Abstract
AIM
To determine the effects of intravitreal resveratrol (RSV) on murine laser-induced choroidal neovascularization (CNV).
METHODS
The toxicity of RSV to choroidal endothelial cell (CEC) was measured using thiazolyl blue tetrazolium bromide (MTT) assay. Effects of RSV on choroidal endothelial cell (CEC) migration were evaluated with a modified Boyden chamber assay, while tube formation was evaluated in a 2-D gel assay. CNV was induced by laser photocoagulation in mice. The effects of intravitreal injection of RSV on CNV development were evaluated by fluorescein angiography (FA), confocal analysis of isolectin B4 labeled choroidal flat mounts, and histologic examination of CNV membranes. Immunostaining was used to analyze the expression and phosphorylation of vascular endothelial growth factor receptor 2 (VEGFR2).
RESULTS
No significant cell toxicity was observed in CEC if the concentration of RSV was less than 200 µmol/L (P>0.05). RSV inhibited vascular endothelial growth factor (VEGF)-induced CEC migration (P<0.05) and tube formation (P<0.05) in vitro. Furthermore, intravitreal injection of RSV significantly inhibited laser induced CNV formation in mice. The FA leakage, CNV volume and CNV area analysis revealed that there were 41%, 45%, and 58% reduction in RSV-treated eyes (1.691±0.1032, 178 163±78 623 µm3 and 6508±619.0 µm2, respectively) compared with those in control (2.724±0.08447, 379 676±98 382 µm3 and 16 576±2646 µm2, respectively; P<0.05). Phospho-VEGFR2 expression was much weaker in the sections of CNV lesions in RSV injected mice compared with that in control (P<0.05).
CONCLUSION
Intravitreal injection of RSV exerts an inhibitory effect on CNV, which may through suppressing endothelial cell migration, tube formation and VEGFR2 phosphorylation.
Keywords: resveratrol, intravitreal injection, choroidal neovascularization, vascular endothelial growth factor receptor 2, tube formation, murine
INTRODUCTION
Age-related macular degeneration (AMD) is a leading cause of blindness among the elderly population in the Western world[1]. Choroidal neovascularization (CNV) is a serious complication of AMD in late phase, in which newly formed choroidal vessels extend into the retinal pigment epithelium (RPE) or subretinal space, leading to exudation and hemorrhage[2]. It has been identified that vascular endothelial growth factor (VEGF) is a key mediator of CNV[2]. Although intravitreal injection of the VEGF inhibitors is the current standard of care for patients with neovascular AMD, not all patients benefit from these therapies[3] and serious ocular and systematic complications have been reported[4]. Moreover, targeting one factor or pathway in such a complex, multifactorial progress may not be sufficient to halt CNV progression in all patients[4]. Thus, development of alternative or combination therapies is needed.
Resveratrol (RSV) is a natural polyphenol compound, and several plants and red wine are enriched with RSV[5]. In addition to RSV's known cardio-protective, neuro-protective and anti-inflammatory effects[6], several studies have revealed anti-angiogenic activities[7]–[8]. Abu-Amero et al[9] recently reviewed the relevance of RSV with ocular diseases. It has been reported RSV down-regulates pro-angiogenic factors such as VEGF in a primate-derived retino-choroidal cell line (RF/6A)[10], human adult RPE cells[11], and other retinal cell types[12]. Furthermore, systemic delivery of RSV inhibits pathologic angiogenesis in animal models of diabetic retinopathy[13], macular telangiectasia[14], and murine CNV[11]–[12],[15]–[17]. However, the effects of RSV on angiogenesis are at times conflicting and situation-dependent[18]. Based on the reported lower bioavailability of systemically administered RSV in humans[19], and the lack of any bio-distribution studies of RSV in the eye after oral delivery, it has been suggested that oral administration of RSV may not be the most effective route of administration for eye diseases[20]. On the other hand, intravitreal injection has the ability to achieve high intravitreous drug concentration and to avoid the toxicities associated with systemic treatment[21]. In order to directly deliver RSV to the posterior segment of the eye and limit its possible adverse systemic effects, we evaluated the use of intravitreous injection of RSV in a murine CNV model, and in vitro assays using choroidal endothelial cells (CECs).
MATERIALS AND METHODS
Ethical Approval
All procedures were performed in compliance with the Keck School of Medicine Institutional Animal Care and Use Committee (IACUC) approved protocols and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cells Culture
Bovine CECs were isolated from bovine eyes through magnetic beads bound to the specific endothelial marker Lycopersicon esculentum (Sigma-Aldrich, MO, USA), as previously described. Bovine CECs was cultured in endothelial growth medium (EGM™ Bullet Kit, #CC-3124, Lonza, Switzerland), and cells from passages 2 to 8 were used.
Thiazolyl Blue Tetrazolium Bromide Assay
CEC cells (2×103) were cultured in 96-well plates in CEC medium with 10% fetal bovine serum (FBS). The CEC cells were treated with different concentrations of RSV (0, 10, 20, 50, 100, and 200 µmol/L) for 48h, and treated with 20 µL thiazolyl blue tetrazolium bromide (MTT; 5 mg/mL; Sigma) for 4h. Then the supernatants were decanted, and the formazan precipitates were solubilized by 150 µL of 100% dimethyl sulfoxide (DMSO; Sigma) for 10min on a plate shaker. Absorbance at 550 nm was detected on a multi-well plate reader (Benchmark Plus; Bio-Rad, Tokyo, Japan).
Migration Assay
The CECs migration assay was performed using a modified Boyden chamber assay system[22]. Briefly, after being serum starved overnight, 5×104 cells were seeded into the upper compartment of a Boyden chamber in 24-well plates. Inserts were coated with fibronectin (2 mg/cm2). After 1-hour attachment and 5-hour incubation with EGM medium containing 50 ng/mL VEGF (R&D Systems Inc., Minneapolis, MN, USA) with or without 50 µmol/L RSV, the inserts were fixed with cold methanol and counterstained with hematoxylin for 25min. The ScanScope digital whole slide scanner (CSO; Aperio, Vista, CA, USA) was used to scan the inserts. The number of migrated cells per insert was counted in four chosen fields randomly[22].
Tube Formation Assay
Two-dimensional tube formation was induced in Geltrex Reduced Growth Factor Basement Membrane Matrix gel (Life Technologies). The gel was deposited in 96-well plates and incubated for 30min at 37°C to reconstitute it into basement membrane-like structure. Totally 5×103 CECs that had been serum starved overnight were seeded onto each well of 96-well plates and incubated in EGM containing 50 ng/mL VEGF with or without 50 µmol/L RSV for 5h. Then the medium was removed and 100 uL of Calcein-AM (2 µg/mL, Life Technologies) was added for 30min at 37°C. Tube formation was documented by fluorescence microscope (IMT-2; Olympus, NY, USA). Adobe Photoshop (CS5) was used to measure tubule length of the newly formed vascular network.
Laser-Induced Choroidal Neovascularization
C57Bl/6 male mice aged 6-8wk were purchased from the National Cancer Institute (Frederick, MD). For all surgical procedures, the mice were anesthetized and their pupils were dilated with topical 2.5% phenylephrine hydrochloride and 1% tropicamide (Alcon, Fort Worth, TX, USA). Four photocoagulation lesions were produced with diode green laser (75-mm spot size, 0.05s duration, 100 mW; IRIDEX, Mountain View, CA, USA) between the retina vessels in both eyes[23]. Production of a subretinal bubble at the time of laser treatment showed the rupture of Bruch's membrane. Any lesion, which did not produce bubble or the lesion coming with obvious bleeding was excluded in the study. Intravitreal injection with RSV (2 µL, 100 µmol/L) or vehicle (0.1% DMSO)[24] was performed immediately after the laser procedure and on post-laser day 3.
Fluorescence Angiography
On day 7 after laser treatment, digital images were taken at 3-5min using an angiography camera (VK2e, KD-2UC; Kowa, Nagoya, Japan) after dilating pupils with 2.5% phenylephrine hydrochloride and 1% tropicamide and subsequent intraperitoneal injection of 0.1 mL of 2.5% fluorescein sodium (Akorn, Decatur, IL). The fluorescence intensity in the angiography was scored using a blinded manner (0, no staining; 1, slight leakage; 2, moderate leakage; and 3, prominent leakage) by two examiners with standardized photographs for each stage[22].
Quantitative Assessment of Choroidal Neovascularization Lesion Volumes
After being stained with fluorescein-labeled isolectin B4 (1:50; Vector Laboratories), RPE-choroid-sclera flat mounts were evaluated using a laser scanning confocal microscope (LSM510; Carl Zeiss, CA, USA). Z stack of images of CNV lesion were taken and rendered in 3D using Volocity imaging software (Improvision Inc., Waltham, USA) and processed to digitally extract the fluorescent lesion volume.
Histologic Analysis of Choroidal Neovascularization Lesions
On day 8 after laser treatment, the mice were euthanized with an overdose of pentobarbital sodium. Then the mouse eyes were enucleated and snap frozen in optimal cutting temperature (OCT) medium (Sakura Finetek USA, Inc., Torrance, CA, USA). Each eye was serially sectioned to look for each individual lesion. Once the lesion was determined at the start, 8 µmol/L sections were then cut through to the end of the lesion. It was then determined from the number of sections where the middle was. That particular slide was identified, fixed and selected for the staining with hematoxylin and eosin (H&E). Digital images were captured using a high resolution ScanScope whole slide scanner (CSO; Aperio, Vista, CA, USA). Aperio software (Images cope) was used to outline the CNV size and measure the CNV lesion area. The data was averaged per lesion.
Fluorescent Immunohistochemical Staining
Thawed tissue sections were air-dried and fixed with acetone, and then rinsed in phosphate buffered saline (PBS) for 10min. After blocked with 5% normal goat serum for 1h, the sections were incubated with primary antibodies (VEGFR2 #2479, 1:200; phospho-VEGFR2 #2478, 1:100; Cell Signaling, MA, USA) at 4°C overnight. After being washed three times in PBS, fluorescein-conjugated goat anti-rabbit IgG (1:500, A11034, Life technologies, CA, USA) was applied for 1h at room temperature. Mounting medium with propidium iodide (Vector Laboratories, CA, USA) was used for mounting and the immunoreactivities were revealed using a confocal microscope (LSM510; Zeiss, Thornwood, NY, USA). The staining of non phospho-VEGFR2 and phospho-VEGFR2 was quantified by spinning disk microscopy with the velocity software (Zeiss, NY, USA); the result was expressed as the percentage of positive staining area.
Statistical Analysis
All experiments were repeated at least three times. Data are shown as mean±SEM. Differences were analyzed by Student's t-test and P<0.05 was considered significant.
RESULTS
Potential Toxicity of Resveratrol on Cultured Bovine Choroidal Endothelial Cell
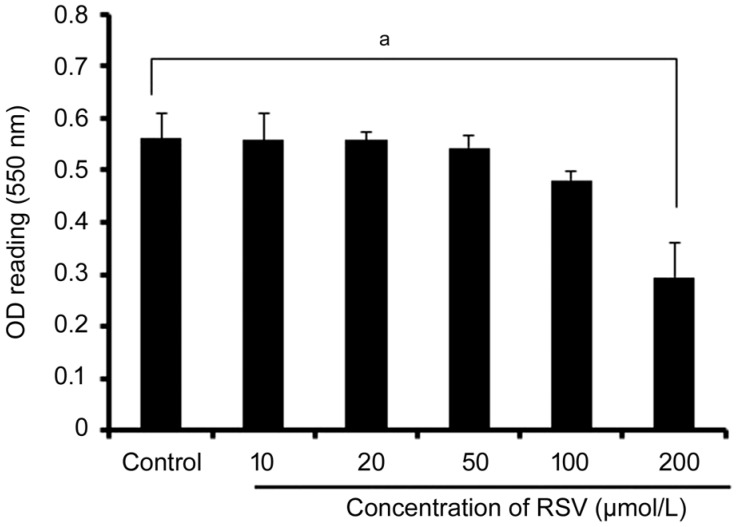
Toxicity was measured using MTT assay. Treatment the cells with 0, 10, 20, 50, 100, and 200 µmol/L RSV, there was no significant toxicity observed in the CEC cells if the concentration of RSV was lower than 200 µmol/L (Figure 1). However, the reduced cell survival and increased apoptosis (Figure 1) were revealed if the concentration of RSV reached to 200 µmol/L (P<0.05).
Figure 1. The effect of RSV on CEC cell survival.
The CEC cells were treated with various concentrations of RSV (0, 10, 20, 50, 100, and 200 µmol/L) for 48h. MTT assay was performed to evaluate the cell survival. No significant differences in OD reading values were seen at the concentration of RSV from 10 to 100 µmol/L compared with control without RSV application (P>0.05). The cell survival was reduced up to 200 µmol/L of RSV addition (aP<0.05). Values represent mean±SEM for 3 separate experiments (n=3).
Resveratrol Inhibits Endothelial Cell Migration and Tube Formation
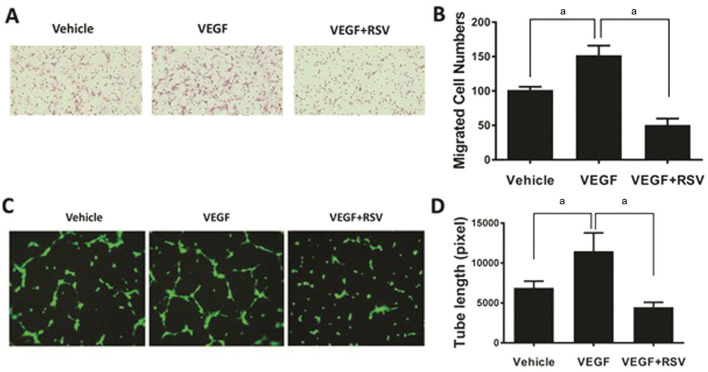
Increased migration of bovine CECs to the lower compartment of a Boyden chamber was displayed in response to VEGF stimulation (Figure 2A, 2B). While treatment with RSV significantly reduced the migration of CECs induced by VEGF (P<0.05; Figure 2A, 2B). The tube formation assay showed that the length of tubular networks was significantly increased upon VEGF treatment, whereas the increase was diminished in presence of RSV (P<0.05; Figure 2C, 2D).
Figure 2. RSV inhibits VEGF-induced CECs migration and tube formation.
The bovine CECs were used for cell migration assay (A) and tube formation assay (C). RSV showed a significant inhibition on cell migration as shown by hematoxylin stained cells on the lower side of the Boyden chamber membrane (B, aP<0.05) and the extent of tube formation after staining with Calcein-AM (green; D, aP<0.05) induced by VEGF (50 ng/mL). In tube formation assay, the result was obtained from 3 gel wells per condition in each experiment.
Suppression of Choroidal Neovascularization in Mice by Intravitreal Injection of Resveratrol
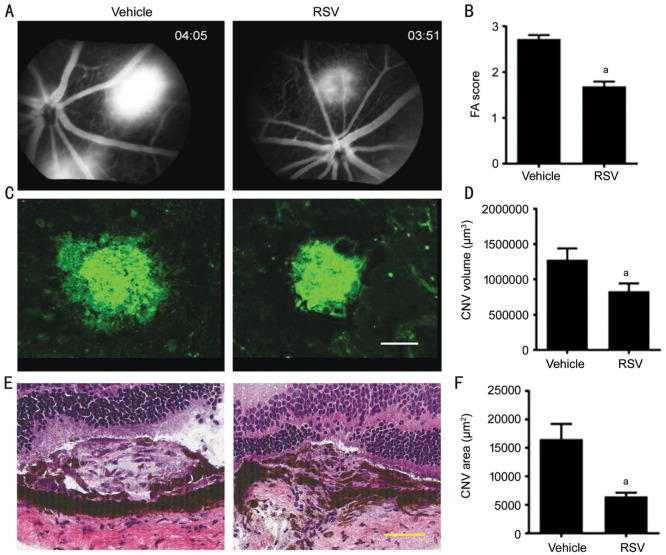
To study the effects of intravitreal injection of RSV on the development of laser-induced CNV in vivo, the extent of CNV was measured by three methods: fluorescein angiography (FA) in vivo, CNV volume by confocal analysis of choroidal flat mounts, and lesion area by histological analysis. At day 7 post-laser, the leakage was assessed by FA score, it was shown that the FA leakage was significantly lower in the RSV group (41% reduction, 1.691±0.1032) compared with control (2.724±0.08447, P<0.05; Figure 3A, 3B). CNV volume analysis revealed that there was a 45% reduction in choroidal lesion volume in RSV-treated eyes (178 163±78 623 µm3) compared with control (379 676±98 382 µm3, P<0.01; Figure 3C, 3D), which is coherent with the FA analysis. The CNV sections stained with H&E showed that RSV treatment resulted in a significant decrease in lesion area (58% reduction, 6508±619.0 µm2) compared with control (16 576±2646 µm2; P<0.05; Figure 3E, 3F).
Figure 3. Intravitreal injection of RSV inhibits laser-induced CNV in mice.
Mice underwent retinal laser photo-coagulation in both eyes and intravitreal injection with RSV (2 µL, 100 µmol/L) or vehicle (0.1% DMSO) directly immediately after laser and on day 3. Fluorescein angiograms (FA) were performed at day 7 after laser surgery. On day 8, the eyes of euthanized mice were evaluated for histology and CNV volume analysis. RSV displayed an inhibitory effect on CNV formation, which was demonstrated by FA (A, B; aP<0.05); A, B: The results were from vehicle vs RSV; CNV volume analysis using isolectin B4-stained choroidal flat mounts (C, D; Scale bar, 100 µm; aP<0.05). C, D: The results were obtained from vehicle vs RSV: CNV lesion area on hematoxylin & eosin stained sections (E, F; Scale bar, 50 µm; aP<0.05), which include vehicle vs RSV.
Resveratrol Inhibits VEGFR2 Phosphorylation in Choroidal Neovascularization Lesions
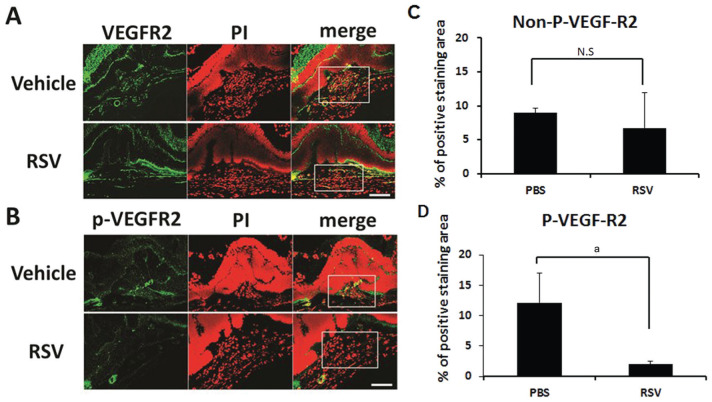
Immunostaining of CNV lesion sections demonstrated that total VEGFR2 expression in RSV-treated eyes was similar to control (Figure 4A). However, phospho-VEGFR2 expression in CNV lesion in RSV injected mice were much weaker compared with lesions in control mice (Figure 4B). Quantification showed that the phospho-VEGFR2 was significantly suppressed by the injection of RSV compared to vehicle application in CNV lesion (P<0.05; Figure 4D). There was no statistic difference in the expression of total VEGFR2 in RSV treated group and control (P>0.05; Figure 4C).
Figure 4. RSV inhibits VEGFR2 phosphorylation in CNV lesions.
Confocal immunofluorescent images of CNV lesion sections showed no significant difference in the total VEGFR2 (green) expression between RSV injection and control (A, C; P>0.05) but phospho-VEGFR2 (green) expression was much weaker in RSV injected mice compared with controls (B, D; aP<0.05). Nuclear counterstain (red) obtained using propidium iodide (PI). CNV lesion area in the sections is indicated by white rectangle. Scale bar: 100 µm.
DISCUSSION
RSV has been of interest for the treatment of ocular disease because of its anti-oxidant, anti-apoptotic, anti-inflammatory, and anti-angiogenic properties[20]. In the current study, we found that treatment with RSV inhibits the cell migration and tube formation of CECs induced by VEGF which is relevant to VEGF-driven angiogenesis as occurs in CNV, comparing with other studies reported that RSV inhibits baseline CEC tube formation in the absence of VEGF[17]. Because cell migration and tube formation are crucial pathological events in the development of new vessels, inhibition cell migration and tube formation is able to suppress the pathological process of angiogenesis. Thus, based on these studies in vitro, we attempted to demonstrate the findings in vivo.
In the current study, we delivered RSV directly into the vitreous, a method of administration by which it is easy to control the concentration of the drug and ensure that the drug will be available to act directly on the retina. Our study demonstrated the effect of RSV on murine CNV using three methods: FA, lesion volume and lesion histology. It is also the first to evaluate the effect of intravitreal administration of RSV. Our results showed significant inhibitory effects of intravitreal FA leakage (41%), CNV volume (45%) and area (58%) compared with control, which is in the same range with previous reports when RSV is given by systemic administration[12],[15]–[17]. Previous studies have showed that RSV may have either pro- or anti-angiogenic effects[16],[18], including CNV animal models in vivo. For example, it was shown that pretreatment with RSV by oral gavage can suppress neovascularization in a CNV mouse model[12]. In addition, osmotic pumps have been used in other experimental CNV[15]. Notably, systemic administration of RSV may have cause sides effect or controversy in term of pro-angiogenesis action[18], promote tumor growth[25] and abnormal of estrogen production[26], although there is no report regarding the sides effects of application of RSV in the eye. Therefore, the route and rate of administration as well as the dosage of RSV determine the outcome of systemic treatment in experimental CNV, and these findings reflect the difficulty of controlling neovascularization when administering RSV by the oral route[17]. Additionally, there is the complication of low bioavailability of oral RSV in humans[19] and uncertain distribution and concentration of RSV in the eye.
It is recognized that VEGFR2 is a major receptor in the induction of angiogenesis, in the current study, we found that phospho-VEGFR2 expression was much weaker in sections of CNV lesions after injection of RSV, while, there were no apparent changes in the expression of total VEGFR2, suggesting that decreased VEGFR2 phosphorylation might play an important role in RSV suppression of CNV. Our and others' previous studies[27]–[29] showed that RSV derivatives exert their anti-angiogenic effects through blockage of the phosphorylation of VEGFR2 and thereby suppress the signaling pathway mediated by VEGFR2 in vitro; our present study provides the first evidence that this may be a mechanism for the anti-angiogenic effect of RSV on CNV in vivo. We showed that intravitreal injection of RSV inhibited pathological angiogenesis in murine laser-induced CNV model. Suppression of VEGFR2 phosphorylation could be one of the mechanisms in which RSV inhibits CNV development.
In term of the frequency of the RSV application, it is shown that RSV is rapidly metabolized, the plasma half-life times of RSV were 9.2h after oral administration and 11.4h after intravenous injection in human[19]. There is no publication about RSV vitreous half-life reported. However, eye is relatively isolated from systemic circulation, RSV might be sustained for longer time than other systemic administrations after vitreous injection, therefore, the effects on local lesion might be more effectively. In addition, the previous study shows that localized accumulation of RSV and its potentially active metabolites may still sustain cardiovascular disease and cancer-preventive effects, although the systemic bioavailability of RSV is very low. We performed RSV (100 µmol/L) intravitreal injection immediately after the laser procedure and on post-laser day 3. Our result showed that the CNV formation was inhibited significantly, suggesting the frequency and doses of the RSV injection were appropriate. However, the metabolism and the pharmacokinetics of RSV intravitreal injection as well as different administration routes, doses, patterns, and safety need to be further studied.
To the best of our information, the current study is the first report showing that intravitreal injection of RSV inhibits laser-induced murine CNV and this treatment is associated with a reduction of phospho-VEGFR2 expression in the CNV lesions. These data add novel evidence that local delivery of RSV may be a potential approach for the neovascular AMD therapy.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81703134; No.81770952); Henan Province Nature Science Foundation of China (No.162300410296); Hunan Province Nature Science Foundation of China (No.2018JJ3772).
Conflicts of Interest: Zhang HM, None; Li XH, None; Chen M, None; Luo J, None.
REFERENCES
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Shao J, Choudhary MM, Schachat AP. Neovascular age-related macular degeneration. Dev Ophthalmol. 2016;55:125–136. doi: 10.1159/000438969. [DOI] [PubMed] [Google Scholar]
- 3.Sun XD, Yang SQ, Zhao JK. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: a comprehensive review. Drug Des Dev Ther. 2016:1857. doi: 10.2147/DDDT.S97653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eandi CM, Alovisi C, de Sanctis U, Grignolo FM. Treatment for neovascular age related macular degeneration: the state of the art. Eur J Pharmacol. 2016;787:78–83. doi: 10.1016/j.ejphar.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018;6(3):E91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guthrie AR, Chow HS, Martinez JA. Effects of resveratrol on drug- and carcinogen-metabolizing enzymes, implications for cancer prevention. Pharmacol Res Perspect. 2017;5(1):e00294. doi: 10.1002/prp2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lançon A, Frazzi R, Latruffe N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules. 2016;21(3):304. doi: 10.3390/molecules21030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol. 2017;15(6):503–519. doi: 10.2174/1570161115666170713094319. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Amero K, Kondkar A, Chalam K. Resveratrol and ophthalmic diseases. Nutrients. 2016;8(4):200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaiya S, Murthy RK, Chalam KV. Resveratrol inhibits proliferation of hypoxic choroidal vascular endothelial cells. Mol Vis. 2013;19:2385–2392. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CS, Choi EY, Lee SC, Koh HJ, Lee JH, Chung JH. Resveratrol inhibits hypoxia-induced vascular endothelial growth factor expression and pathological neovascularization. Yonsei Med J. 2015;56(6):1678–1685. doi: 10.3349/ymj.2015.56.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai N, Kubota S, Tsubota K, Ozawa Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J Nutr Biochem. 2014;25(11):1218–1225. doi: 10.1016/j.jnutbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012;90(1):e31–e37. doi: 10.1111/j.1755-3768.2011.02243.x. [DOI] [PubMed] [Google Scholar]
- 14.Hua J, Guerin KI, Chen J, Michán S, Stahl A, Krah NM, Seaward MR, Dennison RJ, Juan AM, Hatton CJ, Sapieha P, Sinclair DA, Smith LE. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(-/-) mice. Invest Ophthalmol Vis Sci. 2011;52(5):2809–2816. doi: 10.1167/iovs.10-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AA, Dace DS, Ryazanov AG, Kelly J, Apte RS. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am J Pathol. 2010;177(1):481–492. doi: 10.2353/ajpath.2010.090836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheu SJ, Liu NC, Ou CC, Bee YS, Chen SC, Lin HC, Chan JY. Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2013;54(9):6426–6438. doi: 10.1167/iovs.13-12024. [DOI] [PubMed] [Google Scholar]
- 17.Kanavi MR, Darjatmoko S, Wang SJ, Azari AA, Farnoodian M, Kenealey JD, van Ginkel PR, Albert DM, Sheibani N, Polans AS. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules. 2014;19(11):17578–17603. doi: 10.3390/molecules191117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Tseng SH. Review. Pro- and anti-angiogenesis effects of resveratrol. In Vivo. 2007;21(2):365–370. [PubMed] [Google Scholar]
- 19.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 20.Bola C, Bartlett H, Eperjesi F. Resveratrol and the eye: activity and molecular mechanisms. Graefes Arch Clin Exp Ophthalmol. 2014;252(5):699–713. doi: 10.1007/s00417-014-2604-8. [DOI] [PubMed] [Google Scholar]
- 21.Peyman GA, Lad EM, Moshfeghi DM. Intravitreal injection of therapeutic agents. Retina. 2009;29(7):875–912. doi: 10.1097/IAE.0b013e3181a94f01. [DOI] [PubMed] [Google Scholar]
- 22.He SK, Ding Y, Zhou JH, Krasnoperov V, Zozulya S, Kumar SR, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2005;46(12):4772–4779. doi: 10.1167/iovs.05-0502. [DOI] [PubMed] [Google Scholar]
- 23.Giani A, Thanos A, Roh MI, Connolly E, Trichonas G, Kim I, Gragoudas E, Vavvas D, Miller JW. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(6):3880–3887. doi: 10.1167/iovs.10-6266. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizumi MO, Niizawa JM, Meyers-Elliott R. Ocular toxicity of intravitreal vidarabine solubilized in dimethyl sulfoxide. Arch Ophthalmol. 1986;104(3):426–430. doi: 10.1001/archopht.1986.01050150128043. [DOI] [PubMed] [Google Scholar]
- 25.Lin L, Zheng XX, Qiu CP, Dongol S, Lv Q, Jiang J, Kong BH, Wang CG. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol Rep. 2014;32(6):2831–2835. doi: 10.3892/or.2014.3521. [DOI] [PubMed] [Google Scholar]
- 26.Bhat KPL, Kosmeder JW, 2nd, Pezzuto JM. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3(6):1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 27.Alex D, Leong EC, Zhang ZJ, Yan GT, Cheng SH, Leong CW, Li ZH, Lam KH, Chan SW, Lee SM. Resveratrol derivative, trans-3, 5, 4′-trimethoxystilbene, exerts antiangiogenic and vascular-disrupting effects in zebrafish through the downregulation of VEGFR2 and cell-cycle modulation. J Cell Biochem. 2010;109(2):339–346. doi: 10.1002/jcb.22405. [DOI] [PubMed] [Google Scholar]
- 28.Chen LK, Qiang PF, Xu QP, Zhao YH, Dai F, Zhang L. Trans-3, 4, 5, 4′-tetramethoxystilbene, a resveratrol analog, potently inhibits angiogenesis in vitro and in vivo. Acta Pharmacol Sin. 2013;34(9):1174–1182. doi: 10.1038/aps.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang HM, He SK, Spee C, Ishikawa K, Hinton DR. SIRT1 mediated inhibition of VEGF/VEGFR2 signaling by Resveratrol and its relevance to choroidal neovascularization. Cytokine. 2015;76(2):549–552. doi: 10.1016/j.cyto.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]