Abstract
According to the recent report, there are 870 million people suffer from ocular diseases worldwide. The present approaches for diagnosis are morphological examination, imaging examination and immunological examination, regrettably, they lack of sensitivity and difficult to make a definite diagnosis in the early stage. Systemic biology as an effective method has been used in clinical diagnosis and treatment for diseases, especially metabolomics which is more attractive with high sensitivity and accuracy. Although previous researches had been confirmed that endogenous metabolites in the ocular matrix play a crucial role in the progress of diseases related diseases, the standard protocols and systematic summary about the biomarker researches based on ocular matrix has not been established. This review article highlights the pretreatment for ocular matrix and the new biomarkers expressed by the eye diseases, expected to promote the application of biomarkers in the diagnosis and treatment of eye diseases.
Keywords: metabolomics, biomarkers, ocular matrix, ocular diseases, diagnosis
INTRODUCTION
Eye is a complex sensory organ that can be divided into anterior part composed of cornea, conjunctiva, aqueous humor, iris, ciliary body and lens, the posterior part composed of sclera, choroid, retina and vitreous humor (VH). It is able to receive light and convert it into electrical impulses which are transmitted to the brain through the optic nerve for visual perception[1]. Various injuries in the above ocular matrices will result in different ocular diseases companied with impaired vision function, moreover, systemic diseases such as diabetes[2], Alzheimer's disease[3] and inflammatory bowel disease[4] may also cause damages in ocular matrix. Morphological, imaging, and immunological examination are the major diagnosis approaches for ocular diseases. However, when there is a significant change in the structure and function of the eye, the diseases have progressed to an irreversible stage. What's more, affected individuals experience different clinical appearance and progression of the diseases[5]. Given the difficulty in diagnosis and treatment for ocular diseases, there is an urgent need to develop an effective tool.
With the rapid development of analytical technology and bioinformatics and the concept of precision medicine rooted deeply in the people's mind, systems biology which consist of genomics, proteomics, transcriptomics and metabolomics offers a powerful tool to simulate metabolic reactions in the biological system. Metabolomics is a rapidly evolving field of biochemical research following genomics, transcriptomics and proteomics, was defined as global analysis of the small-molecule metabolites present within the internal environment in an identified and quantified manner[6]. Endogenous metabolites as products or substrates in the process of in vivo metabolism, are jointly influenced by gene, environment and daily diet habits and involved in the organism homeostasis that can act as biomarkers to indicate the normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention[7]. In other words, biomarker is conducive to more deeply understand the in vivo abnormal mechanism and can be used to evaluate the course of disease[8]–[10]. At present, biomarker has been applied in prevention, diagnosis and treatment of cancer and diabetes[11]–[12].
Over the last years, increasing number of metabolomics analysis in ocular matrix have performed, and gained a pivotal role in comprehending and explaining ocular diseases and systemic diseases. Nevertheless, it still remains problems in research procedure standardization and the clinical conversion of the achievement. Herein, this article will provide an overview of the pretreatment of ocular matrix and the biomarkers related to ocular diseases and systemic diseases, with a view to further study and application of ocular matrix.
Sample Collection and Pretreatment
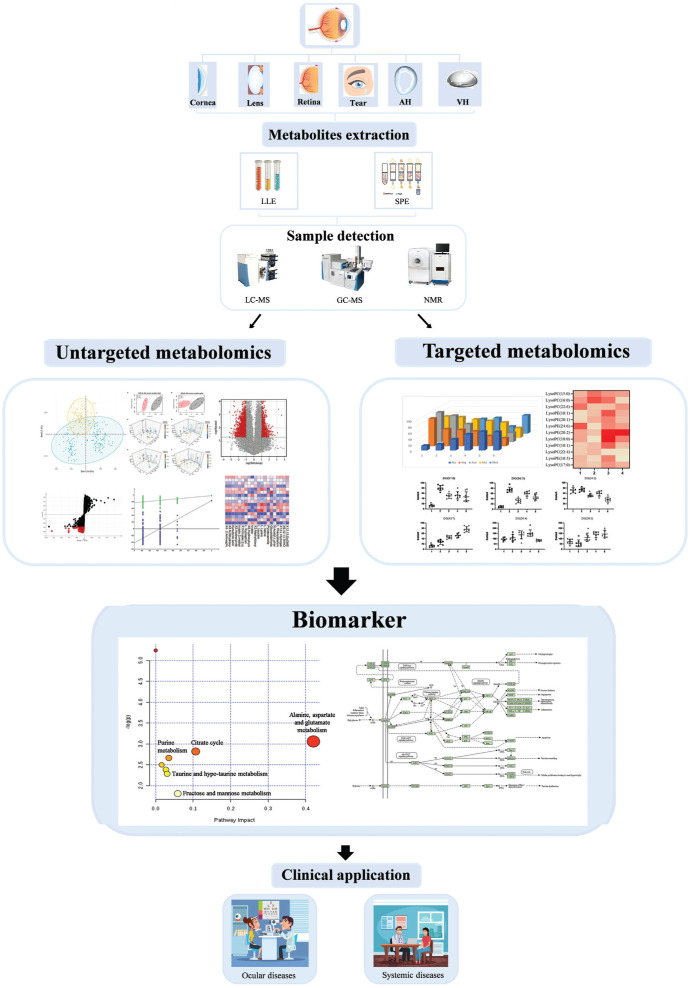
A workflow of metabolomics analysis in ocular matrix is shown in Figure 1. Sample pretreatment is a chief step of any bioanalytical workflow and a good sample preparation is considered as a starting point of successful metabolome analysis[13]. Sample preparation includes all procedures and operations applied to the sample prior to analysis. The existing pretreatment methods for ocular matrix fit to different analysis techniques are summarized in Table 1.
Figure 1. The workflow in metabolomics analysis based on ocular matrix.
Table 1. Pretreatment for ocular matrix.
| Sample | Method | Pretreatment | Ref. |
| Tear | LC-MS | Vortex mixing Schirmer's strips in 9:1 MeOH/H2O; centrifuge and collect supernatant; dry; reconstitute | [30] |
| Tear sample were centrifuged (14000 g, 10min, 4°C) in ice-cold 80% MeOH; supernatants were incubated on dry ice; evaporated; reconstitute | [38] | ||
| AH | LC-MS | Vortex-mixing for 1min equal volumes on the AH sample and freeze cold (-20°C) methanol/ethanol (1:1) mixture; stored on ice for 10min; centrifuge; collect supernatant and filter for analysis | [55] |
| One volume AH mix with four volumes 100% ethanol; centrifuge; collect supernatant; lyophilization; reconstitute with aqueous solution | [23] | ||
| One volume AH mix with five volumes ultrapure water; centrifuge; collect supernatant; analysis | [48] | ||
| GC-MS | One volume AH mix with seven volumes 75% MeOH; vortex; centrifuge; collect supernatant and derivatize for analysis | [49] | |
| NMR | One volume AH mix with four volumes 100% ethanol; centrifuge; collect supernatant; lyophilization; reconstitute with DSS. | [23] | |
| VH | LC-MS | One volume VH mix with four volumes acetone; store at -20°C overnight; centrifuge; collect supernatant; precipitation extract used 80% methanol and merge supernatant; freeze-drying; reconstitute with acetonitrile:methanol:isopropanol (4:4:1); sonic; centrifuge; collect supernatant and analysis | [92] |
| One volume VH mix with four volumes 100% ethanol; centrifuge; collect supernatant; add 1/2 chloroform and same volume water; centrifuge; lyophilization; reconstitute with water | [23] | ||
| NMR | One volume VH mix with four volumes 100% ethanol; centrifuge; collect supernatant; add 1/2 chloroform and same volume water; centrifuge; lyophilization; reconstitute with D2O containing DSS and phosphate buffer | [23] | |
| Cornea | LC-MS | Samples were rinsed in 1×PBS and lysed with ice-cold 80% methanol; incubated on dry ice for 15min and homogenized; centrifuged; collect supernatant; analysis | [80] |
| NMR | The proteins in cornea samples were precipitated by EtOH; the lipids were removed from the protein-free cornea extracts using the chloroform/EtOH/water mixture; centrifuged; lyophilization; reconstitute with D2O containing DSS and phosphate buffer | [67] | |
| Lens | LC-MS | Lens homogenate with pre-cooled EtOH; centrifuged; collect supernatant; pellet extracted again; merge supernatant; dry; reconstitute with water | [72] |
| Lens homogenate with pre-cooled EtOH; centrifuged; collect supernatant; pellet extracted again; merge supernatant; to remove lipids from the extract, H2O and CHCl3 was added to the combined supernatant, shaken, then H2O was added; centrifuged; collect supernatant; lyophilized; re-dissolved in aqueous solution | [71]–[72] | ||
| NMR | Lens homogenate with pre-cooled EtOH; centrifuged; collect supernatant; pellet extracted again; merge supernatant; dry; reconstitute | [72] | |
| Lens homogenate with pre-cooled EtOH; centrifuged; collect supernatant; pellet extracted again; merge supernatant; to remove lipids from the extract; H2O and CHCl3 was added to the combined supernatant, shaken, then H2O was added; centrifuged; collect supernatant; lyophilized; re-dissolved in D2O containing DSS and phosphate buffer | [71]–[72] | ||
| Retina | LC-MS | Retina homogenate with 80% MeOH; incubate on ice; centrifuge; collect supernatant; lyophilized; reconstitute with mobile phase (A:B=4:6) | [26] |
| Add 800 µL of chloroform:methanol (50:50, pre-cooled to -20°C) to retina samples; homogenate; 400 µL of water was added to the mixture; centrifuge; collect bottom lipophilic layer; lyophilized; reconstituted in 200 µL 50:50 | [27] | ||
| Retina homogenization with 40 µL water; centrifuged; 5 µL of the supernatant were transferred for protein quantitation and 140 µL of methanol were added; homogenize; centrifugate; collect supernatant and spin-dried for 24h; reconstitute with water | [17] | ||
| Add 140 µL extraction buffer [methanol:chloroform:H2O (700:200:50)] to the retina sample; homogenize; centrifuge; collect supernatant; spin-dry; suspended in 100 µL of mobile phase (40% of A and 60% of B) with vortex for 10s | [16] | ||
| GC-MS | Retina homogenate with 80% MeOH; incubate on ice; centrifuge; collect supernatant; lyophilized; derivatize | [26] | |
| Add 800 µL of chloroform:methanol (50:50, pre-cooled to -20°C) to retina samples; homogenate; 400 µL of water was added to the mixture; centrifuge; collect top hydrophilic layer; lyophilized; derivatize | [27] |
LC-MS: Liquid chromatograph-mass spectrometer; GC-MS: Gas chromatograph-mass spectrometer; NMR: Nuclear magnetic resonance; AH: Aqueous humor; VH: Vitreous humor.
Sampling involved collected equipment and materials, which depends on samples status. Ocular matrix can be divided into two status, which includes solid and liquid. Collection of solid samples is usually done by operation while collection of liquid samples should be selected based on their location. Schirmer band and capillary are the more procedures for collection of tears, and anterior chamber puncture is utilized to collect aqueous humor (AH), while VH can be taken with 27G needle and surgery.
Owing to the presence of enzymes, metabolites have rapid turnover, and storage environment influence the composition of origin biological samples hugely. For ocular matrix, several studies had proved that cryo-preservation as the most frequently used method can stabilize metabolite composition well. Back in 1998, Sitaramma et al[14] reported that the collected tears stored at -80°C for 1mo with the smallest change in metabolites. In 2013, Kryczka et al[15] also stated that after storing the obtained cornea at -80°C for 8d, metabolites without significantly change.
An ideal sample pretreatment method should be as far as possible to keep the original metabolite composition and determined by the character of the target metabolite and the platform selected. Separating the analytes from protein is the vital procedure for sample pretreatment, generally methanol, ethanol, chloroform, water and their mixtures with different proportion were used in ocular matrix, take retina for example[16]–[17]. Subsequently, liquid-liquid extraction (LLE) and solid phase extraction (SPE)[18] can be employed to extract the targeted metabolites. Up to now, SPE has not been reported in the studies of endogenous metabolites from ocular matrix but applied in exogenous metabolites[19]–[20].
In detection platform, nuclear magnetic resonance (NMR), liquid chromatograph-mass spectrometer (LC-MS) and gas chromatograph-mass spectrometer (GC-MS) are the prevalent techniques.
In the early stage, NMR is the major technology for the researches of ocular matrix. There are some key advantages of NMR such as relative ease sample preparation, high reproducibility and inherently nondestructive. Furthermore, NMR is particularly suited to characterize highly polar compounds such as sugars, organic acids, alcohols, polyols and unique classes of metabolites such as protein-bound metabolites and ions[21]. For ocular matrix, before analysis, the free-protein supernatant was often lyophilized and then reconstituted with D2O containing TSP[22] or DSS[23]. In recent years, a number of emerging NMR technologies are being used to strengthen its utility in metabolomic applications such as solid-state NMR (ssNMR) and magic-angle sample spinning (MAS-NMR) which can offer broader possibilities for detecting intact tissues, organs, and other solid or semisolid samples[21].
Since the robust, reproducible, selectivity and recently increasing number of well-established metabolite libraries as well as new types of GC column, GC-MS is an efficient and well used analytical platform suited for metabolomics. Non-volatile metabolites containing carboxylic acids (-COOH), alcohols (-OH), amines (-NH2), and thiols (-SH) that can be derivatized, low molecular weight compounds (ca. 50-600 Da), and volatile metabolites are amenable to separating and identifying with GC-MS[24]. Yet present metabolomics studies in ocular matrix, GC-MS just applied in AH and retina. Before injecting to detect, endogenous metabolites often should be derivatized to render them volatile. Derivatized will be processed followed with lyophilizing the free-protein supernatant, and the mainstay method is methy-lsilylation used MSTFA and BSA[25]–[27].
Not only LC-MS as the complementary to NMR and GC-MS, but also has particular features to allow it become a powerful metabolomic tool that could be adapted to nearly all kind of compounds and provide rich structure information. Ultra-high performance liquid chromatography (UHPLC) can annotation metabolites within the short spans and possible to analyze a completely different set of metabolites by simply changing the chromatographic column and mobile phase[28]. For LC-MS, following with protein precipitation, either direct injection analysis using protein-removed supernatant, or resolve after lyophilizing in the mobile phase or water[29].
Biomarkers in Ocular Diseases
Current metabolomics studies involved almost all ocular matrices such as tear, AH, VH, cornea, lens and retina. Biomarkers of diseases found in metabolomics based on ocular matrix are listed in Table 2.
Table 2. Biomarkers in ocular matrix.
| Sample | Diseases | Regulation |
|
| Up | Down | ||
| Tear | Dry eye disease | - | Cortisol, 4-Androstene-3,17-dione, 17-α-hydroxyprogesterone |
| KC | Isocitrate, aconitate, malate, acetyl-phosphate, ornithine, aspartate, lactate | GSH | |
| AH | Glaucoma | Alanine, glutamic, glutamine, H-L-proline, lysine, valine, very-low-density lipoproteins | Glucose |
| Myopia | Amino-caprylic, arginine, citrulline, sphingine, amino-decanoic, cis-phytol, thymine, oxalic acid, glutamine | - | |
| Cataract | - | Methyl-tetrahydrofolate, taurine, nicotinamide, xanthine, uric acid | |
| Retinoblastoma | Uric acid | - | |
| DR | Asparagine, histidine, glutamine, threonine, dimethylamine, isoleucine | Lactic acid, succinic acid, 2-hydroxybutyric acid, ascorbic acid, formic acid | |
| VH | PDR | Arginine, ornithine, proline, citrulline | - |
| RRD | Tyrosine, urea ascorbic acid | - | |
| DR | Lactate, glucose | Galactitol, ascorbic acid | |
| RRDCD | Succinic acid, lactic acid, phenylpyruvate, L-carnitine | Sphingosine, sphingosine, dihydro-sphingosine, arachidonic acid | |
| Cornea | DM | Glucosamine, piperonic acid, spermidine, betaine, sphingosine, 2-hydrosphingosine, Indole-3-carboxylic acid, aminoadipic acid | Pyruvate, glyceraldehyde-3-phosphate |
| KC | Acetate, citrate | GSH | |
| Lens | Age-related-cataract | Alanine, arginine, asparagine, glutamic acid, isoleucine, proline, threonine, carnitine, glycerophosphate, AMP, ADP, inositol, GSSG | Choline |
| Retina | Myopia | Mannose, glucose | Tyrosine, threonine, valine, isoleucine, aminobutyric acid |
| Glaucoma | Hypo-taurine, urea, choline phosphate, sorbitol, fructose, N-acylethanolamines | - | |
| Hypoxic ischemic encephalopathy | CDP-choline | - | |
KC: Keratoconus; DR: Diabetic retinopathy; RRD: Rhegmatogenous retinal detachment; RRDCD: Choroidal detached rhegmatogenous retinal detachment; DM: Diabetes mellitus; PDR: Proliferative diabetic retinopathy; GSH: Reduces glutathione; GSSG: Oxidative glutathione; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate. -: Not be detected.
Tear
Tear is extracellular biofluid covers the anterior surface of the eyeball, which provide lubrication, protection and nutrition for the eye and is also the carrier to remove local waste, metabolic drugs and inflammatory mediators generated in eye diseases. It has been proven that tear contains thousands of molecules, including amino acids, amino ketones, amino alcohols, aromatic acids, carbohydrates, acylcarnitine, nucleotides[30].
Dry eye disease (DED)[31] is a multifactorial disorder of the ocular surface with the main feature of imbalance of tear film homeostasis and the instability of extra lacrimal lipids. A research applied HPLC-MS to correlate DED to tear steroid levels[32], the results showed that the content of cortisol (CORT), 4-Androstene-3,17-dione (ADIONE), 17-α-hydroxyprogesterone (17-OHP) in DED patients is decreased. Ocular inflammation is the factor responsible for DED. Cell-based studies have found that cortisol can exert its biological role through several different molecular mechanisms, thereby reducing the production of eicosane-like substances and inhibiting various white blood cell-related inflammations[33]–[34]. In addition, due to cortisol (CORT) is one of the products derived from 17-α-hydroxyprogesterone (17-OHP), the decrease in 17-OHP levels and the CORT levels is interconnected. ADIONE is a precursor of synthetic androgen, and previous literatures have reported that reduced androgen levels may cause structural dysfunction of the glands and meibomian glands[35]–[37]. The research made it possible to study steroid profiling directly in tear for diagnose of DED.
Keratoconus (KC) is a non-inflammatory disease companied with progressive, asymmetric corneal ectasia. The mechanism of KC is complicated and still remain mystery so that the discovery of the differences in the metabolic composition of KC is of great significance. Karamichos et al[38] used LC-MS to identify biomarker between KC patients and normal people, as a result, a total of 296 endogenous polar metabolites were detected of which more than 40 were significantly changes in KC patients. The metabolites involved in glycolysis and gluconeogenesis such as 1,3-glycerophosphate and 3-phosphoglycerate increased significantly in the tear of KC and induced the up-regulated in isocitrate, aconitate, malate, and acetyl-phosphate involved in citric acid cycle (TCA). What's more, ornithine and aspartate were accumulated which indicated that urea cycle was affected. In terms of oxidative stress, the ratio of reduces glutathione (GSH) to oxidative glutathione (GSSG) was decreased while the ratio of lactate to pyruvate was increased. Previous researches reported that the ratio of lactate to pyruvate is positively correlated with oxidative stress while the ratio of reduces GSH to GSSG is adverse[39]. Notably, the metabolites that associated with inflammation was not found, which is in line with the nature of the disease. Overall, the results suggested that KC may alter the metabolites related to urea cycle, TCA cycle and oxidative stress.
Aqueous humor
AH is a transparent liquid with a complex mixture of electrolytes, organics, growth factors, cytokines and proteins[40]. The circulating AH nourishes the cornea and lens and removes the metabolic waste from the avascular tissues. The major types of metabolite contains lipids, amino acids, carnitines, alkaloids, nucleotides, carbohydrates[41], involved in variety of metabolic pathways and related to some kinds of ophthalmopathy.
Glaucoma is a chronic irreversible disease and the leading cause of blindness in human characterized by increased intraocular pressure (IOP), degeneration of retinal ganglion cells (RGC) and optic nerve fibers (ONF)[42]. It is manifested by progressive changes in retinal sensitivity and visual field performance. Mayordomo-Febrer et al[22] analyzed the rat AH in glaucoma model established by injection of sodium hyaluronate solution used NMR spectra. The results indicated the accumulation of alanine, glutamic, glutamine, H-L-proline, lysine, valine and very-low-density lipoproteins (VLDLs) while a significant decreased in glucose on the side of sodium hyaluronate injection. Degeneration of RGC is one of the major mechanisms for the progression of glaucoma. Excessively accumulation of glutamic will lead to the over-expression of N-methyl-D-aspartate (NMDA) receptor and consequently decrease the expression of retinal anti-apoptotic factor Bcl-2 which will reduce pro-apoptotic factor Bax and enzyme caspase-3 related to RGC apoptosis[43]. Abnormal IOP represents the primary risk factor for developing glaucoma and ATP is involved in regulating IOP. The reduction of glucose may elevate IOP by decreasing the generation of ATP and in turn the elevated IOP will lead to less ATP into the capillaries[44]. The changes in VLDLs is also linked with the pathogenesis of glaucoma. According to the previous researches, VLDLs can prompt the expression of fibronectin, laminin and collagen type IV which contributes to reduce cell adhesion to the basement membrane of the trabecular meshwork[45]–[46]. The identified metabolites in this study could enhance our knowledge of glaucoma biomarkers and new biotherapy.
Myopia[47] is a public health problem, moreover the severe myopia (high degree myopia) has more likely to develop into eye disorders overtime. Barbas-Bernardos et al[48] had investigated on the AH in myopia by LC-MS suggesting that higher abundant metabolites, which include amino-caprylic, arginine, citrulline and sphinganine, occurred in highly myopic person. Arginine and citrulline are contacted by the citrulline cycle that jointly regulated by the concentrations of arginine and citrulline. The high concentration of these amino acid will compete for the enzyme center to inhibit NO production and impaired blood flow, ultimately chronic ischemic injury of optic nerve. Surprisingly, the study found that amino-decanoic in AH showed a significant difference between high myopia and low myopia, but did not appear in the metabolic profile of normal human aqueous humor. It suggested that amino-decanoic acid plays a vital role in the progression of myopia. Another study in AH based on GC-MS in 2017 identified four metabolites discriminated normal and myopia groups, cis-phytol, thymine, oxalic acid and glutamine respectively[49]. As reported, thymidine[50] is related to the phenotype of the eye, and oxalic acid[51] concentration is associated with the content of calcium ion. Additionally, altered in glutamine[52] indicated that high myopia may cause changes in active oxygen concentration. These works provided potential biomarkers for the diagnosis of myopia and a new insight into the underlying mechanisms of the high myopia formation.
Cataract mainly caused by crosslinking, aggregation and deposition of proteins in crystalline bodies, is a leading cause to blindness[53]. Epidemiological studies revealed that people with diabetes have five times the risk of cataracts than normal and the incidence increase with age[54]. A metabolomics study of AH in diabetic and non-diabetic cataract patients based on LC-MS was conducted[55] and several antioxidants (methyl-tetrahydrofolic acid, taurine, niacinamide, xanthine, and uric acid) were found decreased in AH of diabetics. It has been found in animal models that taurine can prevent diabetic cataract caused by tetraoxopyrimidine[56]–[57]. Nicotinamide[58] has been reported as an effective inhibitor of protein glycosylation and subsequent advanced glycation end products. Xanthine[59] and uric acid are regulated by xanthine oxidoreductase, catalyzing the oxidation of hypoxanthine to xanthine, xanthine to uric acid, and the reduction of NAD+ or molecular oxygen. The differences in antioxidants observed indicated that increased oxidative stress may be contributed to earlier cataract onset in diabetic patients.
Retinoblastoma (Rb) is a primary intraocular cancer in children with high rate of recurrence, tissue metastasis and fatality rate, which is difficult to diagnose[60]. As early as 1998[61], it was reported that the uric acid in the aqueous humor of patients with retinoblastoma was increased. During cell replacement, nucleic acids and nucleotides are degraded to form xanthine and uric acid. Increased uric acid levels in body fluids are associated with many malignancies and also with the rapid destruction of malignant tissues after chemotherapy or radiotherapy. Uric acid in AH has a potential to be a biomarker for retinoblastoma.
Vitreous humor
VH is a transparent liquid located at the back of the eyeball which the major composition of metabolites are amino acids, sugars, alkaloids, sphingosine and others, able to separates the lens from the retina[62].
Rhegmatogenous retinal detachment (RRD)[63] is a serious eye disease. It had been reported the occurrence of the up-regulated of tyrosine, urea and ascorbic acid in the VH of people with RRD. With development of disease, RRD can complicated with choroidal detachment which named choroidal detached rhegmatogenous retinal detachment (RRDCD)[64]. LC-Q-TOF/MS technology was used in research by Wu[65] on VH. After multiple data analysis, 24 differential metabolites were identified. According to the comparison, in RRDCD patients, the expression levels of succinic acid, lactic acid, and phenylpyruvate that are directly involved in energy metabolism were significantly increased, which indicated that the progression of RRDCD has a greater demand for energy than RRD. In contrast, the concentration of sphingosine, sphingosine, and dihydro-sphingosine were reduced. The most common product in sphingolipid metabolism is ceramide, which play an important role in cell apoptosis and proliferation. Decreasing the concentration of these three compounds may lead to cell proliferation. What's more, arachidonic acid was decreased while L-carnitine was increased in the RRDCD group suggesting a severe inflammatory response in RRDCD patients.
Cornea
Cornea is the outermost structure of the eye without vascular tissue so that the main nutrients are supplied by the aqueous humor. Lipids, amino acids, fatty acids, purines are the major metabolites of cornea[25].
KC is a non-inflammatory disease and had been confirmed that the occurrence of KC is closely related to the process of oxidative stress[66]. Snytnikova et al[67] applied 1H-NMR and LC-MS to perform a quantitative study which aim at comparing the metabolomic compositions of cornea taken from KC patients and normal. The results showed that the metabolomics of the cornea in KC patients was characterized by an increase in acetate and citrate concentrations and a decrease in the ratio of GSH to GSSG which are indicated the enhanced oxidative stress in KC and it is an important angle for the intervention of KC.
Lens
Lens is a transparent tissue that can transmit and focus incident light onto the retina to provide clear vision[68]. There is no vascular system to scatter light and with a lack of nucleus and organelles in the fibrous cells. Based on the special structure, the main energy and nutrition of the lens comes from the VH. Lens plays an important role in maintaining the balance of the intraocular environment[69]. The metabolites in lens mainly include amino acids, nucleotides and sphingolipids[70].
The main lesion of lens is cataract, which is divided into age-related cataract and diabetic cataract. Increasing number of studies showed that the oxidative stress caused by hyperglycemia in diabetic will increase the risk of cataract[71].
Yanshole et al[72] performed a series of metabolomic studies on the lens. In 2014, they performed quantitative metabolomics study on rat lens for the first time that combined LC-MS and NMR. More than 40 low molecular weight compounds were found and quantified in the lens. Among them, the most abundant metabolites in the three-month-old of rat lens are correlated with oxidative stress include taurine, hypo-taurine, lactic acid, choline phosphate, and GSH. The study also performed that with age, alanine and arginine decreased by 300% while asparagine, glutamic acid, isoleucine, proline, threonine, glycine, carnitine, and glycerol phosphate decreased by 100%. What's more, the experiment reported that the statistically significant difference in the OXYS lens is higher concentration of tryptophan, tyrosine, carnitine, glycerophosphate, GSH and GSSG and lower concentration of choline, point out the imbalance of the kynurenine pathway and the compensatory response of the OXYS rat lens to oxidative stress. Afterwards, two other researches seem to be more helpful in understanding the effect of age on the cataracts. The results displayed that the most pronounced difference is observed for compounds playing a key role in the lens cell protection and metabolic activity such as AMP, ADP, inositol, creatine, carnitine and UV filters[73]–[74]. Results from above studies consistently elucidated the influence of age on the metabolic in lens and provided new intervention idea for age-related cataract.
Retina
Retina as a tissue that can receive light stimulation, transform light into pulses of neurons and reach the brain through visual pathways which plays a vital role in the process of forming vision. Metabolites in the retina mainly include amino acids, glucose, purine peptides and lipids, which were involved in multiple metabolic pathways[75].
Yang et al[76] applied the form deprivation myopia animal model and GC-TOF-MS platform to observe how retinal metabolomic changes during the myopia development. The results showed that mannose and glucose levels in the retina are elevated, which suggested that aerobic glycolysis is reduced during the development of myopia. Additionally, seven intermediates that involved in lipid metabolism were decreased indicated fatty acid biosynthesis inhibition. The study also found in amino acid pathway, tyrosine, threonine, valine, isoleucine and aminobutyric acid showed a decrease trend during the progression of myopia. Such changes provided new idea to identify possible drug targets to suppress myopia development.
Optic nerve cell damage and axon degenerative changes leading to vision degradation are the main mechanisms of glaucoma[77]–[78]. A study[79] analyzed the changes of metabolites in retina after optic nerve injury 24h and 14d, which represents two stages after injury and finally identified 9, 19, and 32 regulated metabolites respectively when comparison of 24h versus control, 14d versus control samples, and 24h versus 14d. The metabolites which change significantly in 24h are involved in (L)-proline metabolism and phosphatidylcholine pathway. After 14d, it found that the content of hypo-taurine, urea, choline-phosphate, sorbitol and fructose were significant increased. In addition, at 24h and 14d, an inverse regulation of N-acylethanolamines (NAEs) was observed. NAEs are endogenous lipids that are synthesized and accumulated in response to tissue injury and considered as neuroprotectants[80]. These metabolites showed a clear difference between the early and late stages of degeneration and may have potential to act as prognostic factors or therapeutic target molecules during retinal or neuronal degeneration.
Ocular Biomarkers Indicating Systemic Diseases
Systemic diseases such as diabetes mellitus (DM), multiple sclerosis (MuS), hypoxic brain damage and Alzheimer's disease have been proven that will induce ocular manifestations. Therefore, exploring biomarkers based on the ocular matrix may also be important for understanding systemic diseases.
Diabetes mellitus
Sustained hyperglycemia in DM will lead to microvascular complications and eye is the major organ affected by DM. There are many reports on metabolic behaviors of DM complication.
In diabetic patients, hyperglycemia may change the corneal epithelium basement membrane before more serious lesions[81]. Glucose metabolism disorder in diabetic patients is observed in corneal metabolites. In the glucose metabolism of diabetic patients, the highest concentrations of metabolites were glucosamine, piperonic acid, spermidine and betaine, but the content of glycolysis-related metabolites such as pyruvate and glyceraldehyde-3-phosphate were reduced. Lipidomics found that the levels of sphingosine and 2-hydrosphingosine were significantly increased, which is consistent with the increase in ceramide and sphingomyelin reported in previous research[82]. Additionally, Indole-3-carboxylic acid as a derivative of tryptophan increased significantly in the cornea of patients with type 2 diabetes. Reporter speculated the phenomenon is linked with oxidative stress induced by high glucose status. Due to tryptophan is a vital metabolite that is regulated by the kynurenine pathway and is used to produce NAD, which is the basic substrate for NADH in the glycolysis and citric acid cycle. Another metabolite with significant change is aminoadipic acid, which has been detected in the plasma and skin of diabetic patients[11],[83]. Since aminoadipic acid is derived from lysine metabolism that unlike the easily metabolized glucose derivative, it is considered as a biomarker in cornea.
Diabetic retinopathy (DR) is the most common microvascular complication of DM. This metabolic disorder is a chronic inflammatory state that damages both the photoreceptors and the blood vessels of the retina[84]. One study conducted with 1H-NMR compared the AH between people with DR, DM and age-related cataract[85]. The results illustrated the lower level of lactic acid, succinic acid, and 2-hydroxybutyric acid in patients with DR than those in patients with DM alone, while higher level of asparagine, histidine, glutamine, threonine and dimethylamine. Compared with age-related cataract patients, it showed that lactic acid, succinic acid, ascorbic acid, and formic acid were reduced in DR, and asparagine and isoleucine were increased. Lactic acid and succinic acid are the intermediate products of the tricarboxylic acid cycle[86]. Hyperglycemia induces mitochondrial dysfunction will cause cell division and decreased cellular which explains the decrease in lactic acid and succinic acid content in DR patients. Additionally, hyperglycemia also induces oxidative stress pathways and promotes the consumption of NADPH that contribute to the pathogenesis of DR. This process increases the levels of NADH and NADH/NAD, reduces the TCA cycle of patients with DR, thereby reducing catabolism and increasing asparagine, glutamine, histidine and threonine[87]–[88]. In this study, ascorbic acid was reported significantly different between DR and senile cataract patients, while DR and DM with no differentiate. Another study used VH demonstrated that the main metabolic fingerprints of vitreous fluid are the higher abundance of lactate and glucose and the significant deficiency of galactitol and ascorbic acid[89]. To our knowledge, ascorbic acid can inhibit angiogenesis[90], the modulation of ascorbic acid may be considered as a therapeutic option in DR.
During the process of DR, proliferative retinopathy is severe blinding stages. It has been proven that arginine metabolism shows severe disturbances in the VH of both human and mice during the early DR phase[91]–[92]. Based on the LC-MS method, Paris et al[93] generated and validated two separate patient sample sets and explored the metabolomic features of vitreous samples from the eyes of oxygen-induced-retinopathy (OIR) mouse models. Common imbalance in human and mice revealed by the research are arginine metabolism and urea metabolism. The levels of arginine, ornithine, proline, and citrulline in the VH of PDR patients were significantly increased. In order to metabolize arginine, there are two ways balanced with each other in retina, the first way is the arginase pathway that produces ornithine and urea and the production of citrulline, and the second way is the nitric oxide synthase pathway which produce nitric oxide synthase (NOS). Current knowledge suggests that pathological features of the retina in diabetic rodent models are caused by excessive activity of arginase II[94]. Once arginase pathway is overexpressed, the NOS pathway will be inhibited, which can result in the accumulation of peroxynitrite, polyamines and prolines that may cause cell proliferation and fibrosis. Furthermore, overexpression of the arginase pathway not only leads to hyperoxia-induced retinal neurodegeneration through upregulation of polyamine synthesis, but also induces retinal microvascular edema by increasing oxidative stress[92].
Multiple sclerosis
Multiple sclerosis (MuS) is an autoimmune demyelinating disease. Lipids have been reported to play a role in autoimmune processes and new evidence suggested that lipid metabolism alters the central nervous system (CNS) in MuS subjects. Currently, eye is considered as an extension of nervous system and tear is considered as a substitution for cerebrospinal fluid, which was widely applied in the researches of MuS[95]–[96]. Cicalini et al[97] combined metabolomics and lipid-omics to study tears in patients with multiple sclerosis. Lipid-omics showed that 30 phospholipids were significantly regulated, especially many sphingomyelin, which were significantly reduced in MuS patients. This will open a new path for the diagnosis and treatment of patients with multiple sclerosis.
Hypoxic ischemic encephalopathy
Perinatal asphyxia is a worldwide problem and the subsequent hypoxic ischemic encephalopathy is a leading cause of neurodevelopmental disorders and death in infants. In view of the special vasculature lead to an extraordinarily high oxygen demand of retina and retina is considered as an oxygen sensitive tissue[98], hypoxia will induce adaptive responses in the retina[99]. Solberg et al[100] carried out an experiment to investigate the effects of hypoxia on retinal metabolism in newborn piglets after birth and highlights CDP-choline as a candidate biomarker for hypoxic-induced brain damage. CDP-choline is a restricted intermediate compound in the main pathway of phosphatidylcholine biosynthesis. It had been reported that under normal oxygen conditions, due to the interaction of CDP-choline and diacylglycerol, phosphatidylcholine and monoglycerides are produced; under hypoxic conditions, the normal reaction is reversed due to the increase of monoglycerides[101]. Results from this study could improve the prognosis and therapeutic strategies in hypoxic ischemic encephalopathy caused by perinatal asphyxia.
CONCLUSION
Ocular diseases may be caused by multi-factor and still remain huge problems for diagnose and treatment worldwide. With the continuous development of precision medicine and technology, metabolomics has been widely used in the study of ocular matrix. With literature reviewed, we discovered that metabolites associated with oxidative stress, energy and inflammation may represent a new hope for diagnosis in ocular diseases and systemic diseases. However, there are some challenges remain to be addressed in such studies. Firstly, compared with the common samples such as plasm, urea, feces and other tissues, trace metabolites in ocular matrix is the inherent limitation for pretreatment, detection and analysis. Therefore, continuous researches should be done to develop a standard research protocol. Secondly, in terms of metabolites level, the association and mutual metastasis of ocular diseases and other systemic diseases will be an arduous but deserved challenge. Finally, the majority of current data comes from animal experiment and the cohort of individuals, in order to transform the biomarkers from laboratory to clinic, it is necessary to plan future studies in the condition of large sample, multicenter, blindness and randomization.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81872980; No.81673556); the Guangdong Basic and Applied Basic Research Special Fund (No.2017A030313753); the Administration of Traditional Chinese Medicine of Guangdong Province (No.20192055).
Conflicts of Interest: Luo Y, None; Cui HP, None; Liu Y, None; Chen L, None.
REFERENCES
- 1.Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm Res. 2019;36(3):40. doi: 10.1007/s11095-019-2569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang BS, Congdon N, Bourne R, Li YC, Cao K, Zhao AP, Yusufu M, Dong WL, Zhou MG, Wang NL. Burden of vision loss associated with eye disease in China 1990-2020: findings from the Global Burden of Disease Study 2015. Br J Ophthalmol. 2018;102(2):220–224. doi: 10.1136/bjophthalmol-2017-310333. [DOI] [PubMed] [Google Scholar]
- 3.Wotman KL, Johnson AL. Ocular manifestations of systemic disease in the horse. Vet Clin North Am Equine Pract. 2017;33(3):563–582. doi: 10.1016/j.cveq.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AS, Lin P. Ocular manifestations of inflammatory bowel disease. Curr Opin Ophthalmol. 2016;27(6):552–560. doi: 10.1097/ICU.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 5.Burgess LG, Uppal K, Walker DI, Roberson RM, Tran V, Parks MB, Wade EA, May AT, Umfress AC, Jarrell KL, Stanley BOC, Kuchtey J, Kuchtey RW, Jones DP, Brantley MA., Jr Metabolome-wide association study of primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2015;56(8):5020–5028. doi: 10.1167/iovs.15-16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naz S, Vallejo M, García A, Barbas C. Method validation strategies involved in non-targeted metabolomics. J Chromatogr A. 2014;1353:99–105. doi: 10.1016/j.chroma.2014.04.071. [DOI] [PubMed] [Google Scholar]
- 7.Drouin N, Rudaz S, Schappler J. Sample preparation for polar metabolites in bioanalysis. Analyst. 2017;143(1):16–20. doi: 10.1039/c7an01333g. [DOI] [PubMed] [Google Scholar]
- 8.von Thun Und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013;117:126–137. doi: 10.1016/j.exer.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald GA. Measure for Measure: Biomarker standards and transparency. Sci Transl Med. 2016;8(343):343fs10. doi: 10.1126/scitranslmed.aaf8590. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng SS, Rhee EP, Sinha S, McCabe E, Fox CS, O'Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123(10):4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H, Chen BL. CD147 in ovarian and other cancers. Int J Gynecol Cancer. 2013;23(1):2–8. doi: 10.1097/IGC.0b013e3182749139. [DOI] [PubMed] [Google Scholar]
- 13.Villas-Bôas SG, Højer-Pedersen J, Akesson M, Smedsgaard J, Nielsen J. Global metabolite analysis of yeast: evaluation of sample preparation methods. Yeast. 2005;22(14):1155–1169. doi: 10.1002/yea.1308. [DOI] [PubMed] [Google Scholar]
- 14.Sitaramamma T, Shivaji S, Rao GN. Effect of storage on protein concentration of tear samples. Curr Eye Res. 1998;17(10):1027–1035. doi: 10.1076/ceyr.17.10.1027.5241. [DOI] [PubMed] [Google Scholar]
- 15.Kryczka T, Szaflik JP, Szaflik J, Midelfart A. Influence of donor age, post-mortem time and cold storage on metabolic profile of human cornea. Acta Ophthalmol. 2013;91(1):83–87. doi: 10.1111/j.1755-3768.2011.02271.x. [DOI] [PubMed] [Google Scholar]
- 16.Du JH, Linton JD, Hurley JB. Probing metabolism in the intact retina using stable isotope tracers. Meth Enzymol. 2015;561:149–170. doi: 10.1016/bs.mie.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Barca JMC, Huang NT, Jiao HH, Tessier L, Gadras C, Simard G, Natoli R, Tcherkez G, Reynier P, Valter K. Retinal metabolic events in preconditioning light stress as revealed by wide-spectrum targeted metabolomics. Metabolomics. 2017;13(3):22. doi: 10.1007/s11306-016-1156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal Chem. 2016;88(1):524–545. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
- 19.English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot Essent Fatty Acids. 2017;117:17–27. doi: 10.1016/j.plefa.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunikata H, Ida T, Sato K, Aizawa N, Sawa T, Tawarayama H, Murayama N, Fujii S, Akaike T, Nakazawa T. Metabolomic profiling of reactive persulfides and polysulfides in the aqueous and vitreous humors. Sci Rep. 2017;7:41984. doi: 10.1038/srep41984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emwas AH, Roy R, McKay RT, Tenori L, Saccenti E, Gowda GAN, Raftery D, Alahmari F, Jaremko L, Jaremko M, Wishart DS. NMR spectroscopy for metabolomics research. Metabolites. 2019;9:123. doi: 10.3390/metabo9070123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayordomo-Febrer A, López-Murcia M, Morales-Tatay JM, Monleón-Salvado D, Pinazo-Durán MD. Metabolomics of the aqueous humor in the rat glaucoma model induced by a series of intracamerular sodium hyaluronate injection. Exp Eye Res. 2015;131:84–92. doi: 10.1016/j.exer.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Zelentsova EA, Yanshole LV, Snytnikova OA, Yanshole VV, Tsentalovich YP, Sagdeev RZ. Post-mortem changes in the metabolomic compositions of rabbit blood, aqueous and vitreous humors. Metabolomics. 2016;12:172. [Google Scholar]
- 24.Beale DJ, Pinu FR, Kouremenos KA, Poojary MM, Narayana VK, Boughton BA, Kanojia K, Dayalan S, Jones OAH, Dias DA. Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics. 2018;14(11):152. doi: 10.1007/s11306-018-1449-2. [DOI] [PubMed] [Google Scholar]
- 25.Kryczka T, Ehlers N, Nielsen K, Midelfart A. Impact of organ culturing on metabolic profile of human corneas: preliminary results. Acta Ophthalmol. 2012;90(8):761–767. doi: 10.1111/j.1755-3768.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhu SY, Yam M, Wang YK, Linton JD, Grenell A, Hurley JB, Du JH. Impact of euthanasia, dissection and postmortem delay on metabolic profile in mouse retina and RPE/choroid. Exp Eye Res. 2018;174:113–120. doi: 10.1016/j.exer.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan SZ, Mullard G, Hollywood KA, Dunn WB, Bishop PN. Characterisation of the metabolome of ocular tissues and post-mortem changes in the rat retina. Exp Eye Res. 2016;149:8–15. doi: 10.1016/j.exer.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Perez de Souza L, Alseekh S, Naake T, Fernie A. Mass spectrometry-based untargeted plant metabolomics. Curr Protoc Plant Biol. 2019;4(4):e20100. doi: 10.1002/cppb.20100. [DOI] [PubMed] [Google Scholar]
- 29.Pietrowska K, Dmuchowska DA, Krasnicki P, Mariak Z, Kretowski A, Ciborowski M. Analysis of pharmaceuticals and small molecules in aqueous humor. J Pharm Biomed Anal. 2018;159:23–36. doi: 10.1016/j.jpba.2018.06.049. [DOI] [PubMed] [Google Scholar]
- 30.Chen LY, Zhou L, Chan EC, Neo J, Beuerman RW. Characterization of the human tear metabolome by LC-MS/MS. J Proteome Res. 2011;10(10):4876–4882. doi: 10.1021/pr2004874. [DOI] [PubMed] [Google Scholar]
- 31.Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. quiz 82. doi: 10.3238/arztebl.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieragostino D, Agnifili L, Cicalini I, Calienno R, Zucchelli M, Mastropasqua L, Sacchetta P, Del Boccio P, Rossi C. Tear film steroid profiling in dry eye disease by liquid chromatography tandem mass spectrometry. Int J Mol Sci. 2017;18(7):E1349. doi: 10.3390/ijms18071349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kallab M, Szegedi S, Hommer N, Stegmann H, Kaya S, Werkmeister RM, Schmidl D, Schmetterer L, Garhöfer G. Topical low dose preservative-free hydrocortisone reduces signs and symptoms in patients with chronic dry eye: a randomized clinical trial. Adv Ther. 2020;37(1):329–341. doi: 10.1007/s12325-019-01137-8. [DOI] [PubMed] [Google Scholar]
- 34.Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116(7):849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- 35.Vehof J, Hysi PG, Hammond CJ. A metabolome-wide study of dry eye disease reveals serum androgens as biomarkers. Ophthalmology. 2017;124(4):505–511. doi: 10.1016/j.ophtha.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014;97(4):324–336. doi: 10.1111/cxo.12147. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan DA, Sullivan BD, Evans JE, Schirra F, Yamagami H, Liu M, Richards SM, Suzuki T, Schaumberg DA, Sullivan RM, Dana MR. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 38.Karamichos D, Zieske JD, Sejersen H, Sarker-Nag A, Asara JM, Hjortdal J. Tear metabolite changes in keratoconus. Exp Eye Res. 2015;132:1–8. doi: 10.1016/j.exer.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, Trnkova L, Kruseova J, Eckschlager T, Kizek R. Redox status expressed as GSH: GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4(6):1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snytnikova OA, Khlichkina AA, Yanshole LV, Yanshole VV, Iskakov IA, Egorova EV, Stepakov DA, Novoselov VP, Tsentalovich YP. Metabolomics of the human aqueous humor. Metabolomics. 2017;13:5. [Google Scholar]
- 41.Pietrowska K, Dmuchowska DA, Samczuk P, Kowalczyk T, Krasnicki P, Wojnar M, Skowronska A, Mariak Z, Kretowski A, Ciborowski M. LC-MS-based metabolic fingerprinting of aqueous humor. J Anal Methods Chem. 2017;2017:6745932. doi: 10.1155/2017/6745932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lambuk L, Jafri AJ, Arfuzir NN, Iezhitsa I, Agarwal R, Rozali KN, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Ismail NM. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox Res. 2017;31(1):31–45. doi: 10.1007/s12640-016-9658-9. [DOI] [PubMed] [Google Scholar]
- 44.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Akiba S, Mukaida Y, Hane K, Oka M, Uozumi N, Shimizu T, Sato T. Group IVA phospholipase A2-mediated production of fibronectin by oxidized LDL in mesangial cells. Kidney Int. 2006;70(6):1013–1018. doi: 10.1038/sj.ki.5001631. [DOI] [PubMed] [Google Scholar]
- 46.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Yu L, Li ZK, Gao JR, Liu JR, Xu CT. Epidemiology, genetics and treatments for myopia. Int J Ophthalmol. 2011;4(6):658–669. doi: 10.3980/j.issn.2222-3959.2011.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbas-Bernardos C, Armitage EG, García A, Mérida S, Navea A, Bosch-Morell F, Barbas C. Looking into aqueous humor through metabolomics spectacles - exploring its metabolic characteristics in relation to myopia. J Pharm Biomed Anal. 2016;127:18–25. doi: 10.1016/j.jpba.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Ji YH, Rao J, Rong XF, Lou S, Zheng Z, Lu Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp Eye Res. 2017;159:147–155. doi: 10.1016/j.exer.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Coxam B, Neyt C, Grassini DR, Le Guen L, Smith KA, Schulte-Merker S, Hogan BM. Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (cad) regulates Notch signaling and vascular development in zebrafish. Dev Dyn. 2015;244(1):1–9. doi: 10.1002/dvdy.24209. [DOI] [PubMed] [Google Scholar]
- 51.Heaney RP, Weaver CM. Oxalate: effect on calcium absorbability. Am J Clin Nutr. 1989;50(4):830–832. doi: 10.1093/ajcn/50.4.830. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava N, Kollipara RK, Singh DK, Sudderth J, Hu ZP, Nguyen H, Wang S, Humphries CG, Carstens R, Huffman KE, DeBerardinis RJ, Kittler R. Inhibition of cancer cell proliferation by PPARγ is mediated by a metabolic switch that increases reactive oxygen species levels. Cell Metab. 2014;20(4):650–661. doi: 10.1016/j.cmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes Metab Res Rev. 2010;26(3):172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 54.Haddad NM, Sun JK, Abujaber S, Schlossman DK, Silva PS. Cataract surgery and its complications in diabetic patients. Semin Ophthalmol. 2014;29(5-6):329–337. doi: 10.3109/08820538.2014.959197. [DOI] [PubMed] [Google Scholar]
- 55.Pietrowska K, Dmuchowska DA, Krasnicki P, Bujalska A, Samczuk P, Parfieniuk E, Kowalczyk T, Wojnar M, Mariak Z, Kretowski A, Ciborowski M. An exploratory LC-MS-based metabolomics study reveals differences in aqueous humor composition between diabetic and non-diabetic patients with cataract. Electrophoresis. 2018;39(9-10):1233–1240. doi: 10.1002/elps.201700411. [DOI] [PubMed] [Google Scholar]
- 56.Devamanoharan PS, Ali AH, Varma SD. Prevention of lens protein glycation by taurine. Mol Cell Biochem. 1997;177(1-2):245–250. doi: 10.1023/a:1006863322454. [DOI] [PubMed] [Google Scholar]
- 57.Hsu YW, Yeh SM, Chen YY, Chen YC, Lin SL, Tseng JK. Protective effects of taurine against alloxan-induced diabetic cataracts and refraction changes in New Zealand White rabbits. Exp Eye Res. 2012;103:71–77. doi: 10.1016/j.exer.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ardan T, Kovaceva J, Cejková J. Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium. Acta Histochem. 2004;106(1):69–75. doi: 10.1016/j.acthis.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Singh L, Kashyap S. Update on pathology of retinoblastoma. Int J Ophthalmol. 2018;11(12):2011–2016. doi: 10.18240/ijo.2018.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendelsohn ME, Abramson DH, Senft S, Servodidio CA, Gamache PH. Uric acid in the aqueous humor and tears of retinoblastoma patients. J AAPOS. 1998;2(6):369–371. doi: 10.1016/s1091-8531(98)90037-4. [DOI] [PubMed] [Google Scholar]
- 62.Mains J, Tan LE, Zhang T, Young L, Shi RW, Wilson C. Species variation in small molecule components of animal vitreous. Invest Ophthalmol Vis Sci. 2012;53(8):4778–4786. doi: 10.1167/iovs.12-9998. [DOI] [PubMed] [Google Scholar]
- 63.Feltgen N, Walter P. Rhegmatogenous retinal detachment—an ophthalmologic emergency. Dtsch Arztebl Int. 2014;111(1-2):12–21. quiz 22. doi: 10.3238/arztebl.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu X, Song Z, Sun X. Metabolic analysis of vitreous fluid in patients with rhegmatogenous retinal detachment. COOC. 2014;2014 [Google Scholar]
- 65.Wu Z. The mechanism study and biomarker screening of vitreous for rhegmatogenousretinal detachment associated with choroidal detachment. Nanjing: Nanjing Medical University; 2016. [Google Scholar]
- 66.Karamichos D, Hutcheon AE, Rich CB, Trinkaus-Randall V, Asara JM, Zieske JD. In vitro model suggests oxidative stress involved in keratoconus disease. Sci Rep. 2014;4:4608. doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snytnikova OA, Yanshole LV, Iskakov IA, Yanshole VV, Chernykh VV, Stepakov DA, Novoselov VP, Tsentalovich YP. Quantitative metabolomic analysis of the human cornea and aqueous humor. Metabolomics. 2017;13:152. [Google Scholar]
- 68.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74(1):1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 69.Lim JC, Umapathy A, Grey AC, Vaghefi E, Donaldson PJ. Novel roles for the lens in preserving overall ocular health. Exp Eye Res. 2017;156:117–123. doi: 10.1016/j.exer.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 70.Midelfart A, Dybdahl A, Gribbestad S. Detection of different metabolites in the rabbit lens by high resolution 1H NMR spectroscopy. Curr Eye Res. 1996;15(12):1175–1181. doi: 10.3109/02713689608995153. [DOI] [PubMed] [Google Scholar]
- 71.Henriques J, Vaz-Pereira S, Nascimento J, Rosa PC. Diabetic eye disease. Acta Med Port. 2015;28(1):107–113. [PubMed] [Google Scholar]
- 72.Yanshole VV, Snytnikova OA, Kiryutin AS, Yanshole LV, Sagdeev RZ, Tsentalovich YP. Metabolomics of the rat lens: a combined LC-MS and NMR study. Exp Eye Res. 2014;125:71–78. doi: 10.1016/j.exer.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Tsentalovich YP, Verkhovod TD, Yanshole VV, Kiryutin AS, Yanshole LV, Fursova AZh, Stepakov DA, Novoselov VP, Sagdeev RZ. Metabolomic composition of normal aged and cataractous human lenses. Exp Eye Res. 2015;134:15–23. doi: 10.1016/j.exer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Yanshole VV, Yanshole LV, Snytnikova OA, Tsentalovich YP. Quantitative metabolomic analysis of changes in the lens and aqueous humor under development of age-related nuclear cataract. Metabolomics. 2019;15(3):29. doi: 10.1007/s11306-019-1495-4. [DOI] [PubMed] [Google Scholar]
- 75.Léveillard T, Sahel JA. Metabolic and redox signaling in the retina. Cell Mol Life Sci. 2017;74(20):3649–3665. doi: 10.1007/s00018-016-2318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang JL, Reinach PS, Zhang S, Pan MZ, Sun WF, Liu B, Li F, Li XQ, Zhao AH, Chen TL, Jia W, Qu J, Zhou XT. Changes in retinal metabolic profiles associated with form deprivation myopia development in Guinea pigs. Sci Rep. 2017;7(1):2777. doi: 10.1038/s41598-017-03075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agudo-Barriuso M, Villegas-Pérez MP, de Imperial JM, Vidal-Sanz M. Anatomical and functional damage in experimental glaucoma. Curr Opin Pharmacol. 2013;13(1):5–11. doi: 10.1016/j.coph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Vidal-Sanz M, Salinas-Navarro M, Nadal-Nicolás FM, Alarcón-Martínez L, Valiente-Soriano FJ, de Imperial JM, Avilés-Trigueros M, Agudo-Barriuso M, Villegas-Pérez MP. Understanding glaucomatous damage: anatomical and functional data from ocular hypertensive rodent retinas. Prog Retin Eye Res. 2012;31(1):1–27. doi: 10.1016/j.preteyeres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Agudo-Barriuso M, Lahoz A, Nadal-Nicolás FM, Sobrado-Calvo P, Piquer-Gil M, Díaz-Llopis M, Vidal-Sanz M, Mullor JL. Metabolomic changes in the rat retina after optic nerve crush. Invest Ophthalmol Vis Sci. 2013;54(6):4249–4259. doi: 10.1167/iovs.12-11451. [DOI] [PubMed] [Google Scholar]
- 80.Hansen HH, Ikonomidou C, Bittigau P, Hansen SH, Hansen HS. Accumulation of the anandamide precursor and other N-acylethanolamine phospholipids in infant rat models of in vivo necrotic and apoptotic neuronal death. J Neurochem. 2001;76(1):39–46. doi: 10.1046/j.1471-4159.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 81.Priyadarsini S, McKay TB, Sarker-Nag A, Allegood J, Chalfant C, Ma JX, Karamichos D. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp Eye Res. 2016;153:90–100. doi: 10.1016/j.exer.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Priyadarsini S, Sarker-Nag A, Allegood J, Chalfant C, Karamichos D. Description of the sphingolipid content and subspecies in the diabetic cornea. Curr Eye Res. 2015;40(12):1204–1210. doi: 10.3109/02713683.2014.990984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sell DR, Strauch CM, Shen W, Monnier VM. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem J. 2007;404(2):269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y, Chen H, Su SB. Neuroinflammatory responses in diabetic retinopathy. J Neuroinflammation. 2015;12:141. doi: 10.1186/s12974-015-0368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jin HY, Zhu BJ, Liu X, Jin J, Zou HD. Metabolic characterization of diabetic retinopathy: an 1H-NMR-based metabolomic approach using human aqueous humor. J Pharm Biomed Anal. 2019;174:414–421. doi: 10.1016/j.jpba.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 86.Trudeau K, Molina AJ, Roy S. High glucose induces mitochondrial morphology and metabolic changes in retinal pericytes. Invest Ophthalmol Vis Sci. 2011;52(12):8657–8664. doi: 10.1167/iovs.11-7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53(11):2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- 88.Zhu SS, Ren Y, Zhang M, Cao JQ, Yang Q, Li XY, Bai H, Jiang L, Jiang Q, He ZG, Chen Q. Wld(S) protects against peripheral neuropathy and retinopathy in an experimental model of diabetes in mice. Diabetologia. 2011;54(9):2440–2450. doi: 10.1007/s00125-011-2226-1. [DOI] [PubMed] [Google Scholar]
- 89.Barba I, Garcia-Ramírez M, Hernández C, Alonso MA, Masmiquel L, García-Dorado D, Simó R. Metabolic fingerprints of proliferative diabetic retinopathy: an 1H-NMR-based metabonomic approach using vitreous humor. Invest Ophthalmol Vis Sci. 2010;51(9):4416–4421. doi: 10.1167/iovs.10-5348. [DOI] [PubMed] [Google Scholar]
- 90.May JM. Ascorbic acid repletion: a possible therapy for diabetic macular edema? Free Radic Biol Med. 2016;94:47–54. doi: 10.1016/j.freeradbiomed.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narayanan SP, Rojas M, Suwanpradid J, Toque HA, Caldwell RW, Caldwell RB. Arginase in retinopathy. Prog Retin Eye Res. 2013;36:260–280. doi: 10.1016/j.preteyeres.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narayanan SP, Suwanpradid J, Saul A, Xu ZM, Still A, Caldwell RW, Caldwell RB. Arginase 2 deletion reduces neuro-glial injury and improves retinal function in a model of retinopathy of prematurity. PLoS One. 2011;6(7):e22460. doi: 10.1371/journal.pone.0022460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paris LP, Johnson CH, Aguilar E, Usui Y, Cho K, Hoang LT, Feitelberg D, Benton HP, Westenskow PD, Kurihara T, Trombley J, Tsubota K, Ueda S, Wakabayashi Y, Patti GJ, Ivanisevic J, Siuzdak G, Friedlander M. Global metabolomics reveals metabolic dysregulation in ischemic retinopathy. Metabolomics. 2016;12:15. doi: 10.1007/s11306-015-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel C, Rojas M, Narayanan SP, Zhang WB, Xu ZM, Lemtalsi T, Jittiporn K, Caldwell RW, Caldwell RB. Arginase as a mediator of diabetic retinopathy. Front Immunol. 2013;4:173. doi: 10.3389/fimmu.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quintana FJ, Farez MF, Viglietta V, Iglesias AH, Merbl Y, Izquierdo G, Lucas M, Basso AS, Khoury SJ, Lucchinetti CF, Cohen IR, Weiner HL. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105(48):18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131(Pt 11):3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cicalini I, Rossi C, Pieragostino D, Agnifili L, Mastropasqua L, di Ioia M, De Luca G, Onofrj M, Federici L, Del Boccio P. Integrated lipidomics and metabolomics analysis of tears in multiple sclerosis: an insight into diagnostic potential of lacrimal fluid. Int J Mol Sci. 2019;20(6):E1265. doi: 10.3390/ijms20061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013;34(17):1270–1278. doi: 10.1093/eurheartj/eht023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lange CA, Bainbridge JW. Oxygen sensing in retinal health and disease. Ophthalmologica. 2012;227(3):115–131. doi: 10.1159/000331418. [DOI] [PubMed] [Google Scholar]
- 100.Solberg R, Escobar J, Arduini A, Torres-Cuevas I, Lahoz A, Sastre J, Saugstad OD, Vento M, Kuligowski J, Quintás G. Metabolomic analysis of the effect of postnatal hypoxia on the retina in a newly born piglet model. PLoS One. 2013;8(6):e66540. doi: 10.1371/journal.pone.0066540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gibellini F, Smith TK. The Kennedy pathway—De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010;62(6):414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]