Abstract
Ischemic and neovascular disease is one of the most difficult ocular diseases to deal with nowadays. Redundancy, poor visual acuity and decreased life quality are bothering patients and ophthalmologists for decades. After vascular endothelial growth factor (VEGF) was found to be a primary factor in promoting retinal angiogenesis, intravitreal injection of anti-VEGF drugs has been the first-line treatment. Whereas, some patients are refractory to this therapy and problems of economic burden, local complications and adverse effects promote researches into other possible targets. The vasohibin (VASH) family is a newly-investigated factor in modulating ocular angiogenesis. The family includes VASH1 and VASH2, which show opposite effects of inhibiting and accelerating angiogenesis respectively. Positive results have been reported in cellular and animal experiments. With further researches, it can be a promising future target of treating ocular neovascular diseases.
Keywords: vasohibin, ocular neovascularization, retinal neovascularization
INTRODUCTION
Normally, retinal tissue is protected by blood-retinal barrier (BRB) and separated from blood constituents of capillaries. In pathological conditions, retinal vasculature undergoes abnormal changes, leading to increased retinal vascular permeability (RVP), exudation, hemorrhages, capillary closure, non-perfusion area and neovascularization induced by angiogenic factors. However, new formed vessels are more fenestrated and lack normal tight junctions. Leaked blood can block vision, cause vitreous degeneration, form fibrous membranes and pull on the retina[1].
The process of neovascularization is one of the most difficult and complicated ocular pathological changes. A myriad of ocular diseases are characterized of excess angiogenesis. The most common ones are diabetic retinopathy (DR), retinal vein occlusion (RVO) and wet age-related macular disease (wAMD). Macular edema (ME), accumulation of sub- and intra-retinal fluid in macular area, is one major redundant complication and a result of damaged BRB and leakage of neovascular vessels. Due to distorted macular structure, visual acuity is largely affected and patients often complain of metamorphopsia. Currently, no modality is able to cure ME radically and repeated treatments are required. At times, neovascularization can develop very quickly and get out of control. Neovascular glaucoma (NVG) is one of the late stage complications of DR and RVO[2]. It causes huge distress on patients and is thought to be treated with anti-glaucoma medications and surgical interventions. Less common ocular neovascular diseases are high myopia and retinopathy of prematurity (ROP). These diseases cause severe vision loss and interfere patients' life quality to a large extent.
VASCULAR ENDOTHELIAL GROWTH FACTOR
Angiogenesis is a process being meticulously regulated and balanced by pro-angiogenic and anti-angiogenic factors. A number of angiogenic stimulators have been researched such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), interleukin 8 (IL-8) and etc[3]–[4]. The most important and fully-investigated factor is vascular endothelial growth factor (VEGF). It is produced not only by endothelial cells, but retinal neurons, Müller cells and retinal pigment epithelium (RPE)[5]–[6]. VEGF is considered as a primary element in physiological angiogenesis and survival of vessels[7]. In pathological situations such as hypoxia, which is a proven stimulus for VEGF, VEGF abundantly expresses and upregulates angiogenesis to supply enough oxygen for tissues. In addition, it attracts leukocytes to vessel walls and initiates inflammation[3]. Therefore, it is believed to be the most primary factor contributing to ocular neovascularization. Its receptor VEGFR-2 has been found to express mainly on vascular endothelial cells, activating endothelial cells to proliferate and migrate, promoting angiogenesis and increasing RVP, which is the basic change in angiogenesis[8]. Increased levels of VEGF has been reported in vitreous body and fibrovascular membranes of proliferative diabetic retinopathy (PDR) patients, suggesting its involvement in ocular vascular diseases[9]–[10].
Anti-VEGF Therapy
Anti-VEGF therapy has been the primary and first-line modality in treating ocular neovascular diseases, including ME associated with DR and RVO, choroidal neovascularization (CNV) secondary to wAMD and high myopia, iris neovascularization, NVG, ROP and before vitrectomy. It functions through blocking VEGF and suppressing VEGF-induced neovascularization. Its efficacy has been proved clinically: quick regression of neovascularization and ME. Without treatment, vision acuities of patients persist dropping, and severe complications may occur for DR and RVO patients. The disadvantages of the utility of anti-VEGF drugs should not be neglect neither: economic burden, recurrent intravitreal injections, and refractoriness or unresponsiveness to treatments showed by some patients. Increased fibrosis, traction on the retina and tractional retinal detachment due to angio-fibrotic effect of VEGF, macular atrophy and rare cases of endophthalmitis have been reported as regional complications[3],[8],[10]. VEGF is a significant molecule in remaining the normal structure and function of vasculatures. Persistent use of anti-VEGF drugs may pose a threat on normal vessels and aggravate pre-existed ischemia. Increased foveal avascular zone (FAZ) was found in a DR patient after intravitreal injection of bevacizumab[11]. Systemic side effects of local anti-VEGF uses are rarely reported. However, it was proven that serum VEGF and VEGF in the other eye also decreased after intravitreal injections and diabetic macular edema (DME) of the other eye improved[12]–[16]. Considering the threats caused by systemic use of anti-VEGF drugs in treating cancer, long-term and repeated intravitreal injections may increase the risk of cardiovascular accidents such as strokes and embolisms[3].
VASOHIBIN FAMILY
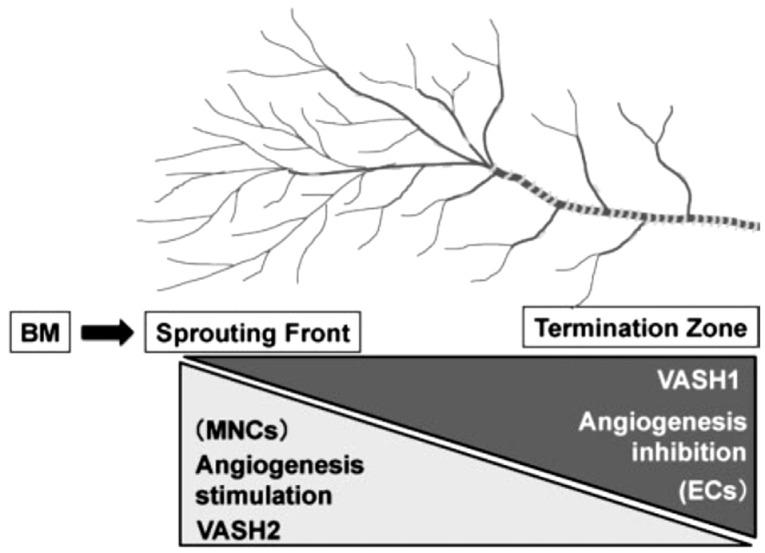
Therefore, looking for new targets of treating ocular neovascularization diseases is necessary. Recently the vasohibin family has been found to be one of the factors in modulating angiogenesis. There are two homologues in the family—vasohibin1 (VASH1) and vasohibin2 (VASH2). They were first isolated and designated by Watanabe et al[17]. VASH1 is a secretory protein composed of 365 amino acids and it is induced by VEGF through protein kinase C (PKC)[18]. Its content is obvious in embryos of human, but reduces gradually[18]–[19]. Though it has been found to express in many organs and tissues such as bone marrow, it preferentially accumulates in endothelium[19]–[20]. It functions as an intrinsic angiogenic inhibitor and expresses in the terminations of angiogenesis to halt the process (Figure 1)[7],[18]. Plenty immature vessels are found in VASH1 (-/-) mouse models[21]. VASH1 also plays a role in maintaining the homeostasis of endothelial cells via regulating enzymes to antagonize reactive oxygen species (ROS) and aging to enhance resistance to stresses[22]–[23]. It is proved to be induced by VEGF and FGF and the induction is interfered in circumstances of hypoxia and inflammation[7],[24]. Its function in cancers has been thoroughly investigated. Its expression limits in vascular endothelium of tumors instead of normal tissues and it takes part in inhibiting angiogenic process and normalizing abnormal vessels as well[4],[25]. It has already been considered as a new marker of tumor angiogenesis and prognosis due to its level is positively correlated with the tumor node metastasis (TNM) stage and metastasis of colorectal cancer[22],[25]–[26].
Figure 1. Function of VASH1 and VASH2 in angiogenesis[7].
VASH1 mainly expresses in the termination of angiogenesis to stop the process. VASH2, however, expresses in the sprouting front of angiogenesis to stimulate the process. BM: Bone marrow; MNC: Mononuclear cell; EC: Endothelial cell.
VASH2 is made up of 355 amino acids with a 52.5% similarity with VASH1, but it seldom expresses in endothelial cells, but rather in mononuclear cells and acts as an angiogenic stimulator at the sprouting front of angiogenesis (Figure 1)[7],[18]. It can stimulate tumor cells to proliferate, migrate and infiltrate. Instead, growth of tumor is suppressed and abnormal vessels normalize with the decrease of VASH2[22],[27]. Additionally, VASH2 can decrease the level of p53 and inhibit the expression of Bax, suggesting its role in tumor development and anti-apoptosis[22].
Vasohibin Family in Ocular Models
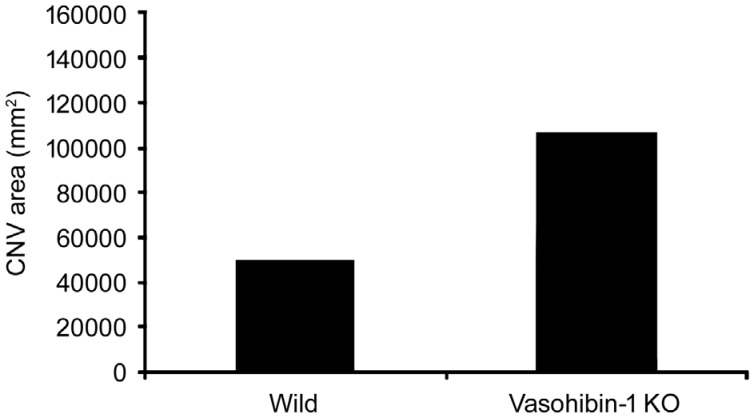
VASH1 has been found in choroidal vessels, retinal vessels and RPE cells in normal mouse eyes[28]. In DR and AMD, its expression in endothelial cells clearly rises[18]. Also, Arnold et al[29] reported that accumulation of VASH1 in retina caused by hyperprolactinemia led to improvement on increased RVP caused by VEGF and DR. In mouse models of oxygen-induced ischemic retinopathy, expression of VASH1 increases in vasoendothelial cells and VASH1-siRNA aggravates retinal neovascularization in ischemic retina, indicating VASH1 is partly responsible for inhibiting angiogenesis[30]. In animal models of laser-induced CNV, intravitreal injection of VASH1 reduces fluorescein leakage and decreases the range and activity of CNV[31]. Vice versa, VASH1 knock-down mice shows larger size of CNV (Figure 2)[32]. Current studies unanimously testify anti-angiogenic function of VASH1 and positive results of improved retinal neovascularization after VASH1 treatment.
Figure 2. Size of CNV lesions in eyes of wild type or VASH1 knock-down mice[32].
CNV area in VASH1 knock-down mice was obviously larger than wild type mice. P<0.0001.
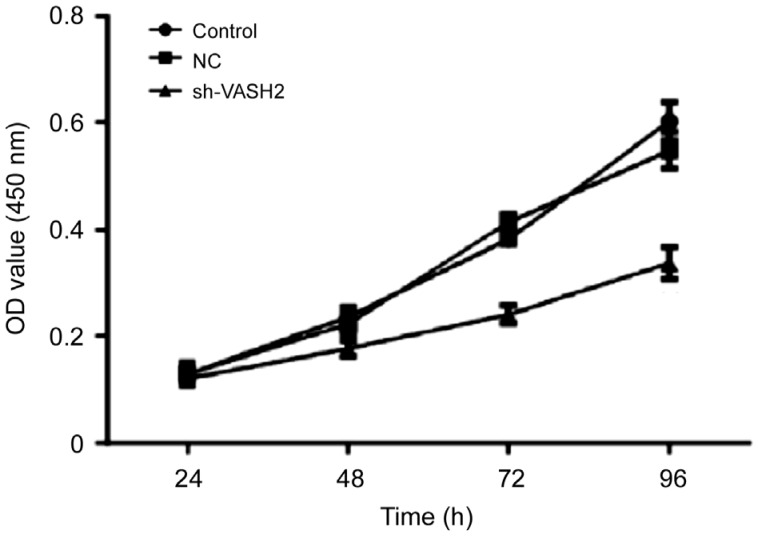
In vitro, VASH2 knock-down by miR-200b/c inhibits cellular growth, migration and activity of human retinal microvascular endothelial cells (HMVEC; Figure 3)[10]. This indicates that suppressing VASH2 can assist in inhibiting neovascularization of retinal vessels. Clinically, in fibrovascular membranes collected from DR patients, vasoendothelial cells widely express VASH2, showing its participation in retinal neovascularization[10]. Suzuki et al[33] reported that VASH2 can promote accumulation and migration of fibroblasts in vitro. Perhaps fibrosis in vitreous hemorrhage patients is partly aggravated by VASH2. In addition, VASH2 facilitates the expression of VEGF and FGF-2 and it cannot be induced by VEGF or other growth factors and cytokines[7],[22]. Therefore, anti-VASH2 may have a synergic function with anti-VEGF therapy clinically. However, when VASH2 was used in the cornea of mice, angiogenesis was inhibited, which is contradictory to previous studies[19]. Hence, the function of VASH2 in eyes needs further research. The discrepancy may be related with different ocular site and pathological condition.
Figure 3. CCK8 assay results in VASH2 knock-down HMVEC[10].
Compared with control groups, level of cell proliferation decreased after VASH2 was knocked down in HMVEC. NC: Nonspecific control; OD: Optic density. P<0.01 in 48, 72, and 96h.
The vasohibin family is a newly-researched family of molecules regulating angiogenesis. Although its structure, location, function and mechanism have been studied a lot in malignant tumors, its role in physiological and pathological circumstances of eyes has not been fully and deeply investigated. One common and primary mechanism behind cancers and ocular neovascularization diseases is neovascularization. Its positive function in inhibiting cancers suggests that vasohibin can be a promising target for treating ocular ischemic diseases as well. Two features of vasohibin should be highlighted. The first characteristic is that vasohibin can antagonize angiogenic process induced by several factors other than VEGF[4],[7]. It has been reported that the intraocular level of some cytokines and factors were meaningfully higher in DME and wAMD patients[34]–[35]. Watanabe et al[17] and Heishi et al[36] both found out that vasohibin could suppress the angiogenic function of VEGF and other factors such as PDGF and FGF to some degree. Hence, multiple targets of vasohibin becomes vital. Its function may cover more widely and strengthen the effect of anti-VEGF drugs. The second attribute is that vasohibin selectively reacts with VEGFR-2[30]–[31]. Currently-used anti-VEGF medications such as bevacizumab and ranibizumab antagonize VEGF directly and show no selection on the receptors[8]. Compared with them, vasohibin can neglect VEGFR-1 to avoid interfering its neuroprotective function[31]. Whereas, detailed mechanism of vasohibin function pathway, efficacy of inhibiting ocular neovascularization and adverse effects need to be further explored to clarify the feasibility and value of its clinical utility.
CONCLUSION
The vasohibin family is a promising future target as a novel choice for dealing with ocular neovascularization. It may exert a synergetic effect with current anti-VEGF therapy with a wider action range, selective function on VEGF receptors and less adverse effects.
Acknowledgments
Foundations: Supported by Natural Science Fund Project in Jiangsu Province (No.BK20180380); Nanjing Health Commission (No.YKK18260).
Conflicts of Interest: Hu XN, None; Ni Y, None; Luan J, None; Ding YZ, None.
REFERENCES
- 1.Campochiaro PA. Ocular neovascularization. J Mol Med. 2013;91(3):311–321. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havens SJ, Gulati V. Neovascular glaucoma. Dev Ophthalmol. 2016;55:196–204. doi: 10.1159/000431196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pożarowska D, Pożarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol. 2016;41(3):311–316. doi: 10.5114/ceji.2016.63132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi Y, Saga Y, Koyanagi T, Takei Y, Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S, Fujiwara H. Vasohibin-1 expression inhibits advancement of ovarian cancer producing various angiogenic factors. Cancer Sci. 2016;107(5):629–637. doi: 10.1111/cas.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Chalam KV, Brar VS, Murthy RK. Human ciliary epithelium as a source of synthesis and secretion of vascular endothelial growth factor in neovascular glaucoma. JAMA Ophthalmol. 2014;132(11):1350–1354. doi: 10.1001/jamaophthalmol.2014.2356. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y. The vasohibin family: a novel family for angiogenesis regulation. J Biochem. 2013;153(1):5–11. doi: 10.1093/jb/mvs128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 2014;28(5):510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato H, Abe T, Wakusawa R, Asai N, Kunikata H, Ohta H, Sonoda H, Sato Y, Nishida K. Vitreous levels of vasohibin-1 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Diabetologia. 2009;52(2):359–361. doi: 10.1007/s00125-008-1229-z. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Hu Z, Luan J, Lv X, Yuan D, Xie P, Yuan S, Liu Q. Protective effect of miR-200b/c by inhibiting vasohibin-2 in human retinal microvascular endothelial cells. Life Sci. 2017;191:245–252. doi: 10.1016/j.lfs.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Koh HJ. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (avastin) with pars Plana vitrectomy. J Ocul Pharmacol Ther. 2009;25(2):173–174. doi: 10.1089/jop.2008.0092. [DOI] [PubMed] [Google Scholar]
- 12.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Le K, Maia M, Visich JE. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98(12):1636–1641. doi: 10.1136/bjophthalmol-2014-305252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158(4):738–744.e1. doi: 10.1016/j.ajo.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yao Z, Kaila N, Kuebler P, Visich J, Maia M, Tuomi L, Ehrlich JS, Rubio RG, Campochiaro PA. Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema. Ophthalmology. 2014;121(11):2237–2246. doi: 10.1016/j.ophtha.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Bakbak B, Ozturk BT, Gonul S, Yilmaz M, Gedik S. Comparison of the effect of unilateral intravitreal bevacizumab and ranibizumab injection on diabetic macular edema of the fellow eye. J Ocul Pharmacol Ther. 2013;29(8):728–732. doi: 10.1089/jop.2013.0049. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama K, Ogata N, Matsuoka M, Wada M, Nishimura T, Takahashi K. Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharmacol Ther. 2011;27(4):379–383. doi: 10.1089/jop.2010.0194. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114(7):898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato Y. The vasohibin family. Pharmaceuticals. 2010;3(2):433–440. doi: 10.3390/ph3020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibuya T, Watanabe K, Yamashita H, Shimizu K, Miyashita H, Abe M, Moriya T, Ohta H, Sonoda H, Shimosegawa T, Tabayashi K, Sato Y. Isolation and characterization of vasohibin-2 as a homologue of VEGF-inducible endothelium-derived angiogenesis inhibitor vasohibin. Arterioscler Thromb Vasc Biol. 2006;26(5):1051–1057. doi: 10.1161/01.ATV.0000216747.66660.26. [DOI] [PubMed] [Google Scholar]
- 20.Naito H, Kidoya H, Sato Y, Takakura N. Induction and expression of anti-angiogenic vasohibins in the hematopoietic stem/progenitor cell population. J Biochem. 2009;145(5):653–659. doi: 10.1093/jb/mvp021. [DOI] [PubMed] [Google Scholar]
- 21.Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T, Sato Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2009;113(19):4810–4818. doi: 10.1182/blood-2008-07-170316. [DOI] [PubMed] [Google Scholar]
- 22.Du H, Zhao J, Hai L, Wu J, Yi H, Shi Y. The roles of vasohibin and its family members: Beyond angiogenesis modulators. Cancer Biol Ther. 2017;18(11):827–832. doi: 10.1080/15384047.2017.1373217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda E, Suzuki Y, Sato Y. Age-associated downregulation of vasohibin-1 in vascular endothelial cells. Aging Cell. 2016;15(5):885–892. doi: 10.1111/acel.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, Sonoda H, Sato Y. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem Biophys Res Commun. 2005;327(3):700–706. doi: 10.1016/j.bbrc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 25.Hosaka T, Kimura H, Heishi T, Suzuki Y, Miyashita H, Ohta H, Sonoda H, Moriya T, Suzuki S, Kondo T, Sato Y. Vasohibin-1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am J Pathol. 2009;175(1):430–439. doi: 10.2353/ajpath.2009.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi G, Hao J, Wang L, He B, Gan J, Wang H, Suo R. Expression of vasohibin-1 in colorectal cancer tissue and its correlation with vascular endothelial growth factor A and microvessei density. Chin J Gastrointest Surg. 2015;18(3):272–276. [PubMed] [Google Scholar]
- 27.Koyanagi T, Suzuki Y, Komori K, Saga Y, Matsubara S, Fujiwara H, Sato Y. Targeting human vasohibin-2 by a neutralizing monoclonal antibody for anti-cancer treatment. Cancer Sci. 2017;108(3):512–519. doi: 10.1111/cas.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakusawa R, Abe T, Sato H, Yoshida M, Kunikata H, Sato Y, Nishida K. Expression of vasohibin, an antiangiogenic factor, in human choroidal neovascular membranes. Am J Ophthalmol. 2008;146(2):235–243. doi: 10.1016/j.ajo.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Arnold E, Rivera JC, Thebault S, Moreno-Páramo D, Quiroz-Mercado H, Quintanar-Stéphano A, Binart N, Martínez de la Escalera G, Clapp C. High levels of serum prolactin protect against diabetic retinopathy by increasing ocular vasoinhibins. Diabetes. 2010;59(12):3192–3197. doi: 10.2337/db10-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Yang X, Xiao WH, Hackett SF, Sato Y, Campochiaro PA. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20(6):723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 31.Onami H, Nagai N, Machida S, Kumasaka N, Wakusawa R, Ishikawa Y, Sonoda H, Sato Y, Abe T. Reduction of laser-induced choroidal neovascularization by intravitreal vasohibin-1 in monkey eyes. Retina. 2012;32(6):1204–1213. doi: 10.1097/IAE.0b013e318233ad0b. [DOI] [PubMed] [Google Scholar]
- 32.Wakusawa R, Abe T, Sato H, Sonoda H, Sato M, Mitsuda Y, Takakura T, Fukushima T, Onami H, Nagai N, Ishikawa Y, Nishida K, Sato Y. Suppression of choroidal neovascularization by vasohibin-1, a vascular endothelium-derived angiogenic inhibitor. Invest Ophthalmol Vis Sci. 2011;52(6):3272–3280. doi: 10.1167/iovs.10-6295. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Kitahara S, Suematsu T, Oshima M, Sato Y. Requisite role of vasohibin-2 in spontaneous gastric cancer formation and accumulation of cancer-associated fibroblasts. Cancer Sci. 2017;108(12):2342–2351. doi: 10.1111/cas.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon JW, Jee D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS One. 2018;13(9):e0203408. doi: 10.1371/journal.pone.0203408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato T, Takeuchi M, Karasawa Y, Enoki T, Ito M. Intraocular inflammatory cytokines in patients with neovascular age-related macular degeneration before and after initiation of intravitreal injection of anti-VEGF inhibitor. Sci Rep. 2018;8(1):1098. doi: 10.1038/s41598-018-19594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M, Yamauchi C, Ohta H, Sonoda H, Sato Y. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176(4):1950–1958. doi: 10.2353/ajpath.2010.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]