Abstract
Purpose
Diabetes and its complications such as diabetic cardiomyopathy still account for significant morbidity and mortality. High-quality evidence was shown the importance of exercise in controlling diabetes complications, but the molecular mechanism on diabetic cardiomyopathy is not yet fully understood. This study aimed to compare and investigate the effect of high intensity interval training (HIIT) and continuous endurance training (CET) on the signaling pathway of diabetic cardiomyopathy.
Methods
Hence, 21 Wistar rats with an average weight of 260 ± 10 g, after induction of diabetes (STZ 50 mg/kg BW) were randomly divided into three groups (control, CET and HIIT; n = 7). Training programs were conducted 5 days a week for 5 weeks. CET program was defined as running at 60% vVO2max for 30 min in each session and the HIIT program was defined as running at 85–90% vVO2max for 3 min followed by 1 min recovery (30–35% vVO2max), that was repeated four times in each session. The cardiac performance was analyzed via determination of end systolic and diastolic dimensions and the ejection fraction by echocardiography. To elucidate the responsible molecular mechanism of miR-1, IGF-1 and IGF-1R mRNA and apoptosis marker protein expression were investigated.
Results
Both training programs specifically HIIT, significantly reduced the blood glucose, enhanced heart performance, reduced miR-1 expression, induced IGF-1 and IGF-1R expression and reduced apoptotic protein expression.
Conclusion
We showed that HIIT is more effective than CET for reduction of diabetic cardiomyopathy as a complication of diabetes in animal models through suppressing miR-1 and its downstream apoptosis pathway.
Keywords: High intensity interval training; Continuous aerobic training; Diabetic cardiomyopathy,miR-1; IGF-1; IGF-1R
Introduction
Diabetes mellitus as the most common metabolic disorders in the world contributes to damage of different organs [1, 2]. Life expectancy in diabetic patients estimated 7–10 years less than the general population [2, 3]. Sedentary lifestyle, high-caloric diet and obesity increase the risk of diabetes in the worldwide [4]. Cardiovascular and cardiomyopathy diseases have reported as the highest risks for heart diseases in diabetic population [1]. The American Heart Association explains that the major risk factors for mortality of diabetic patients occur with no symptoms of high blood pressure, heart failure, coronary artery disease, heart valve disease, or inborn heart defects [4] including diabetic-induced cardiomyopathy, which directly effects on the function and structure of myocardial. Although the molecular mechanisms of diabetic cardiomyopathy-induced left ventricular dysfunction is not yet fully understood, apoptotic death of heart cells is possibly responsible for it as a key trigger of pathogenesis [5, 6]. Glucotoxicity in hyperglycemia accounts as one of the most important cardiomyocyte apoptosis inducer in diabetic patients [7, 8]. Glucose cytotoxic reactions lead to oxidative stress, imbalanced calcium homeostasis, mitochondrial changes and reactive oxygen spices production lead to apoptosis activation. [9]. Although, non-coding RNAs, regulate various processes, including differentiation, proliferation and apoptosis [10, 11], the role of miRNAs in development of apoptosis and diabetes complications is unclear completely [10]. Among miRNAs, miRNA-1 (miR-1) is specifically expressed in skeletal and cardiac muscle cells [12, 13]. Evidences show that there is a different pattern of miR-1 gene expression between healthy subjects and diabetic population, which leads to make some structural and functional changes in the heart of the diabetic patients [14]. miR-1-induced suppression of anti-apoptotic capacity of insulin like growth factor 1(IGF-1) and Hsp60 shows the important role of this miR in diabetic cardiomyopathy [14–16]. It was shown that inhibition of IGF-1 induces the activation of cardiomycyte apoptosis through the induction of miR-1 and leads to cardiomyopathy in diabetic patients [14, 17]. On the other hand, regular physical activity not only is one of the leading factors of life style to reduce the harmful effects of pathological conditions such as diabetes [18], but also is an important option to reduce the financial burden of health system as a preventive strategy [19]. Nevertheless the effects of exercise intervention on miR-1 and its downstream pathways in diabetic animal and human are not fully understood. Volume and intensity of the exercise as the bases of the exercise training protocol account as an important factor to control the risk of diseases such as diabetes and its complications. Although 30 min low or mediate-intensity aerobic exercise was recommended to apply in diabetic patients [20], high intensity interval training (HIIT) reported to be more effective on the heart renewal, control of blood glucose, and many other clinical diabetes-induced complications [21–23]. Given the effective role of HIIT in hypoglycemia control, the present study was undertaken to answer whether the performance of HIIT in comparison with the traditional continuous endurance training (CET) could effectively influence the myocardial apoptosis signaling pathway at the molecular level and be beneficial for diabetic cardiomyopathy treatment in streptozotocin (STZ)-induced diabetic rats.
Material and methods
Chemicals and drugs
To induce diabetes in rat, STZ (Sigma-Alderich) was used. Glucose detection kit (Pars Azmon Iran), miRNeasy Mini Kit, miScript II RT Kit, miScript SYBR Green PCR Kit, miR-1 miScript Primer Assay and SNORD-61 miScript Primer Assay (Qiagen), Dnase Treatment (Fermentas), Transcriptor first strand cDNA synthesis kit (Roche), PCR Master Mix (2X) (ampliqon), GAPDH, IGF-1Primer and IGF-1RPrimer (Nika Zist Gene), Beta actin antibody (abcam), and Caspase-3 antibody (Cell Signaling) were used to lab assay.
Animals
Male Wistar rats (n = 25) with an initial body weight (260 ± 10 g) were purchased from the Pasteur Institute of Iran. Animals were housed individually in cages in a room that was controlled for temperature (22 °C), humidity and light (12 h light: dark cycle). All rats had free access to laboratory chow and tap water ad libitum. All animal treatments were considered humanly and in compliance with the recommendation of the animal care committee of Tehran University of Medical Sciences and the principles of laboratory animal care (Code: EC 00312).
Induction of diabetes
In order to induce diabetes, STZ (55 mg/kg IP; Sigma-Aldrich, St. Louis, MO) in a 2% solution of cold 0.1 M citrate buffer (pH 4.5) was injected to all rats. To verify diabetes, 3 days after STZ treatment, blood sugar levels were determined by glucometer. Blood sugar more than 300 mg/dl (16.5 mm/L) was used as a criterion to diagnose diabetes. Rat body mass and tail vein glucose weekly (every saturday) was determined by glucometer but at the end of fifth week following 4 h fasting blood glucose determined in serum by glucose oxidase in blood obtained by heart puncture.
Training protocol
After 1 week, diabetic rats were randomly divided in 3 groups including control (n = 7), CET (n = 7) and HIIT (n = 7). During this week, rats were adopted to the treadmill. Then, vVO2max was measured by ramp test protocol at the end of the week as previously described in detail [23]. Training protocols were performed at 15% inclination, 5 days a week for 5 weeks. Then on the sixth day of every week the velocity at maximal oxygen uptake (vVO2max) was determined to monitor the ability of diabetic rats to perform exercise protocols. HIIT program was including 5 min warming-up running on treadmill at 30–40% of vVO2max, 15 min running at 85–90% of vVO2max and 5 min cooling down by running at 30–40% of vVO2max. HIIT protocol contained 4 intervals 3 min running session at 85–90% of vVO2max followed by 1 min running at 30–40% of vVO2max between each session. The CET protocol was including 5 min running at 30–40% of vVO2max to warm up, followed by 30 min constant running at 60–65% of vVO2max and terminated by 5 min cooling down by running at 30–40% of vVO2max. The protocols were briefly explained in Table 1. The control group was put on the switched-off treadmill during the all five weeks like the experimental groups and did not perform any exercise training protocol.
Table 1.
HIIT training protocol
| Practice procedures | Warming up | Exercise protocol | Cooling down | |
|---|---|---|---|---|
| Recovery | Exercise | |||
| Practice time (min) | 5 min | 3 min | 1 min | 5 min |
| Intensity of practice (% of vVO2max) | 30–40% | 85–90% | 30–35% | 30–40% |
Echocardiography and left ventricular extraction
After 24 h of the last training session and after an overnight fasting, the rats were anesthetized by intra peritoneal injection of ketamine (90 mg per kg of body weight) and xylazine (10 mg per kg of body weight). The two-dimensional M mode echocardiography was performed In the short axis (the papillary), left ventricular end-diastolic dimension, left ventricular end-systolic dimensions and ejection fractions were measured. The main echocardiographic measurements were performed at least in three separate cardiac cycles. Then, the left ventricular tissue was immediately frozen in liquid nitrogen after being washed in saline and stored for later analysis. Blood samples also were collected directly from the heart of rats and the serum was isolated by centrifugation at 10 °C in 3000 g for 4 min for glucose analysis.
mRNA expression by real-time PCR
Left ventricular tissue (50–60 mg) was homogenized in trizol. Then, the total RNA was extracted and its quality and quantities were confirmed. Total RNA was reverse transcribed using the MMulv reverse transcriptase and random Hexamer primer (miScript II RT Kit, Qiagen). Gene expression level was quantified using specific primers for miR-1, IGF-1 and IGF-1R with SYBR Green PCR Master Mix (miScript SYBR Green PCR Kit, Qiagen). The levels of target gene transcript were normalized relative to GAPDH and SNORD-61. The amplification protocol for 40 cycles was including 10s at 95 °C for initial activation, 5 s at 95 °C for denaturation, and 20s at 60 °C for annealing/extension.
Western blot analysis
Cell lysate was prepared by homogenization of 70–100 mg of left ventricular tissue in modified RIPA buffer (50 mm Tris–HCl, pH 7.4, 1% Triton X-100, and 0.2% sodium deoxycholate, 0.2% SDS, 1 mm Na-EDTA, and 1 mm PMSF) supplemented with protease inhibitor cocktail and PMSF (Roche). After determining protein concentrations, equal amounts of protein was subjected to SDS–PAGE, followed by transfer onto PVDF membrane. Blocking was carried out through 2 h incubation at room temperature with 5% nonfat dry milk or BSA for unbinding proteins sits in TBS with 0.5% Tween 20. Blots were incubated overnight with primary antibodies against caspase 3 and cleaved caspase 3, (Cell Signaling Technology, Beverly, MA, USA) and β-actin (Abcam, Cambridge, MA, USA) at 4 °C. The bands were visualized using an enhanced chemiluminescent (ECL) substrate after incubating with second HRP-conjugated antibodies. The band density was normalized by Image J software.
Statistical analysis
Distribution parameters such as mean and standard deviation were used as descriptive analysis. Kolmogorov-Smirnov (KS) was used to examine the normality of the data and Leven test was also used to determine homogeneity of variances. The one-way analysis of variance (ANOVA) and LSD tests were used to assess the difference between the groups. The significance level for all statistical tests was at 0.05 level. Analysis and statistical analyzes were conducted using SPSS 19.
Results
General characteristics of animals
The general specification of the animals used in this study is shown in Table 2. Both CET and HIIT groups had higher weight than their control group. Although the differences between CET and HIIT were significant (p < 0.05), no significant differences between CET and the control group were observed. Both training protocols significantly reduced blood glucose levels when compared with the control group, 12.9% and 10.2% respectively (p < 0.05). Surprisingly the glucose level reduction was significantly higher in the HIIT group in comparison with the CET (p < 0.05).
Table 2.
General Characteristics of Animals at the end of the fifth week
| Variable | Control | CET | HIIT |
|---|---|---|---|
| Weight (g) | 238.17 ± 17.9 | 250.1 ± 13.1 | 276.4 ± 16.5 *# |
| Glucose (mg/dl) | 577.6 ± 11.5 | 518.4 ± 13.4* | 502.8 ± 10.2 *# |
The data presented as mean ± SD *: significant differences between control and exercise training groups, #: significant differences between CET and HIIT. CET Continuous Endurance Training, HIIT High Intensity Interval Training
In vivo left ventricular function
The M-mode echocardiograms data (Table 3) showed that the mean left ventricular end-diastolic diameter in the control group was significantly bigger than CET and HIIT animals (7.6 ± 0. 46 versus 6.31 ± 0.5 and 5.89 ± 0.44 mm, respectively p < 0.05). Importantly, the mean left ventricular end-systolic diameter in CET and HIIT trained diabetic animals were significantly smaller than control diabetic animals (3.07 ± 0.15 and 2.93 ± 0.15 versus 4.43 ± 1.17, P < 0.05). Furthermore, there was a significant difference in ejection fraction (66.73 ± 0.95 versus 75.68 ± 3.76 and 80.33 ± 2.62 p < 0.05) between control and exercise trained animals. Given these, we showed the beneficial effects of both exercise training protocols (CET and HIIT) on the heart of diabetic rats. In addition, our findings indicated that there was a significant difference in all determined electrocardiography parameters (LVEDD, LVESD and Ejection fraction p < 0.05) between CET and HIIT group. Taken together, HIIT protocol may enhance heart efficiency more than traditional CET protocol.
Table 3.
Ecocardiographic data
| Variable | Control | CET | HIIT |
|---|---|---|---|
| LVEDD (mm) | 7.6 ± 0.46 | 6.31 ± 0.5* | 5.89 ± 0.44*# |
| LVESD (mm) | 4.43 ± 1.17 | 3.07 ± 0.15* | 2.93 ± 0.15*# |
| Ejection fraction (%) | 66.73 ± 0.95 | 75.68 ± 3.76* | 80.33 ± 2.62*# |
The data presented as mean ± SD *: significant differences between control and exercise training groups, #: significant differences between CET and HIIT. CET Continuous Endurance Training, HIIT High Intensity Interval Training. LVEDD Left ventricular end-diastolic diameter, LVESD Left ventricular end-systolic diameter
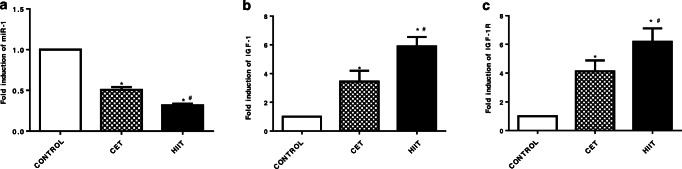
HIIT protocol reduces miR-1 expression
To address molecular mechanisms the effect of HIIT protocol, we assessed miRNA expression in left ventricular tissue. Our data demonstrated that both exercise trainings significantly reduce the miR-1 expression. Conventional CET and HIIT protocol diminished miR-1 expression 50% and 69% respectively in comparison with the control group. Importantly, HIIT protocol reduced miR-1 expression an additional 19% (Fig. 1a). Because of the role of miR-1 in apoptosis, we hypothesized that HIIT may suppress myocardial apoptosis through reduction of miR-1. To test this hypothesis, we assayed HIIT effects on IGF-1 and IGF-1R expression. Our results indicated that HIIT significantly enhances IGF-1 and IGF-1R expression around 5.8 and 6.19 fold versus control diabetic animals. Interestingly, HIIT significantly enhanced IGF-1 and IGF-1R expression by 1.71 and 1.5 fold P < 0.001 when compared to CET group (Fig. 1b, c).
Fig. 1.
Effect of CET and HIIT program on gene expression at mRNA level. a miR-1 expression, b IGF1 expression, c IGF-1R expression. The data presented as mean ± SD *: significant differences between control and exercise training groups, #: significant differences between CET and HIIT. CET: Continuous Endurance Training, HIIT: High Intensity Interval Training
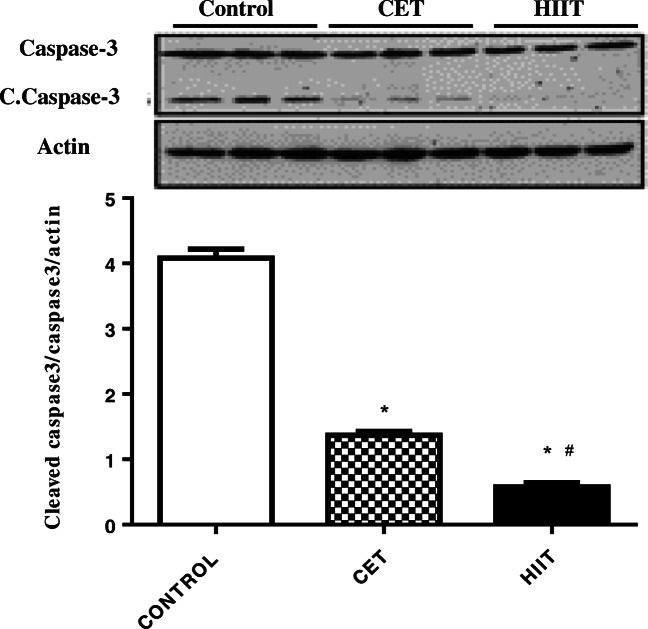
HIIT protocol alleviates diabetes-induced ventricular apoptosis
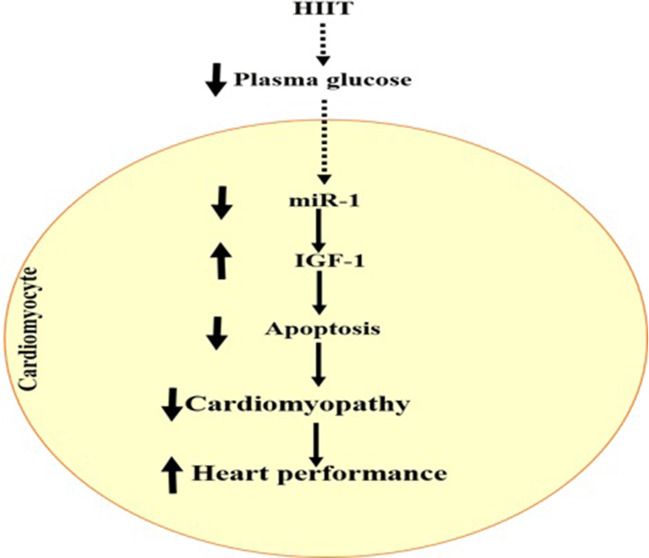
In order to show the preventive effects of HIIT on cardiomyocyte apoptosis at the molecular level, key intermediates in apoptosis pathway were investigated. To address this, we analyzed the protein expressions of caspase 3 and cleaved caspase 3. As shown in Fig. 2, cleaved caspase 3, as an indicator of apoptosis, was reduced by approximately 73% in CET group versus the control group (p < 0.000). In addition, HIIT protocol reduced cleaved caspase 3 protein levels by approximately 70% (p < 0.001) in comparison with CET. Our findings demonstrated that both exercise protocols significantly reduce apoptotic protein expression when compared to the diabetic control animals. Importantly, our data suggest that HIIT protocol significantly exerts more preventive effects on diabetes-induced apoptosis than CET. Molecular mechanisms linking HIIT to heart performance in diabetic animal model has been depicted in Figure 3.
Fig. 2.
Effect of HIIT and CET protocol on caspase 3 as apoptosis marker at protein level. The data presented as mean ± SD *: significant differences between control and exercise training groups, #: significant differences between CET and HIIT. CET: Continuous Endurance Training, HIIT: High Intensity Interval Training
Fig. 3.
Molecular mechanisms linking HIIT to heart performance in diabetic animal model. It appears that HIIT reduces plasma glucose levels, which leads to the suppression of miR-1 expression in cardiomyocyte. Reduction of miR-1 expression via induction of IGF-1 and inhibition of apoptosis improves cardiomyopathy and enhances heart performance
Discussion
Based on the results, both intervention protocols significantly decreased plasma glucose (577.6 mg/dl in control group, 518.4 mg/dl in CET group and 502.8 mg/dl in HIIT group). This result confirms the finding of Little et al. that showed HIIT decreases hyperglycemia in diabetic patients [24]. Snowling et al. also reported that the HIIT reduces post prandial glucose and hyperglycemia in diabetic patients [24]. Overall, the evidences suggest that the high intensity in HIIT training can be used to control blood sugar more efficiently in diabetic patients [25, 26]. Interestingly, the results of our study also confirmed these findings. In this study, only one miR (miR-1) was selected for evaluation because of its importance in diabetes and exercise intervention also for its downstream molecules which connects it to apoptosis [24].
Another important finding of this study was the improvement of the left ventricular function in HIIT and CET groups in comparison with the control group. Our findings show that the end diastolic and systolic dimensions in both groups significantly reduced, but the ejection fraction increased. In line with our results Shao et al. showed that 3 weeks aerobic training in STZ-induced diabetic rats reduces end-diastolic and end-systolic dimensions and increases ejection fractions in rat trained groups versus control [25]. Bidasee et al. also demonstrated that 4 weeks aerobic exercise leads to the reduction of end-diastolic and end-systolic dimensions and an induction of ejection fraction in diabetic rats (injection of 65 mg/kg body weight STZ) [27]. Other studies also emphasized the role of exercise in improving cardiac function of diabetic rats [26]. It is worth noting that HIIT protocol compared with CET clearly reduces the end-diastolic and end-systolic dimensions and enhances ejection fraction in diabetic rats. In spite of the huge body of evidences which show the importance of CETand HIIT on heart beneficial effects in the context of diabetes, no evidences were found which compared these two methods.
In order the molecular basis of the HIIT hypoglycemic effect and its cardiac protective effect, we investigated the role of microRNAs in the apoptosis pathway. The results show that both HIIT and CET protocol significantly reverses diabetes-induced elevation of miR-1 expression. Importantly, HIIT exerted additional effects and reduced miR-1 expression more than CET (HIIT decreased miR-1 expression by 69%, but CET reduced it by 50% in comparison with the control group, Fig. 1). It is possible that the reduction of plasma glucose may be a responsible factor in changing the pattern of miR-1 gene expression in both groups. In support of this claim, Yu et al. showed that 24 h treatment of C2C12 cells and rat cardiomyocytes by 25 mm glucose increased the expression of miR-1 [6]. In addition to Yu et al. study, other studies in some way indicate the same results [6, 27]. The reduction of miR-1 expression likely alleviates its suppression on downstream targets. To address the molecular link between suppression of miR-1 expression and cardiac function enhancement by training, we focused on apoptosis pathway. Our data show that the HIIT group enhances IGF-1 expression by 5.89 fold and CET enhances its expression by 3.44 fold compared to the control. Elia et al. demonstrated that the over expression of miR-1 leads to the reduction of IGF-1 [14]. Moreover Yu et al. reported that IGF-1 expression can be inhibited by miR-1 over expression therefore, they concluded IGF-1 as the target gene of miR-1 [6]. Because of IGF-1 anti-apoptotic capacity, its suppression by miR-1 accelerates cardiomyocyte apoptosis and eventually diabetic cardiomyopathy [6, 17, 28]. It seems that the decrease in expression of miR-1 genes and the increase in its downstream target, IGF-1, reduces apoptosis process in training animals (HIIT and CET). Importantly, our results on the key apoptosis protein expression confirmed this notion. As shown in Fig. 2, HIIT and CET decreased conversion of pro-caspase-3 to its active forms, the caspase-3 (cleaved caspase 3). This finding implies that exercise training reduces diabetes-induced cardiomyocyte apoptosis. In support of our data, Feng, et al. and Lu Cai et al. reported that high glucose induces apoptosis in human endothelial cells and in mouse cardiomyocyte [29–31]. Interestingly, Shan et al. confirmed our findings in in vitro study. They showed that treatment with high glucose (25 mm) via miR-1/miR-206 alleviates glucose-mediated apoptosis in cardiomyocytes [8]. The limitation of this study was restricted research budget and in vitro interventional study. In addition future clinical usage of sport intervention specially HIIT need a comprehensive clinical trial.
In conclusion the present study provides direct evidences, which show both CET and HIIT through suppression of miR-1 expression and its downstream targets leads to inhibit cardiomyocyte apoptosis and subsequently improves diabetes-induced cardiomyopathy in animal models. We suggest exercising; especially HIIT due to its less training time (15 min to 30 min in this study) and other benefits is an effective, efficient and affordable intervention to reduce the complications of diabetes, especially diabetic cardiomyopathy. However, to confirm the results further researches must be carried out and the authors hope that this training model will be administered to diabetic patients in the future.
Acknowledgments
This work was financially supported by grants from Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (1392-02-97-1677). We thank Nandini Nair, Professor at Texas Tech University for her technical contribution and assist.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Failure Rev. 2012;17:325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 2.Skyler JS. Diabetic complications: the importance of glucose control. Endocrinol Metab Clin N Am. 1996;25:243–254. doi: 10.1016/S0889-8529(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 3.Khodabandeloo H, Gorgani-Firuzjaee S, Panahi S, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta cell dysfunction. Transl Res. 2016;167:228–256. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugger H. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci. 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 6.Yu X-Y, Song Y-H, Geng Y-J, Lin Q-X, Shan Z-X, Lin S-G, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–552. doi: 10.1016/j.bbrc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Kuo W-W, Wang W-J, Tsai C-Y, Way C-L, Hsu H-H, Chen L-M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int J Cardiol. 2013;168:270–280. doi: 10.1016/j.ijcard.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 8.Shan Z-X, Lin Q-X, Deng C-Y, Zhu J-N, Mai L-P, Liu J-L, Fu Y-H, Liu X-Y, Li Y-X, Zhang Y-Y. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med. 2013;65:1435–1446. doi: 10.1016/j.freeradbiomed.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Mazloom H, Alizadeh S, Pasalar P, Esfahani EN, Meshkani R. Downregulated microRNA-155 expression in peripheral blood mononuclear cells of type 2 diabetic patients is not correlated with increased inflammatory cytokine production. Cytokine. 2015;76:403–408. doi: 10.1016/j.cyto.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townley-Tilson WD, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42:1252–1255. doi: 10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 2018:1–8. [DOI] [PubMed]
- 14.Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120:2377–2385. doi: 10.1161/CIRCULATIONAHA.109.879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghareghani P, Shanaki M, Ahmadi S, Khoshdel AR, Rezvan N, Meshkani R, Delfan M, Gorgani-Firuzjaee S. Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes Res Clin Pract. 2018;12:80–89. doi: 10.1016/j.orcp.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–593. doi: 10.1093/cvr/cvr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X-Y, Geng Y-J, Liang J-L, Lin Q-X, Lin S-G, Zhang S, Li Y. High levels of glucose induce apoptosis in cardiomyocyte via epigenetic regulation of the insulin-like growth factor receptor. Exp Cell Res. 2010;316:2903–2909. doi: 10.1016/j.yexcr.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S-M, Ho T-J, Yang A-L, Chen I-J, Kao C-L, Wu F-N, Lin JA, Kuo C-H, Ou H-C, Huang C-Y. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167:478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Miller Y, Dunstan D. The effectiveness of physical activity interventions for the treatment of overweight and obesity and type 2 diabetes. J Sci Med Sport. 2004;7:52–59. doi: 10.1016/S1440-2440(04)80278-0. [DOI] [PubMed] [Google Scholar]
- 20.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. Exercise and type 2 diabetes the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–2696. doi: 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchan DS, Ollis S, Young JD, Thomas NE, Cooper SM, Tong TK, Nie J, Malina RM, Baker JS. The effects of time and intensity of exercise on novel and established markers of CVD in adolescent youth. Am J Hum Biol. 2011;23:517–526. doi: 10.1002/ajhb.21166. [DOI] [PubMed] [Google Scholar]
- 22.Meyer P, Normandin E, Gayda M, Billon G, Guiraud T, Bosquet L, Fortier A, Juneau M, White M, Nigam A. High-intensity interval exercise in chronic heart failure: protocol optimization. J Card Fail. 2012;18:126–133. doi: 10.1016/j.cardfail.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Khakdan S, Delfan M, Heydarpour Meymeh M, Kazerouni F, Ghaedi H, Shanaki M, et al. High-intensity interval training (HIIT) effectively enhances heart function via miR-195 dependent cardiomyopathy reduction in high-fat high-fructose diet-induced diabetic rats. Arch Physiol Biochem. 2018:1–8. [DOI] [PubMed]
- 24.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111:1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 25.Gorgani-Firuzjaee S, Khatami S, Meshkani R. SH2 domain-containing inositol 5-phosphatase (SHIP2) regulates de-novo lipogenesis and secretion of apoB100 containing lipoproteins in HepG2 cells. Biochem Biophys Res Commun. 2015;464:1028–1033. doi: 10.1016/j.bbrc.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 26.Epp RA, Susser SE, Morissette MP, Kehler DS, Jassal DS, Duhamel TA. Exercise training prevents the development of cardiac dysfunction in the low-dose streptozotocin diabetic rats fed a high-fat diet 1. Can J Physiol Pharmacol. 2012;91:80–89. doi: 10.1139/cjpp-2012-0294. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 28.Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Therapu. 2007;114:307–317. doi: 10.1016/j.pharmthera.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium mitochondrial cytochrome c–mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 30.Ho FM, Liu SH, Liau CS, Huang J, Lin-Shiau SY. High glucose–induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.CIR.101.22.2618. [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, et al. (2017) ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2017;70(6):776–803. [DOI] [PubMed]