Abstract
Background
Previous studies have shown thylakoids, the membrane proteins which are extracted from green leaves like spinach, can induce satiety through homeostatic and non-homeostatic pathways. In this study, we reviewed the current human literature on thylakoids’ characteristics and their relationship to satiety regulation and weight loss.
Methods
A systematic search of literature published between January 1990 and May 2019 was conducted on the electronic databases; including WEB OF SCIENCE, Cochrane Library, MEDLINE, Scopus, and EMBASE databases. We included all clinical trials that addressed the effects of thylakoids or chloroplast intake on satiety and weight loss.
Results
After excluding non-human studies, non-RCTs, duplications, studies with irrelevant data and interventions, eight studies were included in the qualitative synthesis. All studies supported this hypothesis that thylakoids reduce the feeling of hunger by increasing postprandial cholecystokinin and leptin and decreasing serum ghrelin, but the consequences of thylakoid intake on anthropometric characteristics were controversial.
Conclusion
In conclusion, our results may approve this postulation that receiving a thylakoid-enriched meal can decrease appetite and probably food intake in short term; however, more studies are needed to explore the effects of long term supplementation with thylakoids on weight loss in human subjects.
Electronic supplementary material
The online version of this article (10.1007/s40200-019-00443-w) contains supplementary material, which is available to authorized users.
Keywords: Appetite, Obesity, Satiety, Spinach, Thylakoid, Weight loss
Introduction
The World Health Organization (WHO) defines obesity as a condition of excess body fat to the extent that health is impaired [1, 2]. Currently, obesity is highly prevalent in both developed and developing countries. The number of overweight and obese individuals has increased at an alarming rate recently as a result of which at least 2.8 million people die each year (http://www.who.int/gho/ncd/risk_factors/obesity_text/en/). In 2016, more than 1.9 billion adults (39% of world population), 18 years and older, were overweight among whom 650 million (13%) were obese [3]. Threatening trends in obesity prevalence during the last decades could be explained by the negative influences of industrialization on both diet and physical activity levels [4, 5].
Obesity is a disorder of multifactorial pathogeneses [6]. Many psychological and physiological factors, such as taste and composition of the meal, gastrointestinal motility, digestion and absorption of nutrients, secretion of satiety hormones and suppression of hunger hormones, can affect appetite [7]. Thylakoids are the internal photosynthetic membrane systems of chloroplasts which can be extracted from green leaves like spinach [8]. Thylakoids have a two-layer membrane which separate their lumen from stroma of cytosol. The stacks of 10 to 20 thylakoids held together are called grana. The grana are interconnected by stroma lamellae through junctions at the margins of the granum [9]. Thylakoids consist of various membrane-bound proteins, galactolipids, phospholipids, and antioxidants such as chlorophyll, carotenoids, zeaxanthin, and lutein [8]. Hunger-suppressing and lipid- and glucose-lowering properties of thylakoids have been shown in previous studies. The satiety-promoting effects of thylakoids, in part, could be due to decreasing fat digestion through inhibition of pancreatic lipase/colipase activity which promotes the release of hormones like cholecystokinin and glucagon-like peptide-1(GLP-1) that signal satiety [10–13]. Administration of thylakoids for 10 days modulated gut microbiota in the rat [14]. Gut microbiota affects metabolism by fermenting carbohydrates into short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. It has been found that SCFAs have beneficial effects on satiety and insulin sensitivity since they act as a ligand to free fatty acid receptors [15, 16].
Various animal and human studies have been conducted to examine the effects of thylakoids on suppressing hunger and weight loss. Erlanson-Albertsson et al. [17] also published a review article about the same relationship, but they did not use a systematic approach. Therefore, we decided to examine this relationship more thoroughly using systematic criteria to draw a more definitive conclusion.
Methods
Search strategy
A systematic search for articles published between January 1990 and May 2019 was conducted on the electronic databases. WEB OF SCIENCE, Cochrane Library, MEDLINE, Scopus and EMBASE databases were searched for related studies by using the specified keywords (refer to search strategy appendix). The bibliographies of the papers found in the primary search were also investigated to recognize other possible related studies. The search was limited to human studies.
Selection criteria
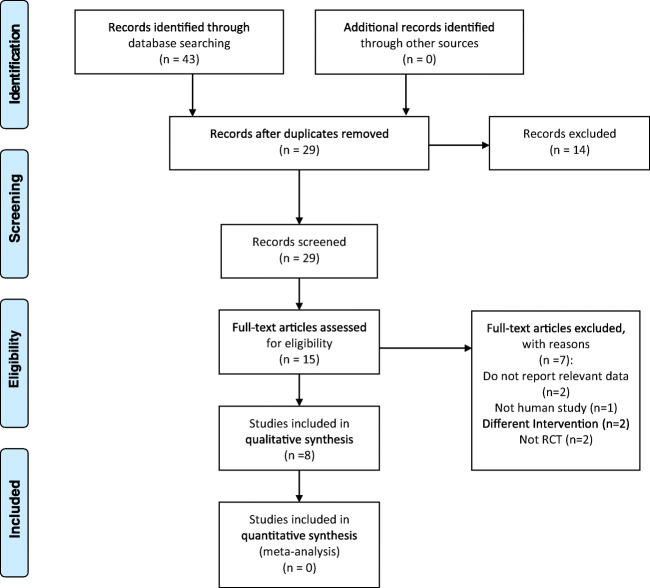
We included articles in this review if they had the following criteria: [1] were randomized double-blind, single-blind or not blinded controlled trial (RCT) in their design, [2] were published as original paper, [3] had a sample size of >5 subjects per arm, [4] were performed on adult subjects aged >18 years old, [5] used thylakoids or spinach extract alone (not in combination) for intervention, [6] clinically investigated the effects of thylakoids or spinach extract in any dosages in human subjects, [7] assessed any marker of weight loss and appetite as the primary outcome variable, and [8] no other special dietary regimen or supplementation were used in control or intervention group. The final literature search yielded a total of 43 articles. After excluding non-human studies, non-RCTs, duplications, studies with irrelevant data and studies with different interventions, eight studies were included in qualitative synthesis (Fig. 1). We contacted the authors by email to ask for further explanation if we found any ambiguity regarding the papers meeting our inclusion criteria.
Fig. 1.
PRISMA flow diagram
Quality assessment
The quality of the selected studies was assessed by means of a five-point Jadad score conducted by two researchers separately. Jadad is a quality assessment tool in which critical evaluation is made over three different domains: [1] randomization, [2] blinding, and [3] an account of all patients. One point would be assigned if the randomization and blinding were mentioned in the paper and one extra point if their methods were proper. Also one more point is assigned if the fate of all patients were known. The minimum score for qualification of the papers in this review was one point (one point for randomization) and the maximum score was five points.
Data extraction
Primary outcomes of interest in this systematic review were weight and appetite. Two reviewers independently extracted and cross-checked the following data from eligible papers: name of the first author, publication date, the location of the study, study design, participants’ characteristics, sample size, composition and dosage of thylakoids, intervention duration, variables measured, and the main outcomes of studies. Articles were consulted again if there were any discrepancies between the extracted data. In one study by Stenblom et al. [12], same populations participating as the treatment group, received three different thylakoids dosages as 3.7, 5 and 7.4 g/day; therefore, we reported their results as three separate pieces of evidence in this review.
Results
Eight studies met the criteria and were included in the qualitative synthesis. The quality of the selected studies in terms of Jadad score is shown in Table 1.
Table 1.
Quality of the selected studies in terms of JADAD score
| Study | Randomization | Blinding | An account of all patients | Total score |
|---|---|---|---|---|
| Stenbolm 2016 | 2 | 2 | 1 | 5 |
| Montelius 2014 | 2 | 2 | 1 | 5 |
| Kohnke 2009 | 0 | 2 | 1 | 3 |
| Maruyama 2013 | 1 | 0 | 1 | 2 |
| Rebello 2015 | 2 | 2 | 1 | 5 |
| Stenblom 2013 | 1 | 2 | 1 | 4 |
| Stenblom 2014 | 1 | 2 | 1 | 4 |
| Stenblom 2015 | 2 | 2 | 1 | 5 |
The included studies are summarized in Table 2. Three studies investigated variables related to appetites such as food intake, hunger and desire for food and anthropometric features in the long-term and five studies measured them in the postprandial state after thylakoids intake (acute studies). Due to differences in study design and high heterogeneity, none of these studies were eligible for meta-analysis.
Table 2.
Characteristics of the included studies; study design, population, intervention and comparison descriptions; outcome measures and JADAD score
| Author | Country | Study design | Subjects | Age of participants Intervention(control) | Sex | Sample | Dose | Duration | Main results | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Stenblom et al., 2016 [23] | Sweden | RCT | Overweight women | 50.7(54.6) | F | 34 | Thylakoids | 12 weeks | ↔faecal fat | 5 |
| (Appethyl) | ↑ Total faecal bacteria | |||||||||
| (5 g/day) | ↑ Bacteroides fragilis group | |||||||||
| Kohnke et al., 2009 [21] | Sweden | RCT | Individuals with normal weight | NR | M/F | 11 | Thylakoid powder in 2 doses: | In one meal | ↔ glucosea | 3 |
| 25 g/50 g | ↓ FFAsb | |||||||||
| Thylakoid delipidated: | ↑ Leptinc | |||||||||
| 25 g | ↓ Ghrelind | |||||||||
| ↓ Insulina | ||||||||||
| ↑ CCKa | ||||||||||
| Maruyama, et al., 2013 [22] | Japan | RCT | Men with normal weight | 25.6 | M | 14 | 75 g of boiled spinach | In one meal | ↔ glucose | 2 |
| ↔ insulin | ||||||||||
| ↔TC | ||||||||||
| ↔LDL-C | ||||||||||
| ↔HDL-C | ||||||||||
| ↔TG | ||||||||||
| Maruyama, et al., 2013 [22] | Japan | RCT | Obese men | 31.2 | M | 10 | 75 g of boiled spinach | In one meal | ↔ glucose | 2 |
| ↔ insulin | ||||||||||
| ↔TC | ||||||||||
| ↔LDL-C | ||||||||||
| ↔HDL-C | ||||||||||
| ↔TG | ||||||||||
| Montelius et al., 2014 [11] | Sweden | RCT | Women with BMI 25–33 | 50.7(54.6) | F | 38 | Thylakoid | 12 weeks | ↓weight | 5 |
| 5 g/day | ↔BMI | |||||||||
| ↔waist circumference | ||||||||||
| ↔ FFM | ||||||||||
| ↔body fat | ||||||||||
| ↔ leptin | ||||||||||
| ↔ glucose | ||||||||||
| ↔ insulin | ||||||||||
| ↓TC | ||||||||||
| ↓LDL-C | ||||||||||
| ↔HDL-C | ||||||||||
| ↔TG | ||||||||||
| ↔ Postprandial ghrelin | ||||||||||
| ↑ GLP1 | ||||||||||
| ↓urge for sweets and chocolate | ||||||||||
| Rebello et al., 2015 [14] | USA | Cross-over RCT | overweight or obese males and females | 35.3 | M/F | 60 | Thylakoids | In one meal | ↑ Fullness | 4 |
| 5 g/day | ↓Hunger | |||||||||
| ↔satisfaction | ||||||||||
| ↓ Desire for salty, savory and thirst | ||||||||||
| ↔ Desire for sweets | ||||||||||
| ↔TG | ||||||||||
| ↔TC | ||||||||||
| ↔LDL-C | ||||||||||
| ↔HDL-C | ||||||||||
| ↔FFA | ||||||||||
| ↑ glucose | ||||||||||
| Stenblom et al., 2013 [20] | Sweden | RCT | Healthy women | 53.3 | F | 20 | Thylakoids in twe doses: | In one meal | ↓hunger | 4 |
| 3.7 g/day | ↑CCK | |||||||||
| 7.4 g/day | ↔ TNF-a | |||||||||
| ↔ glucose | ||||||||||
| ↑ Insulin | ||||||||||
| Stenblom et al., 2014 [19] | Sweden | RCT | Overweight women | 51.6(53) | F | 26 | Thylakoids | 8 weeks | ↔weight | 4 |
| 5.6 g/day | ↔Body fat | |||||||||
| ↔Trunk fat | ||||||||||
| ↓hunger | ||||||||||
| ↔ Waist circumference | ||||||||||
| ↓Hip circumference | ||||||||||
| ↓ leptin | ||||||||||
| ↓ LDL-c | ||||||||||
| ↔ TC | ||||||||||
| ↓ apo B1 | ||||||||||
| ↔ TG | ||||||||||
| ↓ glucose | ||||||||||
| ↔ Hemoglobin A1c | ||||||||||
| ↔ insulin | ||||||||||
| Stenblom et al., 2015 [13] | Sweden | Cross-over RCT | Normal or overweight women | 54.5e | F | 22 | Thylakoids | In one meal | ↓Hunger | 4 |
| 5 g/day | ||||||||||
| ↑Fullness | ||||||||||
| ↓Salty snacks desire | ||||||||||
| ↓Sweet snacks desire | ||||||||||
| ↓Sweet and fat desire | ||||||||||
| ↓All snacks desire | ||||||||||
| ↓Food intake |
Abbreviations:RCT Randomized Controlled Trial, FFA Free Fatty Acids, CCK Cholecystokinin, TC Total Cholesterol, TG Triglyceride, BMI Body Mass Index, FFM Fat Free Mass
↔ = No significant difference compared to control
↓ = significantly decreased compared to control
↑ = significantly increased compared to control
aIn all groups
bIn 50 g thylakoids group
cIn 25 g and 50 g thylakoids group
d In 25 g thylakoid powder and 25 g thylakoid delipidated
emedian
Anthropometric characteristics
Only two studies measured the anthropometric features. In one study thylakoids consumption (5 g/day) decreased body weight as much as 1.5 kg on average more than the control group after 3 months [10]. Stenblom et al. reported only a reduction in hip circumference, but no significant weight change, after 8 weeks of supplementation with thylakoids (5.6 g/day) [18]. Both studies were not able to show any significant changes in on other anthropometric features like total body fat, fat free mass (FFM) or waist circumference.
Appetite and food intake
Five studies investigated the effects of thylakoids on appetite by the use of the Visual Analog Scales (VAS) questionnaire. Among them, two studies showed a decreased urge for sweets and chocolate and a reduction in hunger feeling after long-term intervention [10, 18]. The duration and dosage of thylakoids have been described in the previous section. Three acute studies also support the hunger-suppressing and satiety-promoting effects of thylakoids. Rebello et al. showed that a single meal supplemented with 5 g of thylakoids increases fullness and reduces hunger and prospective intake over the 2-h period following consumption [13]. Stenblom et al. examined two dosages of thylakoids (3.7 g and 7.4 g) paired with a high carbohydrate breakfast; VAS questionnaire was filled out every 15 min after the start of the meal. Thylakoid intake was associated with hunger suppression after 180 min compared to the control group. There were no statistically significant differences in the same parameters between the groups treated by the two dosages of thylakoids [19]. Stenblom et al. conducted a more thorough investigation on the impact of thylakoids on appetite in another trial with cross-over design. They found that supplementation with thylakoids (5 g) in the morning reduces the feeling of hunger, desiring palatable food, and carving sweet, salty and all types of snacks and also increased the feeling of satiety during the rest of the day [12].
Insulin, leptin, cholecystokinin, ghrelin and GLP-1
Elevated postprandial cholecystokinin (CCK) levels were reported in two trials. No study investigated the long-term effects of thylakoids on CCK. Kohnke et al. used different dosages of thylakoids (25 g, 50 g, and 25 g of delipidated thylakoid) in one meal. Serum CCK levels increased after all doses of thylakoids compared to the control. Ghrelin was decreased after consuming 25 g of thylakoid. Also, postprandial serum leptin was increased after thylakoids consumption [20], but long-term supplementation in overweight women had the opposite effect [18]. One study reported that long-term supplementation with thylakoids elevates postprandial circulating levels of GLP-1 [10]. Thylakoids intake in three studies had no effects on serum insulin [10, 18, 21] and the results were controversial in another two trials [19, 20].
Other effects
Serum glucose was increased in the thylakoids group in an acute study [13], while consumption of thylakoids in long-term lead to a lower glucose level in another trial [18]. No significant changes were found in other studies. A minor positive effect on the amount and composition of the gut microbiota was reported after a 12-week intervention with five grams of thylakoids [22].
Adverse effects
No significant adverse effects were reported in none of the trials included in this article.
Discussion
We systematically reviewed the studies which examined the effects of thylakoids on appetite. To the best of the authors’ knowledge, this is the first systematic review of clinical trials on this topic. Hunger-suppressing effects of thylakoids were reported in all studies that directly measured hunger and fullness (Table 2). This in part is due to inhibition of pancreatic lipase/colipase activity and retardation of fat digestion as shown in vitro studies [11, 23]; however, heat treatment could reduce thylakoids’ efficiency in doing so [24]. Colipase knockout mice had impaired weight gain compared to normal mice [25], declaring that lipase/colipase inhibition can lead to weight loss. Thylakoids like other biological membrane inhibit lipase/colipase complex by two types of mechanisms working together:
Thylakoids bind to the triacylglycerol/water interface; as a result, it forms a sterical barrier impeding the lipase-colipase complex from reaching its substrate. This mechanism is supported by electron microscopy.
Thylakoids bind to the lipase/colipase complex; thereby, blocking its active site and preventing getting in touch with the triglyceride substrate. This linkage involves both ionic and hydrophobic interactions [11].
This suppression stimulates a compensatory release of endogenous lipase/colipase and has been suggested as a mechanism for increasing the enterostatin which is an appetite suppressing pentapeptide [11, 26]. Gastrointestinal complications such as steatorrhea are the common side-effects of lipase inhibitors. However, supplementation with thylakoid for 10 days didn’t change fecal fat in the rats treated with high-fat diet [14]. In another study, no significant differences were seen in fecal fat content of healthy human subjects after a three-month supplementation with thylakoid [22]. These results suggest that thylakoids may inhibit lipase activity in a reversible manner that does not cause steatorrhea.
Animal studies showed that CCK serum elevates postprandially after thylakoids intake [11, 27] and these results are supported by two clinical trials included in this review [19, 20]. Montelius et al. showed for the first time that GLP-1 secretion increases after the consumption of a thylakoid-rich breakfast in overweight women [10]. The most potent factors in releasing GLP-1 are carbohydrate and fat. Retarded fat digestion and CCK secretion that occur after thylakoids intake are two probable mechanisms for the raise observed in serum GLP-1 [28]. Delayed fat digestion leads to a reduced gastric emptying and an increased secretion of CCK and GLP-1 [10, 19, 20, 22]. These two hormones themselves inhibit gut motility [29, 30]. Also, serum levels of ghrelin, a potent orexigenic hormone [31], were lowered after thylakoids intake [20]. The reason why Montelius et al. were not able to observe any significant changes in serum ghrelin levels could be the low dose of thylakoids they used (5 g), compared to 25 g of thylakoid/day applied by Kohnke et al. Intestinal fat with the mediation of CCK signaling could suppress ghrelin [32].
Leptin has a critical role in appetite and body weight [33]. Supplementation with thylakoids for 8 weeks caused a reduction in leptin serum in overweight women [18]. This is probably because of weight loss and reduction in body fat following a thylakoid-rich diet. Clearly, the decline in leptin serum is a natural consequence of weight loss [34]. This outcome was different when studied in the short-term. Kohnke et al. reported that postprandial leptin levels increased after receiving a thylakoid-rich meal, describing another mechanism for satiety-inducing features of thylakoid. This increase was not observed after receiving delipidated thylakoids, suggesting that these effects are only attributable to thylakoids with lipid or lipid/protein properties, such as galactolipids, chlorophyll, carotenoids and, antioxidants like tocopherols [20]. Since no more studies investigated the short-term effects of thylakoids on serum leptin, the reason of this increase is still unclear.
The reduction in postprandial plasma insulin reported in one trial [20] could be due to beneficial effects of thylakoids on modifying the composition of intestinal microbiota which may, in turn, decrease insulin and blood glucose concentrations [14, 35]. Conversely, postprandial plasma insulin levels were raised by thylakoids consumption in another clinical trial; they hypothesized that CCK and GLP-1 could increase insulin secretion and thylakoids may exert an incretin-like effect [36]. Nonetheless, there were more RCTs investigating plasma insulin after thylakoids intake, but they didn not report any significant changes (Table 2). Overall, it is too soon to draw a conclusion in this regard and this area warrants more investigations.
Suppression of hedonic hunger which can be described as an individual’s tendency to experience appetitive thoughts, feelings, and urges about food in response to palatable food [37], is another factor that induces satiety. Stenblom et al. showed in three separate studies that receiving thylakoids decreases hunger and desire for salty, sweet and fatty snacks, both after a single meal and a long-term supplementation [12, 18, 19]. These results are also supported by other RCTs [10, 13]. The synergistic effects of appetite-regulating hormones, like CCK, GLP1, and ghrelin, contribute to the food reward system [38–40] and could suppress hedonic hunger. Moreover, the role of glucose and insulin homeostasis in controlling reward system, particularly in urge for sweet, had been determined [41], but studies examining effects of thylakoids on serum insulin and glucose are still inconsistent to regard that as a certain mechanism.
Animal studies demonstrate that rats and mice receiving thylakoids had a significant weight loss or lower weight gain compared to the control groups [11, 27, 42, 43], but only two human studies measured this variable directly. Weight was significantly decreased after 12 weeks of supplementation with 5 g of thylakoids per day in one of these trials [10]. In another study, although no significant difference in weight loss was seen between the groups, based on appetite-suppressing effects of thylakoids, it seems that weight loss was achieved with less effort in the thylakoid-treated group compared to the control [18].
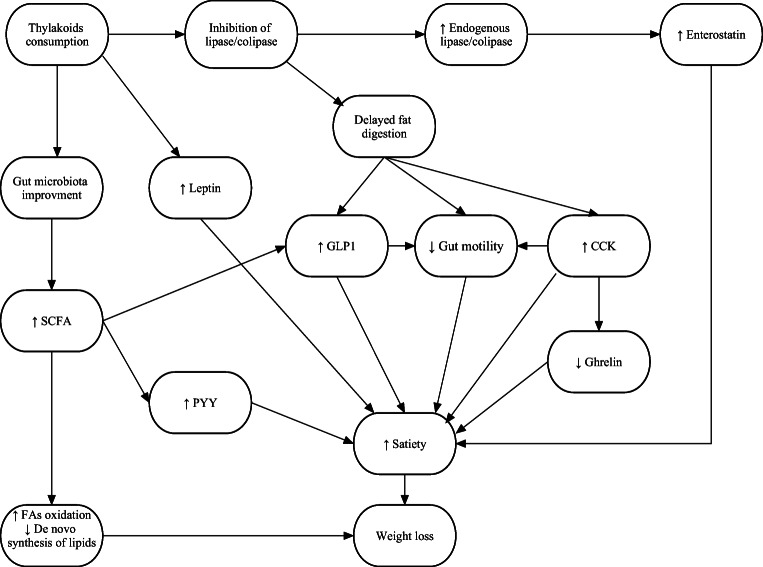
Both population and diversion of gut microbiota can influence energy balance, weight loss, and glucose and lipid metabolism [44–46]. Some Bifidobacterium and Lactobacillus species showed anti-obesity effects in previous studies [47–49]. Lactobacillus reuteri protected against the development of diet-induced obesity in apo E-deficient mice [50]. This bacterium was increased in the distal ileum in rats receiving thylakoids compared to the control [14]. In the only human trial, the count of total and fragilis group bacteria was increased in the feces of individuals after receiving thylakoids for 3 months [22]. SCFAs are produced by microbiota in the distal small intestine and colon from low-digestible carbohydrates. SCFAs receptors are located in colonic L cells causing them to secret GLP-1 and PYY [51]. This suggests that gut microbiota could regulate appetite through secretion of hormones, such as GLP-1 and PYY. Additionally, SCFAs induce fatty acid oxidation and inhibit de novo synthesis of lipids leading to a reduction in plasma free fatty acids and body weight [52]. Further studies are needed to investigate the effects of thylakoids on population and diversion of gut microbiota, especially those with anti-obesity features. The probable mechanisms of thylakoids effects on appetite are summarized in Fig. 2.
Fig. 2.
Probable mechanisms of thylakoids on appetite
We were able to recognize the beneficial role of thylakoids in controlling appetite and food intake, both in the short- and the long-term, in the existing literature. But there is insufficient evidence to support the role of thylakoids in the prevention or treatment of obesity and more human studies are needed to ratify this possible capability. Systematic search and assessing the quality of different trials were the strengths of our study; however, scarcity of human studies and high heterogeneity of results rendered a meta-analysis impossible. Our results are in line with the previous review by Erlanson-Albertsson [17], with this difference that for the first time we used systematic criteria for study inclusion.
Conclusion
In conclusion, our results show that, based on the existing publications so far, receiving a thylakoid-enriched meal decreases appetite and probably food intake in the short term. Both homeostatic and non-homeostatic factors are involved in this reduction. But whether these appetite-lowering effects of thylakoids could be a useful strategy in controlling obesity in the long term is still ambiguous. More studies are needed to investigate the effects of long-term supplementation with thylakoids on weight loss in human subjects.
Electronic supplementary material
(DOC 82 kb)
Funding information
This study was supported by Student Research Committee, Iran University of Medical Sciences (IUMS), Tehran, Iran.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH. Obesity: preventing and managing the global epidemic report of a WHO Consultation (WHO Technical Report Series 894)2000. 252 p. [PubMed]
- 2.Garrow JS. Obesity and related diseases. London: Churchill Livingstone; 1988. [Google Scholar]
- 3.(World Healtrh Organization (WHO) Obesity and Overweight, 2017 http://www.who.int/mediacentre/factsheets/fs311/en. Accessed 18 Dec 2017.
- 4.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. 1971. Milbank Q. 2005;83(4):731–757. doi: 10.1111/j.1468-0009.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan S, Niswender KD. Neuroendocrine regulation of food intake. Pediatr Blood Cancer. 2012;58(1):149–153. doi: 10.1002/pbc.23376. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juhler RK, Andreasson E, Yu SG, Albertsson PK. Composition of photosynthetic pigments in thylakoid membrane vesicles from spinach. Photosynth Res. 1993;35(2):171–178. doi: 10.1007/BF00014747. [DOI] [PubMed] [Google Scholar]
- 9.Mustárdy L, Buttle K, Steinbach G, Garab G. The three-dimensional network of the thylakoid membranes in plants: quasihelical model of the granum-stroma assembly. Plant Cell. 2008;20(10):2552–2557. doi: 10.1105/tpc.108.059147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu E, Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite. 2014;81:295–304. doi: 10.1016/j.appet.2014.06.101. [DOI] [PubMed] [Google Scholar]
- 11.Albertsson PA, Kohnke R, Emek SC, Mei J, Rehfeld JF, Akerlund HE, et al. Chloroplast membranes retard fat digestion and induce satiety: effect of biological membranes on pancreatic lipase/co-lipase. Biochem J. 2007;401(3):727–733. doi: 10.1042/BJ20061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenblom EL, Egecioglu E, Landin-Olsson M, Erlanson-Albertsson C. Consumption of thylakoid-rich spinach extract reduces hunger, increases satiety and reduces cravings for palatable food in overweight women. Appetite. 2015;91:209–219. doi: 10.1016/j.appet.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Rebello CJ, Chu J, Beyl R, Edwall D, Erlanson-Albertsson C, Greenway FL. Acute effects of a spinach extract rich in thylakoids on satiety: a randomized controlled crossover trial. J Am Coll Nutr. 2015;34(6):470–477. doi: 10.1080/07315724.2014.1003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montelius C, Osman N, Westrom B, Ahrne S, Molin G, Albertsson PA, et al. Feeding spinach thylakoids to rats modulates the gut microbiota, decreases food intake and affects the insulin response. J Nutr Sci. 2013;24:2. doi: 10.1017/jns.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74(3):227–234. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva-Millan MJ, Perez-Matute P, Oteo JA. Gut microbiota: a key player in health and disease. A review focused on obesity. J Physiol Biochem. 2015;71(3):509–525. doi: 10.1007/s13105-015-0390-3. [DOI] [PubMed] [Google Scholar]
- 17.Erlanson-Albertsson C, Albertsson PA. The use of green leaf membranes to promote appetite control, suppress hedonic hunger and loose body weight. Plant Foods Hum Nutr. 2015;70(3):281–290. doi: 10.1007/s11130-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenblom E-L, Montelius C, Erlandsson D, Skarping L, Fransson M, Egecioglu E, et al. Decreased urge for palatable food after a two-month dietary intervention with green-plant membranes in overweight women. J Obes Weight Loss Ther. 2014;4:8. [Google Scholar]
- 19.Stenblom EL, Montelius C, Ostbring K, Hakansson M, Nilsson S, Rehfeld JF, et al. Supplementation by thylakoids to a high carbohydrate meal decreases feelings of hunger, elevates CCK levels and prevents postprandial hypoglycaemia in overweight women. Appetite. 2013;68:118–123. doi: 10.1016/j.appet.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Kohnke R, Lindbo A, Larsson T, Lindqvist A, Rayner M, Emek SC, et al. Thylakoids promote release of the satiety hormone cholecystokinin while reducing insulin in healthy humans. Scand J Gastroenterol. 2009;44(6):712–719. doi: 10.1080/00365520902803499. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama C, Kikuchi N, Masuya Y, Hirota S, Araki R, Maruyama T. Effects of green-leafy vegetable intake on postprandial glycemic and lipidemic responses and alpha-tocopherol concentration in normal weight and obese men. J Nutr Sci Vitaminol. 2013;59(4):264–271. doi: 10.3177/jnsv.59.264. [DOI] [PubMed] [Google Scholar]
- 22.Stenblom E-L, Weström B, Linninge C, Bonn P, Farrell M, Rehfeld JF, Montelius C. Dietary green-plant thylakoids decrease gastric emptying and gut transit, promote changes in the gut microbial flora, but does not cause steatorrhea. Nutr Metab. 2016;13(1):67. doi: 10.1186/s12986-016-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emek SC, Akerlund HE, Erlanson-Albertsson C, Albertsson PA. Pancreatic lipase-colipase binds strongly to the thylakoid membrane surface. J Sci Food Agric. 2013;93(9):2254–2258. doi: 10.1002/jsfa.6034. [DOI] [PubMed] [Google Scholar]
- 24.Ostbring K, Rayner M, Sjoholm I, Otterstrom J, Albertsson PA, Emek SC, et al. The effect of heat treatment of thylakoids on their ability to inhibit in vitro lipase/co-lipase activity. Food Funct. 2014;5(9):2157–2165. doi: 10.1039/c3fo60651a. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino D, Cordle RA, Kullman J, Erlanson-Albertsson C, Muglia LJ, Lowe ME. Decreased postnatal survival and altered body weight regulation in procolipase-deficient mice. J Biol Chem. 2002;277(9):7170–7177. doi: 10.1074/jbc.M108328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erlanson-Albertsson C, York D. Enterostatin--a peptide regulating fat intake. Obes Res. 1997;5(4):360–372. doi: 10.1002/j.1550-8528.1997.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 27.Panda V, Shinde P. Appetite suppressing effect of Spinacia oleracea in rats: involvement of the short term satiety signal cholecystokinin. Appetite. 2017;113:224–230. doi: 10.1016/j.appet.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Beglinger S, Drewe J, Schirra J, Goke B, D'Amato M, Beglinger C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J Clin Endocrinol Metab. 2010;95(2):879–886. doi: 10.1210/jc.2009-1062. [DOI] [PubMed] [Google Scholar]
- 29.Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol. 2014;592(14):2927–2941. doi: 10.1113/jphysiol.2014.270850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Phys. 1997;273(5):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 31.Kirchner H, Heppner KM, Tschop MH. The role of ghrelin in the control of energy balance. Handb Exp Pharmacol. 2012;209:161–184. doi: 10.1007/978-3-642-24716-3_7. [DOI] [PubMed] [Google Scholar]
- 32.Degen L, Drewe J, Piccoli F, Grani K, Oesch S, Bunea R, et al. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):30. doi: 10.1152/ajpregu.00734.2006. [DOI] [PubMed] [Google Scholar]
- 33.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in Ob/Ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 34.Blundell JE, Gillett A. Control of food intake in the obese. Obes Res. 2001;9(4):129. doi: 10.1038/oby.2001.129. [DOI] [PubMed] [Google Scholar]
- 35.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 36.Hardikar AA. Role of incretins in pancreas growth and development. JOP. 2004;5(6):454–456. [PubMed] [Google Scholar]
- 37.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM, et al. Ghrelin increases intake of rewarding food in rodents. Addict Biol. 2010;15(3):304–311. doi: 10.1111/j.1369-1600.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7(181):00181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi E, Yasoshima Y, Shimura T. Systemic administration of anorexic gut peptide hormones impairs hedonic-driven sucrose consumption in mice. Physiol Behav. 2017;171:158–164. doi: 10.1016/j.physbeh.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kohnke R, Lindqvist A, Goransson N, Emek SC, Albertsson PA, Rehfeld JF, et al. Thylakoids suppress appetite by increasing cholecystokinin resulting in lower food intake and body weight in high-fat fed mice. Phytother Res. 2009;23(12):1778–1783. doi: 10.1002/ptr.2855. [DOI] [PubMed] [Google Scholar]
- 43.Stenkula KG, Stenblom EL, Montelius C, Egecioglu E, Erlanson-Albertsson C. Thylakoids reduce body fat and fat cell size by binding to dietary fat making it less available for absorption in high-fat fed mice. Nutr Metab. 2017;14(4):016–0160. doi: 10.1186/s12986-016-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagliabue A, Elli M. The role of gut microbiota in human obesity: recent findings and future perspectives. Nutr Metab Cardiovasc Dis. 2013;23(3):160–168. doi: 10.1016/j.numecd.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Tehrani AB, Nezami BG, Gewirtz A, Srinivasan S. Obesity and its associated disease: a role for microbiota? Neurogastroenterol Motil. 2012;24(4):305–311. doi: 10.1111/j.1365-2982.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani PD, Geurts L, Matamoros S, Plovier H, Duparc T. Glucose metabolism: focus on gut microbiota, the endocannabinoid system and beyond. Diabetes Metab. 2014;40(4):246–257. doi: 10.1016/j.diabet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Moya-Perez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One. 2015;10(7):e0126976. doi: 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity. 2013;21(11):2310–2321. doi: 10.1002/oby.20330. [DOI] [PubMed] [Google Scholar]
- 49.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53(2):100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Fak F, Backhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One. 2012;7(10):9. doi: 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89(1):31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 52.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 82 kb)