple comparisons and evaluation of the differences among the groups, one way ANOVA (post-hoc: Tukey) was used. For analysis of plasma glucose and insulin data during IPGTT, two-way repeated measures ANOVA (post-hoc: Bonferroni) was used. Data are presented as expressed as means ± SEM. P < 0.05 was considered statistically significant.
Results
Effect of nitrate and L-NAME on body weight
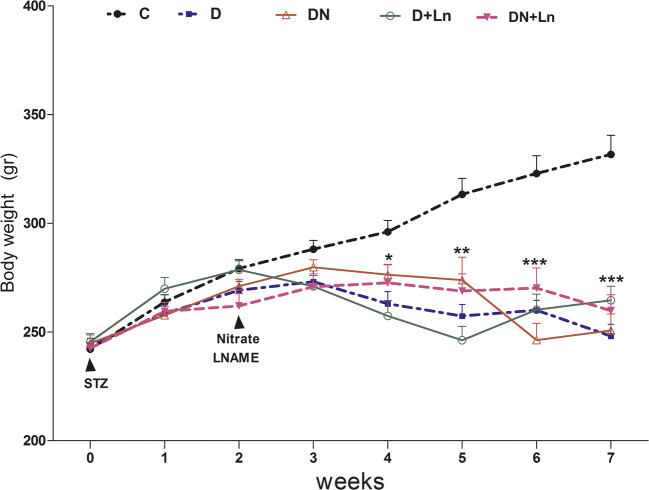
The results of body weight are shown in Fig. 1. There were no differences in the initial body weights of animals among the experimental groups at the start of the experiment. From the fourth week after STZ injection until the end of the experiment, a significant difference in the body weight was observed in the STZ induced diabetic rats compared to the control group. Compared to the diabetic group, nitrate and L-NAME alone or together did not alter the body weight gain in DN, D + Ln and DN + Ln groups. This study also showed significant differences in the body weight from the fourth week until the end of the experiment among the treated diabetic groups.
Fig. 1.
Effect of nitrate and L-NAME on body weights in experimental groups: Data are expressed as means ± SEM, n = 10 animals per group. Differences were analyzed by Student’s unpaired t test
*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control (c) group.
Effect of nitrate and L-NAME on plasma glucose concentrations during glucose tolerance test
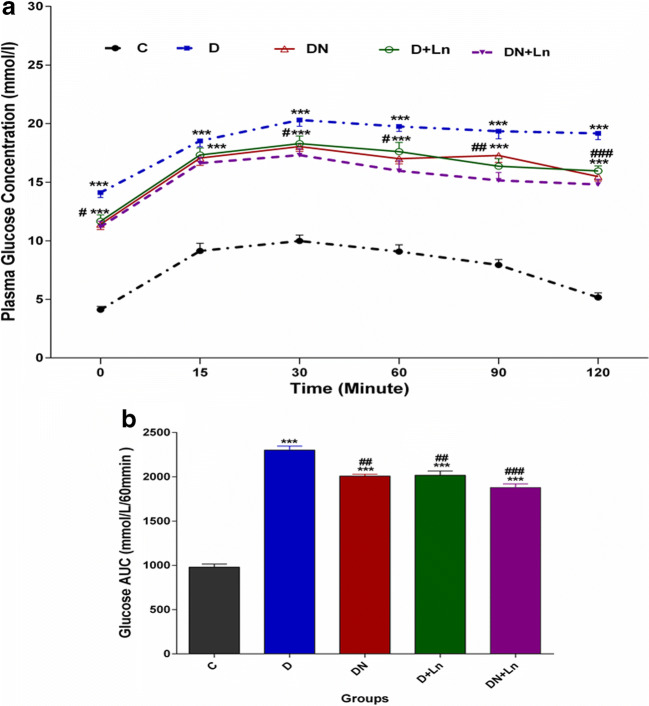
The plasma concentrations of glucose were assayed during intraperitoneal glucose tolerance test (IPGTT). As shown in Fig. 2a and b, the mean plasma concentrations of glucose at the time zero (fasting conditions) and after injection of glucose (2 g/kg body weight) during IPGTT (Fig.2a) and the AUC of plasma glucose levels (Fig. 2b) in the D group were significantly higher than those in the C group. In diabetic rats receiving oral nitrate (DN), the plasma glucose concentrations at all times of glucose tolerance test except for 15 min after the injection of glucose and plasma glucose area under the curve (AUC) were decreased compared to the STZ-diabetic rats, but these parameters were still significantly higher than control group. Plasma glucose concentrations during IPGTT and AUC for D + Ln and DN + Ln groups were similar to those in the DN group (Fig. 2a, b).
Fig. 2.
Mean plasma glucose levels (a) and AUC (b) during intravenous glucose tolerance test in experimental groups: The histograms represent the total area under the glucose curve. Data are expressed as means ± SEM of three independent replicates. n = 10 animals per group. Significant differences were assessed by two-way ANOVA and Bonferoni post hoc test
***P < 0.001 vs. Control (c) group.
#P < 0.05, ##P < 0.01, ###P < 0.001 vs Diabetic (D) group.
Effect of nitrate and L-NAME on plasma insulin concentrations during glucose tolerance test
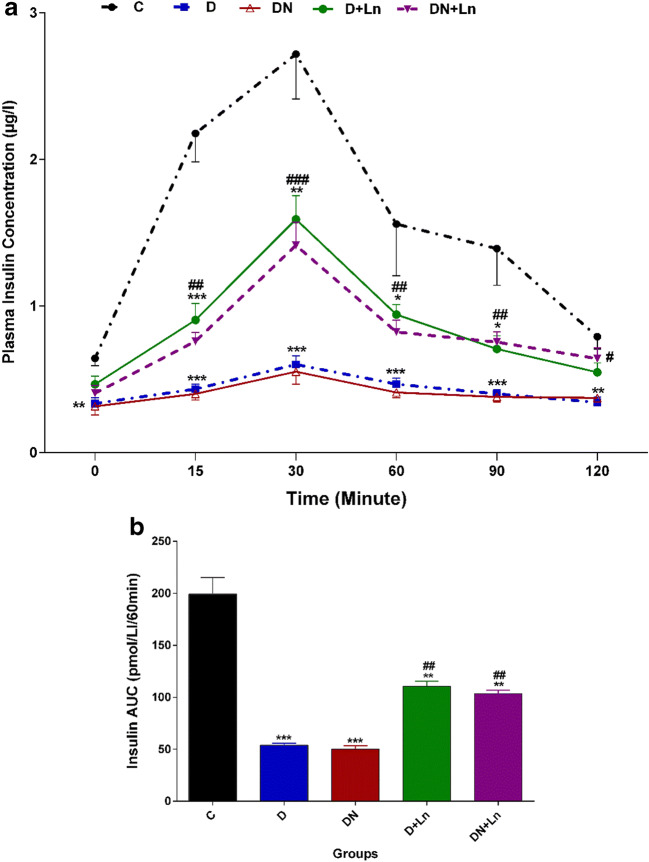
Our results showed that in D and DN groups, the mean plasma insulin levels after intraperitoneal injection of glucose during IPGTT (Fig.3a) and plasma insulin AUC (Fig.3b) were significantly lower than the C group. Compared to the D group, the plasma insulin concentrations were increased at 15, 30, 60 and 90 min in the D + Ln group and at 15, 30, 60, 90 and 120 min in the DN + Ln group. The plasma insulin AUC for these groups were significantly higher and lower than those of the D and C groups, respectively (Fig. 3a, b).
Fig. 3.
Mean plasma insulin levels (a) and AUC (b) during intravenous glucose tolerance test in experimental groups: The histograms represent the total area under the insulin curve. Data are expressed as means ± SEM of three independent replicates. n = 10 animals per group. Significant differences were assessed by two-way ANOVA and Bonferoni post hoc test
*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control (c) group.
#P < 0.05, ##P < 0.01, ###P < 0.001 vs Diabetic (D) group.
Effect of nitrate and L-NAME on the insulin content and secretion from the isolated islets
The insulin secretion and content of the isolated islets in different experimental groups are presented in Fig. 4. Data showed that the insulin secretion from the pancreatic isolated islets in response to 5 mM glucose in the D and DN groups was significantly lower than that of C group. L-NAME alone (D + Ln) or in combination with nitrate (DN + Ln) significantly increased the glucose stimulated insulin secretion from the islets compared to the diabetic islets, but it was significantly lower than that of the control islets.
Fig. 4.
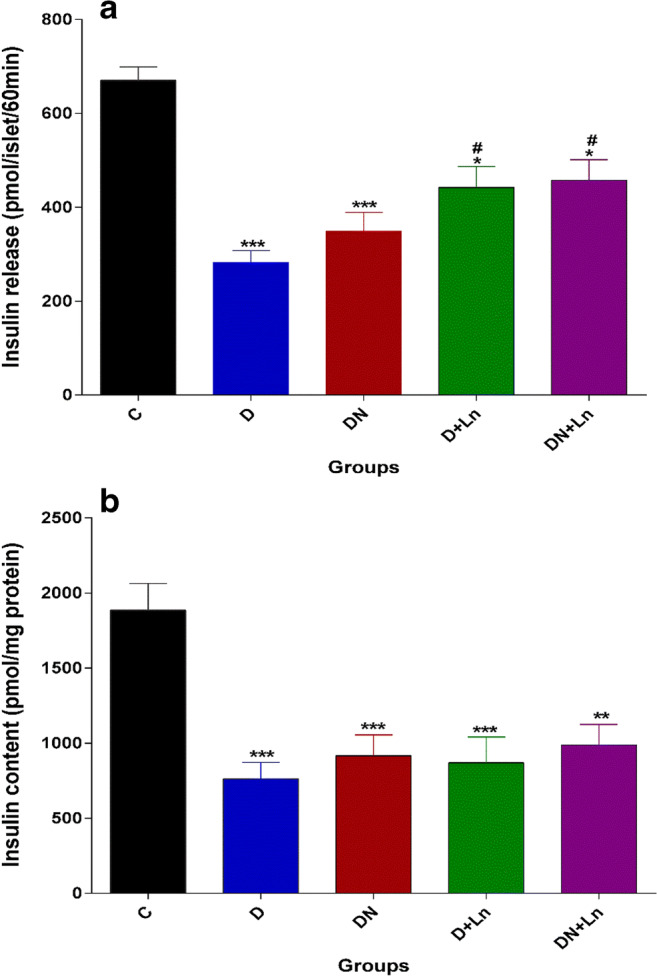
Effect of nitrate and L-NAME on insulin release (a) and content (b) in experimental groups: Data are expressed as means + SEM of three independent replicates. n = 10 batches of eight islets incubated for 60 min in 1 ml RPMI-1640 media supplemented with 5 mmol/l glucose. Differences were analyzed by One way ANOVA (post Hoc:Tukey)
*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control (c) group.
# P < 0.05 vs Diabetic (D) group.
The insulin content of the pancreatic islets in the D group was significantly lower than that of pancreatic islets in the C group. Nitrate and L-NAME alone or in combination did not affect the islet insulin content (Fig.4b).
Effect of nitrate and L-NAME on fasting glucose and insulin and HOMA-IR
The one way ANOVA analysis showed that in the D group, fasting plasma glucose and HOMA-IR index were significantly higher and fasting plasma insulin was significantly lower than those of the C group (Table 1). Oral administration of nitrate in the DN group only significantly reduced the fasting plasma glucose compared to the D group; however, there were no differences in the fasting plasma levels of insulin and HOMA-IR. In the D + Ln and DN + Ln groups, fasting plasma glucose concentration was significantly lower than that of the D group, but fasting plasma insulin level and HOMA-IR were not significantly different from the D group, but these groups demonstrated significant differences in these values compared with the C group.
Table 1.
Effects of nitrate and L-NAME on fasting plasma glucose and insulin, HOMA-IR
| Group | Fasting glucose (mmol/l) | Fasting insulin (U/ml) | HOMA-IR |
|---|---|---|---|
| C | 4.11 ± 0.27 | 111.99 ± 8.85 | 3.64 ± 0.29 |
| D | 14.11 ± 0.43*** | 58.55 ± 6.93*** | 5.31 ± 0.64 ** |
| DN | 11.41 ± 0.45 *** # | 55.20 ± 10.21 *** | 3.91 ± 0.59 |
| D + Ln | 11.66 ± 0.55 *** # | 75.56 ± 9.99 *** | 5.95 ± 0.81 ** |
| DN + Ln | 11.19 ± 0.47 *** # | 70.66 ± 9.13 *** | 5.01 ± 0.58 ** |
Values are expressed as means ± SEM of three independent replicates. n = 8 animals per group.
** P < 0.01, *** P < 0.001 vs Control (C) values
#P < 0.05 vs Diabetic (D) values.
Effect of nitrate and L-NAME on nitric oxide metabolites (NOx) and oxidative stress parmeters in the pancreas
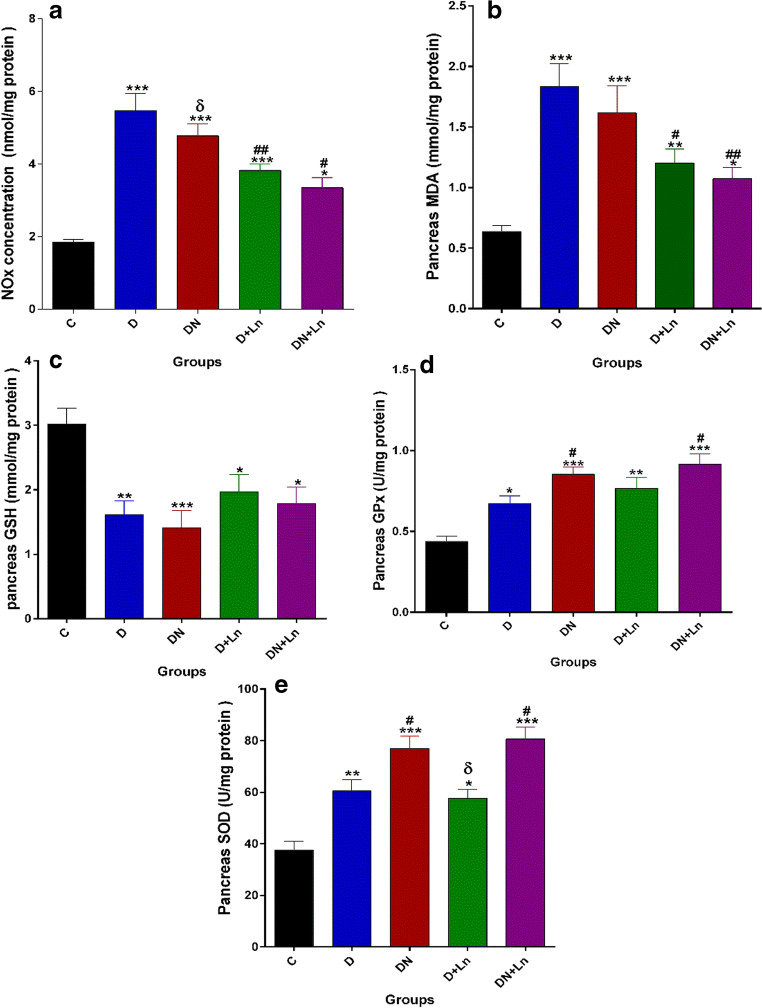
Pancreatic levels of NOx and MDA, GSH, SOD and GPx in all of the experimental groups are presented in Fig. 5 A-E. The results of this study showed that pancreatic tissue NOx and MDA levels and specific activities of GPx and SOD in the D, DN, D + Ln and DN + Ln groups were significantly (P < 0.05) higher than those in the C group. The pancreatic concentration of GSH was significantly decreased in these groups compared to the control group. No significant differences were observed between the DN group and D group in pancreatic concentrations of Nox, MDA and GSH. Antioxidant enzyme activities of GPx and SOD were not significantly increased in the pancreas of DN rats compared to D rats. Compared to the D group, in groups that received L-NAME alone (D + Ln) or in combination with nitrate (DN + Ln), significant decreases in the pancreatic levels of Nox, and MDA and significant increases in specific activities of SOD and GPx in the DN + Ln group were observed. The pancreatic concentration of GSH in the D + Ln and DN + Ln groups was not significantly different from the D group. There were the statistically significant differences between DN and DN + Ln in pancreatic NOx levels, as well between D + Ln and DN + Ln in pancreatic SOD activity.
Fig. 5.
Effect of nitrate and L-NAME on NOx level (a), MDA concentration (b), GSH content (c), enzymatic activities of GPx (d), and SOD (e) in pancreas of experimental rats. Each bar presents the mean ± SEM of three independent replicates. n = 8 animals per group. Differences were analyzed by one-way ANOVA
*P < 0.05, **P < 0.01, ***P < 0.001 vs. Control (c) group.
#P < 0.05, ##P < 0.01 vs Diabetic (D) group.
δ P < 0.05 vs DN + Ln group.
Discussion
In this study, we investigated the effect inorganic nitrate in the presence or absence nitric oxide synthase inhibitor on oxidative stress status and secretory function of the pancreatic islet in STZ- induced diabetic rats. The results of this study showed that although inorganic nitrate alone did not alter the plasma insulin level in diabetic rat, this compound could slightly improve the glucose intolerance and decrease the plasma glucose level. L-NAME as nitric oxide synthase inhibitor alone or in combination with nitrate increased plasma inulin level and islet insulin secretion that led to a decrease in the plasma glucose. The effect of nitrate in the presence of L-NAME reduced the oxidative stress more effectively than that of nitrate alone.
In STZ induced diabetic rats, there was a significant decrease in the body weight compared to the control group. Weight loss in the STZ-induced diabetic rats was shown in some studies, which could be due to a decrease in the plasma insulin concentration that resulted in reduced body protein synthesis and weight loss [23, 24]. In diabetic rats, no difference was observed in the weight gain percentage before and after administration of nitrate. Similar to our result, some studies have indicated that administration of sodium nitrate (200 and 400 mg / l in drinking water) for 60 days [25] and sodium nitrite (500, 1500 mg / kg of body weight)) for 18 days in rats [26] did not alter the weight gain. In the studies performed on both male and female rats by Til et al. [27] and on streptozotocin-induced diabetic rats by Khalif et al. [28], no significant differences in the weight gain were observed between the nitrate treated group and control group. In contrast to our data, some of the studies reported a decline in weight gain after oral administration of high dose of nitrate [29]. In our study, L-NAME alone or combination with nitrate consumption did not alter the weight gain in diabetic groups. According to our results, Broulík et al. [24] showed that L-NAME had no effect on the weight gain in the diabetic and control groups.
The present study showed that the levels of NOx metabolites in diabetic rats were higher than those of in the control rats. It is reported that expression of iNOS in diabetic rats is higher than the controls [30, 31]. It has been shown that factors such as anti-inflammatory cytokines, obesity, free fatty acids, hyperglycemia, and oxidative stress increase the iNOS expression [13, 32, 33]. It has been suggested that the dysfunction and destruction of pancreatic islet beta cells in diabetes is due to high expression of iNOS isoform and excessive production of NO [9, 31, 33].
Although nitrate and nitrite anions could act as substrates for NO generation and other nitrogenous forms of bioactive oxides, contrary to expectation, there were no significant differences in the NOx metabolites between nitrate treated diabetic group and diabetic control group. Some studies have shown that mineral nitrite significantly reduces iNOS mRNA levels in the mouse and human active macrophages through reduced NADPH oxidase activity or through nitrite-derived NO that have negative feedback on iNOS mRNA expression [14, 34]. It seems that nitrate, on the one hand, increased NO generation from nitrate-nitrite- NO pathway, and on the other hand, it decreased NO production from endogenous L-arginine–NOS pathway by reducing the expression or activity of iNOS. Therefore, in the nitrate treated diabetic group, reduction of iNOS activity/expression by nitrate and increase in NO production by nitrate-nitrite- NO pathway may neutralize each other. In the diabetic group, the use of L-NAME, as inhibitor of nitric oxide synthases (NOSs), significantly reduced NO production in the pancreatic tissue. The simultaneous administration of nitrate and L-NAME resulted in further reduction of NOx levels in the pancreas of diabetic rats due to the inhibitory effects of L-NAME on NOSs and reduction of iNOS activity/expression by nitrate/nitrite compounds that prevent excessive NO production [14, 34, 35].
In the present study, our results indicated that fasting plasma glucose and insulin levels significantly were increased and decreased respectively in STZ-diabetic rats. It was demonstrated that insulin secretion and content were significantly decreased in diabetic islets. Reduced insulin secretion may be due to increased pancreatic iNOS activity and STZ- induced oxidative stress that resulted in the pancreatic β-cells damage. Administration of sodium nitrate in diabetic rats significantly decreased the plasma glucose level with no significant alterations in the plasma insulin concentration. Nitrate sodium also had no effect on insulin secretion and islet insulin content in diabetic rats. Studies have shown that nitrate/nitrite compounds independent of insulin can improve the insulin resistance and enhance the glucose uptake in the peripheral tissue by increasing the GLUT4 gene expression and the translocation of GLUT4 to plasma membrane of the skeletal muscle and adipose tissue [14, 36, 37]. It seems that nitrate could reduce hyperglycemia in diabetic rats through increase in insulin-independent glucose uptake in the peripheral tissues, rather than alteration in the pancreatic islet insulin secretion and content.
Compared to the diabetic control group, in the diabetic rats which had received L-NAME, simultaneously with reduction of pancreatic concentrations of NO, plasma insulin levels were significantly increased; consequently, plasma glucose concentrations were significantly decreased. The role NO in the physiology of insulin secretion is still being debated. It seems that the low concentrations of NO produced by eNOS act as survival factor in pancreatic β –cells, while high levels of NO induced by iNOS are involved in the development of both type 1 and type 2 diabetes [38]. A number of studies have shown that glucotoxicity and lipotoxicity induce iNOS expression and enhance NO production [32, 39] .Therefore, pancreatic β-cell dysfunction and impaired insulin secretion in diabetes may be due to the expression of the iNOS isoform and excessive production of NO induced by hyperglycemia [31, 40]. It has been shown that inhibition of iNOS leads to an increase in β -cell stability in the pancreatic islets and regular insulin secretion in type 2 diabetes [32]. In current study, L-NAME, as a non-specific NOS inhibitor, prevented excessive NO production in the pancreatic islets that resulted in increased islet insulin secretion and plasma insulin levels and partially improved glucose intolerance in diabetic rats. In diabetic rats, co-administration of nitrate and L-NAME improved the glucose intolerance and regulated the plasma glucose and insulin concentrations which may be related to the inhibitory effect of L-NAME on iNOS and the effect of nitrate/nitrite compounds on insulin signaling pathways in the peripheral tissues.
In the present study, the pancreatic lipid peroxidation and antioxidant enzymes activities were measured. A significant increase in MDA concentration was observed in the pancreas of control the diabetic rats compared with the control rats. Oxidative stress plays an important role in the onset and progression of diabetes and pancreatic beta cells dysfunction [41]. It has been shown that excessive ROS production and oxidative stress in diabetes mellitus can be associated with bioavailability of nitric oxide. Increased oxidative and nitrosative stress is closely attributed to hyperglycemia that leads to increased superoxide anion formation. In diabetes, nitric oxide produced by iNOS can interact with superoxide anion to form the potent cytotoxic peroxynitrite [42]. The pancreatic antioxidant enzymes activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in diabetic rats were higher than those in control ones. Some studies reported reduced activities of SOD and GPx while others found increase in antioxidant enzymes activities [43, 44]. The hyperglycemia in the diabetic rats might enhance production of superoxide anion. Superoxide anion is a highly reactive oxygen compound that is produced as a by-product of oxygen metabolism. High concentration of superoxide anions causes oxidative stress and cell damage. SOD is an important antioxidant enzyme that breaks down harmful oxygen molecules specially superoxide anion in cells [45]. This enzyme has important role in the regulation of oxidative stress in diabetes, therefore, the elevation of SOD activity in diabetic rats might be due to increased production of superoxide anions. The elevation of GPx activity in diabetic rats might also be due to attenuate the oxidative stress. It has been shown that with increased oxidative stress, the activities of antioxidant enzymes as a compensatory mechanism were increased in diabetic rats [44, 46].
GSH level in diabetic rats was reduced. The decreased GSH levels in diabetic rat might be due to the increased activity of GPx that convert reduced glutathione (GSH) to oxidized glutathione (GSSG) to alleviate the oxidative stress [47].
In the diabetic group, the oral consumption of sodium nitrate did not alter the level of plasma insulin. Nitrate consumption also led to increases in antioxidant enzymes activities of the pancreas with no significantl decrease in the MDA level. It was expected that in the nitrate treated diabetic rats, the production of more NO by nitrate compounds resulted in increased proxynitrite formation and oxidative stress, but in this group, to prevent further increase of oxidative stress and more production of MDA in the pancreas of diabetic rats receiving nitrate, the activities of the pancreatic antioxidant enzymes as a compensatory mechanism were increased.
Some studies have reported that components of nitrate and nitrite decrease oxidative stress by reducing NADPH activity and superoxide production [34, 48]. Intraperitoneal injection of L-NAME decreased NO and MDA concentrations in the pancreases of diabetic rats. According to our study, Seven et al. [42] have shown that L-NAME resulted in decreased oxidative stress due to the inhibition of iNOS enzyme in the STZ-diabetic rats. In comparison to the diabetic group, in diabetic rats receiving nitrate and L-NAME, increased pancreatic antioxidant activities and decreased MDA concentrations were observed. It seems that increased activities of antioxidant enzymes and possible reduction of iNOS activity by L-NAME have led to reduced lipid peroxidation and oxidative stress.
In conclusion, our results demonstrated that nitrate decreased the blood glucose and increased antioxidant enzymes activities but did not affect the pancreatic insulin secretion and content. It seems that reduced blood glucose by nitrate is probably through increased glucose uptake in the peripheral tissue. Our observations also showed that effects of combination of nitrate and L-NAME on reduced oxidative stress and increased secretory function of pancreas and improved glucose intolerance were more than nitrate alone.
Acknowledgements
The present work was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (grant no. 12977); it is a part of Ms. Thesis by Zahra Shabgard Shahraki. The funders had no role in study design, data collection and analysis and preparation of the manuscript.
Author contributions
All authors have contributed to the conception and design of the research. Z.S.S, M.N. performed the experiments/analysis. N.K. conceived the study. All authors have participated in drafting the article or revising it and approved the final version.
Compliance with ethical standards
Disclosure statement
The authors report no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.Omar SA, Webb AJ, Lundberg JO, Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J Intern Med. 2016;279(4):315–336. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 3.McNally B, Griffin JL, Roberts LD. Dietary inorganic nitrate: from villain to hero in metabolic disease? Mol Nutr Food Res. 2016;60(1):67–78. doi: 10.1002/mnfr.201500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5(12):865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghasemi A, Zahediasl S. Potential therapeutic effects of nitrate/nitrite and type 2 diabetes mellitus. Int J Endocrinol Metab. 2013;11(2):63–64. doi: 10.5812/ijem.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Wakf AM, Hassan HA, Mahmoud AZ, Habza MN. Fenugreek potent activity against nitrate-induced diabetes in young and adult male rats. Cytotechnology. 2015;67(3):437–447. doi: 10.1007/s10616-014-9702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novelli M, Pocai A, Lajoix AD, Beffy P, Bezzi D, Marchetti P, Gross R, Masiello P. Alteration of beta-cell constitutive NO synthase activity is involved in the abnormal insulin response to arginine in a new rat model of type 2 diabetes. Mol Cell Endocrinol. 2004;219(1–2):77–82. doi: 10.1016/j.mce.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Lajoix AD, Reggio H, Chardes T, Peraldi-Roux S, Tribillac F, Roye M, Dietz S, Broca C, Manteghetti M, Ribes G, Wollheim CB, Gross R. A neuronal isoform of nitric oxide synthase expressed in pancreatic beta-cells controls insulin secretion. Diabetes. 2001;50(6):1311–1323. doi: 10.2337/diabetes.50.6.1311. [DOI] [PubMed] [Google Scholar]
- 9.Broniowska KA, Oleson BJ, Corbett JA. Chapter Twelve - β-Cell Responses to Nitric Oxide. In: Litwack G, editor. Vitamins & Hormones. Cambridge: Academic Press; 2014. pp. 299–322. [DOI] [PubMed] [Google Scholar]
- 10.Smukler SR, Tang L, Wheeler MB, Salapatek AM. Exogenous nitric oxide and endogenous glucose-stimulated beta-cell nitric oxide augment insulin release. Diabetes. 2002;51(12):3450–3460. doi: 10.2337/diabetes.51.12.3450. [DOI] [PubMed] [Google Scholar]
- 11.Henningsson R, Salehi A, Lundquist I. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am J Physiol Cell Physiol. 2002;283(1):C296–C304. doi: 10.1152/ajpcell.00537.2001. [DOI] [PubMed] [Google Scholar]
- 12.Jones PM, Persaud SJ, Bjaaland T, Pearson JD, Howell SL. Nitric oxide is not involved in the initiation of insulin secretion from rat islets of Langerhans. Diabetologia. 1992;35(11):1020–1027. doi: 10.1007/BF02221676. [DOI] [PubMed] [Google Scholar]
- 13.Salehi A, Meidute Abaraviciene S, Jimenez-Feltstrom J, Ostenson CG, Efendic S, Lundquist I. Excessive islet NO generation in type 2 diabetic GK rats coincides with abnormal hormone secretion and is counteracted by GLP-1. PLoS One. 2008;3(5):e2165. doi: 10.1371/journal.pone.0002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghasemi A, Jeddi S. Anti-obesity and anti-diabetic effects of nitrate and nitrite. Nitric Oxide. 2017;70:9–24. doi: 10.1016/j.niox.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14(11):21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Wang H. Oxidative stress in pancreatic Beta cell regeneration. Oxidative Med Cell Longev. 2017;2017:1930261–1930269. doi: 10.1155/2017/1930261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlström M, Larsen FJ, Nyström T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A. 2010;107(41):17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadek-Michalska A, Bugajski J. Role of nitric oxide in the nicotine-induced pituitary-adrenocortical response. J Physiol Pharmacol. 2004;55(2):443–455. [PubMed] [Google Scholar]
- 19.Farrokhfall K, Khoshbaten A, Zahediasl S, Mehrani H, Karbalaei N. Improved islet function is associated with anti-inflammatory, antioxidant and hypoglycemic potential of cinnamaldehyde on metabolic syndrome induced by high tail fat in rats. J Funct Foods. 2014;10:397–406. [Google Scholar]
- 20.Safayee S, Karbalaei N, Noorafshan A, Nadimi E. Induction of oxidative stress, suppression of glucose-induced insulin release, ATP production, glucokinase activity, and histomorphometric changes in pancreatic islets of hypothyroid rat. Eur J Pharmacol. 2016;791:147–156. doi: 10.1016/j.ejphar.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Karbalaei N, Noorafshan A, Hoshmandi E. Impaired glucose-stimulated insulin secretion and reduced beta-cell mass in pancreatic islets of hyperthyroid rats. Exp Physiol. 2016;101(8):1114–1127. doi: 10.1113/EP085627. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Chen M, Shou Q, Li Y, Hu F. Biological activities of Chinese Propolis and Brazilian Propolis on Streptozotocin-induced type 1 diabetes mellitus in rats. Evid Based Complement Alternat Med. 2011;2011:1–8. doi: 10.1093/ecam/neq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broulik PD, Haluzik M, Skrha J. The influence of nitric oxide synthase inhibitor L-NAME on bones of male rats with streptozotocin-induced diabetes. Physiol Res. 2003;52(6):729–734. [PubMed] [Google Scholar]
- 25.Ogur R, Coskun O, Korkmaz A, Oter S, Yaren H, Hasde M. High nitrate intake impairs liver functions and morphology in rats; protective effects of alpha-tocopherol. Environ Toxicol Pharmacol. 2005;20(1):161–166. doi: 10.1016/j.etap.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 26.National TP. Toxicology and carcinogenesis studies of sodium nitrite (CAS NO. 7632-00-0) in F344/N rats and B6C3F1 mice (drinking water studies) Natl Toxicol Program Tech Rep Ser. 2001;495:7–273. [PubMed] [Google Scholar]
- 27.Til HP, Kuper CF, Falke HE. Nitrite-induced adrenal effects in rats and the consequences for the no-observed-effect level. Food Chem Toxicol. 1997;35(3–4):349–355. doi: 10.1016/s0278-6915(97)00122-1. [DOI] [PubMed] [Google Scholar]
- 28.Khalifi S, Rahimipour A, Jeddi S, Ghanbari M, Kazerouni F, Ghasemi A. Dietary nitrate improves glucose tolerance and lipid profile in an animal model of hyperglycemia. Nitric Oxide. 2015;44:24–30. doi: 10.1016/j.niox.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Akasha M, A. K, A. AS Effect of Nitrate on the Body Weight, Food and Water Consumption and Thyroid Hormone in Hybrid Female Rabbits. J Vet Adv. 2015;5(5):912–918. [Google Scholar]
- 30.Kato Y, Miura Y, Yamamoto N, Ozaki N, Oiso Y. Suppressive effects of a selective inducible nitric oxide synthase (iNOS) inhibitor on pancreatic beta-cell dysfunction. Diabetologia. 2003;46(9):1228–1233. doi: 10.1007/s00125-003-1173-x. [DOI] [PubMed] [Google Scholar]
- 31.Muhammed SJ, Lundquist I, Salehi A. Pancreatic beta-cell dysfunction, expression of iNOS and the effect of phosphodiesterase inhibitors in human pancreatic islets of type 2 diabetes. Diabetes Obes Metab. 2012;14(11):1010–1019. doi: 10.1111/j.1463-1326.2012.01632.x. [DOI] [PubMed] [Google Scholar]
- 32.Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, Tejedo JR. Regulation of pancreatic beta-cell survival by nitric oxide: clinical relevance. Islets. 2012;4(2):108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- 33.McDaniel ML, Kwon G, Hill JR, Marshall CA, Corbett JA. Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med. 1996;211(1):24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 34.Yang T, Peleli M, Zollbrecht C, Giulietti A, Terrando N, Lundberg JO, Weitzberg E, Carlstrom M. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radic Biol Med. 2015;83:159–166. doi: 10.1016/j.freeradbiomed.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Carlström M, Liu M, Yang T, Zollbrecht C, Huang L, Peleli M, Borniquel S, Kishikawa H, Hezel M, Persson AEG, Weitzberg E, Lundberg JO. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid Redox Signal. 2015;23(4):295–306. doi: 10.1089/ars.2013.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H, Torregrossa AC, Potts A, Pierini D, Aranke M, Garg HK. Dietary nitrite improves insulin signaling through GLUT4 translocation. Free Radic Biol Med. 2014;67:51–57. doi: 10.1016/j.freeradbiomed.2013.10.809. [DOI] [PubMed] [Google Scholar]
- 37.Ohtake K, Nakano G, Ehara N, Sonoda K, Ito J, Uchida H. Dietary nitrite supplementation improves insulin resistance in type 2 diabetic KKA(y) mice. Nitric Oxide. 2015;44:31–38. doi: 10.1016/j.niox.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Nino Fong R, Fatehi-Hassanabad Z, Lee SC, Lu H, Wheeler MB, Chan CB. Uncoupling protein-2 increases nitric oxide production and TNFAIP3 pathway activation in pancreatic islets. J Mol Endocrinol. 2011;46(3):193–204. doi: 10.1530/JME-10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 40.Qader SS, Ekelund M, Andersson R, Obermuller S, Salehi A. Acute pancreatitis, expression of inducible nitric oxide synthase and defective insulin secretion. Cell Tissue Res. 2003;313(3):271–279. doi: 10.1007/s00441-003-0764-7. [DOI] [PubMed] [Google Scholar]
- 41.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacher P, Obrosova IG, Mabley JG, Szabo C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12(3):267–275. doi: 10.2174/0929867053363207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 44.Tiedge M, Lortz S, Munday R, Lenzen S. Protection against the co-operative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia. 1999;42(7):849–855. doi: 10.1007/s001250051237. [DOI] [PubMed] [Google Scholar]
- 45.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seven A, Guzel S, Seymen O, Civelek S, Bolayirli M, Yigit G, Burcak G. Nitric oxide synthase inhibition by L-NAME in streptozotocin induced diabetic rats: impacts on oxidative stress. Tohoku J Exp Med. 2003;199(4):205–210. doi: 10.1620/tjem.199.205. [DOI] [PubMed] [Google Scholar]
- 47.Sheweita SA, Mashaly S, Newairy AA, Abdou HM, Eweda SM. Changes in oxidative stress and antioxidant enzyme activities in Streptozotocin-induced diabetes mellitus in rats: role of Alhagi maurorum extracts. Oxidative Med Cell Longev. 2016;2016:5264064. doi: 10.1155/2016/5264064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]