Abstract
Inflammation plays an important role in pathogenesis and progression of many chronic diseases. Although, anti-inflammatory activities of mungbean have been suggested, the underlying mechanism have not been fully understood. The present study aimed to reveal the anti-inflammatory effects of mungbean seed coat water extract (MSWE) in lipopolysaccharide (LPS)-stimulated inflammation in RAW 246.7 macrophages and LPS-induced acute liver injury mice. MSWE pretreatment downregulated the elevated expression of inflammatory markers induced by LPS in the transcriptional and protein level. MSWE inhibited NF-κB activation through the suppression of phosphorylated p65 subunit, IκBα degradation, and transforming growth factor-β-activated kinases 1 (TAK1) phosphorylation in LPS-stimulated RAW 246.7 cells. Vitexin, the major flavonoid in MSWE showed similar effects. In in vivo experiments, we found that oral administration of MSWE downregulated iNOS expression in LPS-induced acute liver injury mice. The mRNA expression of inflammatory markers and macrophage infiltration was also decreased in the livers. Collectively, MSWE exerts anti-inflammatory role, in part possibly through its active compound vitexin, by inhibiting NF-κB activation via inhibition of TAK1 phosphorylation and IκBα degradation. This suggests that MSWE is beneficial to combat various inflammatory diseases.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04302-y) contains supplementary material, which is available to authorized users.
Keywords: Anti-inflammation, Mungbean seed coat, NF-κB, RAW 246.7 macrophages
Introduction
Inflammation is a conventional route that is expressed during the progression of many chronic diseases such as diabetes, neurodegenerative diseases, atherosclerosis, and cancer. Inflammatory response is the pathological reaction of the body against detrimental stimuli such as pathogens, and macrophages play a crucial role in these responses (Ko et al. 2017). Lipopolysaccharide (LPS), endotoxin derived from gram-negative bacteria, is widely used to investigate induced inflammation in many cell types such as macrophages. Toll-like receptor 4 (TLR4) is the primary signaling receptor for LPS in mammals (Leon et al. 2008). Binding of LPS to TLR4 induces macrophages and activates nuclear factor-κB (NF-κB). Furthermore, activated NF-κB regulates the transcription of genes related to immunity and inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), and adhesion enzymes such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (Leon et al. 2008). Thus, inhibition of inflammatory response in macrophages via suppression of NF-κB activation can serve as a basis for the potential development of food supplements with anti-inflammatory activity.
Mungbean (Vigna radiata) is one of the important legumes consumed all over the world, especially in Asian countries. It is consumed as a grain or processed into high-value glass noodles or confectionery (Nair et al. 2013). Mungbean is a rich source of proteins with essential amino acids and considerable amounts of minerals, vitamins, and phenolic compounds. Many researchers have investigated mungbean for its health benefits which include anti-oxidant activity (Randhir and Shetty 2007, Singh et al. 2017), anti-inflammatory activity (Kang et al. 2015, Lee et al. 2011), inhibitory activity against formation of advanced glycation end products (Peng et al. 2008), anti-diabetic activity (Jang et al. 2014; Yao et al. 2008). Mungbean protein has also been reported to exhibit angiotensin I-converting enzyme (ACE) inhibitory activity (Li et al. 2006) and efficacy to reduce lipid accumulation (Watanabe et al. 2016). However, few studies have reported the biological activity of mungbean seed coat (Li et al. 2016; Luo et al. 2016). Ethanolic extract of mungbean seed coat has shown more efficient anti-oxidant and inhibitory effects on protease and aldose reductase compared to the cotyledon (Luo et al. 2016). Similarly, seed coats from other beans have also been reported for its biological activities. Polyphenol-rich black soybean seed coat extract has shown preventive effect against 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH)-induced oxidative DNA damage in human hepatocellular carcinoma cell line, HepG2 (Yoshioka et al. 2017). Faba bean seed coat has been reported to possess intense anti-oxidant activity with high phenolic compounds and condensed tannins, and a binding capacity to bile acid (Çalışkantürk Karataş et al. 2017). Based on various reports on polyphenol and flavonoid content in mungbean seed coat (Li et al. 2016; Luo et al. 2016), we hypothesized that mungbean seed coat extract might possess significant and interesting biological roles. In the present study, we investigate the anti-inflammatory effects of mungbean seed coat water extract (MSWE) and its associated mechanism in LPS-induced RAW 246.7 macrophages. Moreover, we anticipate that if mungbean seed coat shows great potential on inflammatory activity then it would enhance the significance of mungbean seed coat utilization as functional food ingredient.
Materials and methods
Chemical and reagents
Bacterial LPS from Escherichia coli O55:B5 was purchased from Wako Pure Chemical Corporation (Japan). DMEM high glucose was obtained from Nacalai Tesque (Japan) and fetal bovine serum was obtained from Biological Industries (USA). Penicillin–Streptomycin was obtained from Invitrogen (USA). Primary antibodies against NF-κB p65, ERK1/2 and JNK were obtained from Santa Cruz Biotechnology and primary antibodies against phospho-NF-κB p65, phospho-ERK1/2, phospho-p38, and phospho-JNK were obtained from Cell Signaling Technology. Anti-iNOS and anti-COX2 antibodies were obtained from BD Transduction Laboratories. Phospho-transforming growth factor-β-activated kinases 1 (TAK1) antibody was obtained from Professor Hiroaki Sakurai, University of Toyama, Japan. Anti-β-actin antibody was purchased from Wako Pure Chemical Corporation (Japan). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse were purchased from Cell Signaling. Vitexin and isovitexin were obtained from Sigma Aldrich. All other chemicals were of analytical grade and obtained from reputable suppliers.
Preparation of MSWE
Mungbean seed coat was obtained from Kittitat Co., Ltd. (Thailand). The seed coat was stored at − 80 °C before use. Impurities were sorted out and mungbean seed coat was then washed with tap water followed by deionized water, respectively. Sixty grams of mungbean seed coat was boiled in deionized water for 30 min and then filtered through Whatman filter paper No. 1. The filtrate was collected, concentrated by rotary evaporator, and lyophilized to obtain MSWE. The extract was stored at − 80 °C until further analysis.
Identification of major compounds in MSWE
MSWE was dissolved with deionized water and subjected to high performance liquid chromatography (HPLC) equipped with diode array detector (DAD) (Waters 600, USA) (Li et al. 2012). The spectra were recorded from 210 to 600 nm and UV absorbance at 337 nm was used to monitor flavonoids. An analytical column C18 (4.6 × 250 mm, 5 µm, Waters Symmetry Column, Ireland) was used and kept at 30 °C. The injection volume of sample was 10 µL and the flow rate was 1 mL/min. An event Elution was done using two solvent gradients: solvent A (1% acetic acid in deionized water, v/v) and solvent B (1% acetic acid in methanol, v/v). The elution program started with 10–35% B (10 min), 35–42% B (15 min), 42–75% B (10 min), 75% B (5 min), 75–10% B (5 min) and 10% B (5 min). Standard vitexin and isovitesin were used to identify the compound in MSWE. Vitexin and isovitexin at the concentration 25, 50, 75 and 100 mg/kg were used to make standard curve and to determine the concentration of vitexin and isovitexin in MSWE.
Cell culture
Mouse RAW 264.7 macrophages (American Type Culture Collection) were routinely maintained in DMEM medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin and 100 µg/mL streptomycin at 37 °C in 5% CO2.
RNA preparation and real-time PCR analysis
RAW 264.7 cells were cultured in a 12-well plate at a density of 5 × 105 cells/well. The cells were pretreated with MSWE for 1 h followed by LPS (1 µg/mL) for 4 h. The total RNA was then isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA concentration was quantified using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). The isolated RNA was used as a template to synthesize cDNA using ReverTra Ace (TOYOBO, Osaka, Japan) and random hexamers (Takara Bio, Kyoto, Japan) following the manufacturer’s instructions. Quantitative PCR analysis was performed by using 2 × Brilliant III SYBR Green QPCR Master Mix (Agilent) on StepOnePlus™ Real-Time PCR system (Applied Biosystems, Foster City, CA). PCR conditions were set as follows: 2 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C as previous protocol (Sanada et al. 2016). Total RNA was extracted from mouse liver using the same protocol. The primer sequences used in this study are listed in Online Resource.
Protein extraction and western blot analysis
RAW 264.7 cells were cultured in a 6-well plate at density of 1 × 106 cells/well. The cells were pretreated with MSWE or vitexin for 1 h followed by LPS (1 µg/mL) for 8 h and then collected by RIPA lysis buffer containing protease inhibitor and phosphatase inhibitor cocktails. Cell debris were removed by centrifugation at 15,000 rpm for 5 min at 4 °C. The protein concentration of samples was determined using Bio-Rad protein assay reagent. Equal amount of protein (16 μg) was loaded on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 2% skim milk for 1 h. After overnight incubation with specific primary antibody at 4 °C, the membrane was washed three times with phosphate buffer solution-Tween 20 (PBS-T) and incubated with HRP-conjugated secondary antibody for 1 h at room temperature. Blots were then washed three times with PBS-T and the signal was visualized with enhanced chemiluminescence (ECL; Perkin Elmer, MA, USA) (Sanada et al. 2016).
Immunofluorescence staining
For staining, RAW 264.7 cells were cultured in a 4-well chamber slide (Lab-Tek®, NY, USA) at density of 5 × 105 cells/well. The cells were pretreated with MSWE or vitexin for 1 h followed by LPS (1 µg/mL) for 5, 10, and 15 min. The cells were then fixed in 4% paraformaldehyde for 15 min, permeabilized with 1% Triton X-100 for 5 min, and blocked with BlockAce solution for 30 min. The slide was incubated with primary antibody (1:1000) overnight at 4 °C, washed with PBS-T, and then incubated for 1 h at room temperature with Cy3-labeled secondary antibody (1:1000) along with DAPI (Invitrogen) to stain the nuclei (1:1000). After washing, the slide was mounted with coverslip. The cells were visualized using fluorescence microscope (Olympus, Milan, Italy) (Sanada et al. 2016).
Animals
All animal experiments were approved by the Animal Ethical and Welfare Committee of Hiroshima University (Permit Number C18-15). Male ICR mice (8-weeks old, Charles Rive Japan) were sheltered in metal cages in a group of three with controlled room temperature (24 ± 1 °C) and a daily 12 h light/dark cycle (light from 0800 h to 2000 h). All mice were acclimated for 7 days before use.
Experiment protocol
The mice were randomly divided into the following 3 groups (n = 3 per group): (a) control group, (b) acute liver injury (ALI) group and (c) MSWE pretreated group. Thirty min before LPS treatment, MSWE (150 mg/kg body weight in 200 μL) was orally administered to the mice. ALI was induced in ICR mice by intraperitoneal (IP) injection of LPS (10 μg/kg body weight in 200 μL). The same volume of saline was administered to the mice of the control group and ALI group instead of MSWE using the same treatment protocol. Same volume of saline was IP injected to the mice of the control group instead of LPS. All mice were sacrificed by decapitation 6 h after LPS challenge. Blood and liver samples were harvested for further analysis. Liver was homogenized in RIPA lysis buffer for protein determination and RNeasy Mini Kit for mRNA analysis using similar protocol as previously described.
Statistical analysis
Values are presented as mean ± standard deviation. Statistical analyses were determined by one-way analysis of variance (ANOVA) and Tukey’s post-test. Statistical differences were considered significant at p < 0.05.
Results
Effects of MSWE on mRNA levels of inflammatory markers in LPS-stimulated RAW 264.7 macrophages
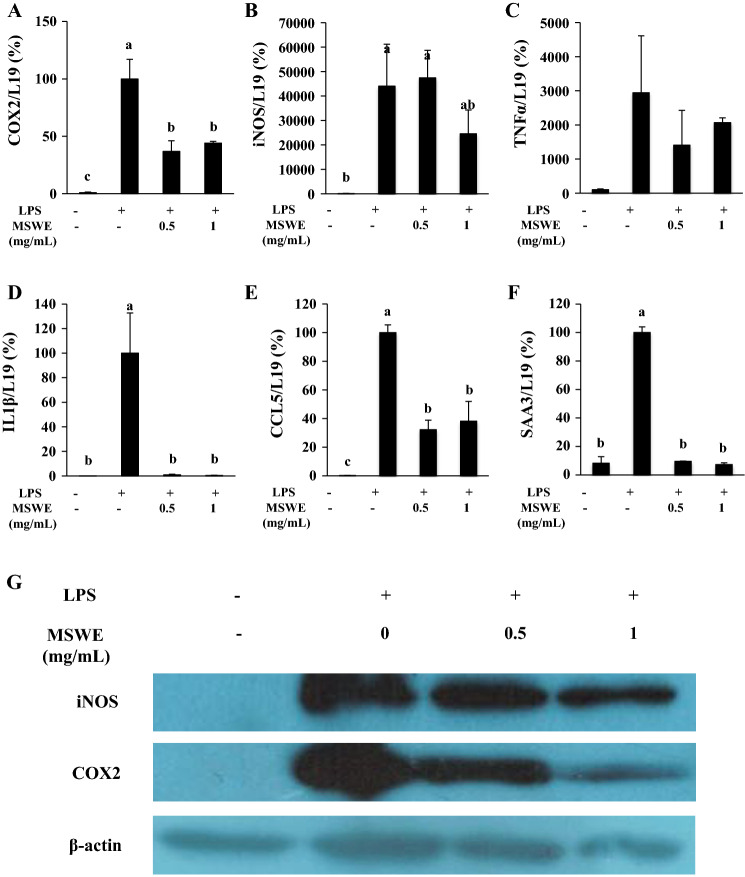
Pro-inflammatory mediators, inflammatory cytokines, and chemokines such as COX2, iNOS, TNFα, IL-1β, and CCL5 play critical roles in inflammatory response (Takeuchi and Akira, 2010). To investigate the effects of MSWE on inflammatory markers in RAW 264.7 macrophages, mRNA expression of COX2, iNOS, TNFα, IL-1β, CCL5 and SAA3 were measured by qRT-PCR. We found that mRNA levels of pro-inflammatory mediators, COX2 and iNOS were significantly upregulated in response to LPS treatment. Although MSWE was found to significantly inhibit the mRNA expression of COX2 (Fig. 1a), it did not significantly inhibit iNOS expression, and a decreasing trend was observed with high dose of MSWE (Fig. 1b). Stimulation with LPS also upregulated the mRNA expression of inflammatory cytokines such as TNFα and IL-1β. Contrastingly, MSWE pretreatment showed marked reduction in the mRNA expression of IL-1β and lowered the expression of TNFα (Fig. 1c, d). LPS also upregulated the pro-inflammatory chemokine, CCL5 (RANTES). However, both the doses of MSWE (0.5 mg/mL and 1 mg/mL) were found to inhibit the mRNA expression of CCL5 (Fig. 1e). SAA3 has been reported as a key factor that responds to activated macrophages (Sanada et al. 2016). It has also been reported that SAA3 acts as an endogenous ligand of TLR4 to activate NF-κB (Hiratsuka et al. 2008). In this study, mRNA expression of SAA3 was found to be upregulated on LPS stimulation while it was downregulated in MSWE pretreated cells (Fig. 1f). Overall, these results illustrated that MSWE suppresses the transcriptional expression of COX2, iNOS, TNFα, IL-1β, CCL5, and SAA3. Subsequently, the effects of MSWE was further determined at translational levels.
Fig. 1.
MSWE pretreatment suppressed inflammation in LPS-stimulated RAW 246.7 cells in transcriptional level. Relative mRNA expressions of aCOX2, biNOS, cTNFα, dIL-1β, eCCL5, and fSAA3 were determined by real-time PCR and protein expressions of g iNOS and COX 2 were determined by western blot. Columns represent mean ± SD. Different letters are statistically differences (p < 0.05)
Effects of MSWE on the protein levels of iNOS and COX2 in LPS-stimulated RAW 264.7 macrophages
To confirm the inhibitory effects of MSWE on inflammation in LPS-stimulated RAW 264.7 cells, we evaluated the protein expression of pro-inflammatory mediators, iNOS and COX2. After 8 h of LPS treatment, iNOS and COX2 expression were determined by western blot analysis. As shown in Fig. 1g, pretreatment with MSWE down-regulated the expression levels of iNOS and COX2 in a concentration-dependent manner compared to iNOS and COX2 expression in LPS-stimulated cells without MSWE pretreatment. The results confirmed the effects of MSWE at protein levels. Thus, MSWE was further investigated for its underlying mechanism of anti-inflammatory activity.
Effects of MSWE on NF-κB activation via suppression of IκBα degradation and TAK1 phosphorylation, and nuclear translocation of the p65 subunit in LPS-stimulated RAW 264.7 macrophages
Since NF-κB is a major transcription factor that regulates the expression of iNOS and COX2 in response to LPS stimulation (Yanaka et al. 2005), we further examined whether MSWE influenced NF-κB activation in RAW 264.7 macrophages. We found that pretreatment with MSWE did not alter the total NF-κB p65 subunit expression. However, it resulted in a significant inhibition in the phosphorylation of NF-κB p65 subunit (Fig. 2a). It is already known that the heteromeric NF-κB complex is sequestered in the cytoplasm as an inactive precursor and complexed with an inhibitory IκBα. On LPS stimulation, IκBα gets phosphorylated, ubiquitinated, and then rapidly degrades thereby activating NF-κB complex. On activation, NF-κB p65 subunit is released and translocated into the nucleus where it binds to specific DNA sequences to induce target gene expressions (Takaesu et al. 2003). In the current study, RAW 264.7 cells were pretreated with MSWE and then challenged with LPS for 5, 10 and 15 min. It was found that MSWE pretreatment suppressed IκBα degradation which was induced by LPS at all the three time points compared to IκBα degradation in LPS-stimulated MSWE-untreated cells (Fig. 2a). Phospho-NF-κB p65 subunit, the activated form of NF-κB, was also found to be decreased in MSWE-treated cells in a time-dependent manner. Furthermore, we confirmed the translocation of NF-κB p65 subunit from cytoplasm to nucleus after being release from IκBα using immunofluorescence staining. As shown in Fig. 2b, MSWE pretreatment inhibited the translocation of NF-κB-p65 subunit from cytoplasm to nucleus compared to the basal level of translocation observed in the LPS-treated cells without MSWE pretreatment. To further study the regulation of IκBα degradation, we investigated the phosphorylation of TAK1 which is an upstream regulator of IκBα. It has already been reported that phosphorylation of TAK1 activates the degradation of IκBα via IκB kinase α/β (IKKα/β) (Srivastava et al. 2010). As shown in Fig. 2c, p-TAK1 was markedly reduced by MSWE pretreatment in LPS-treated cells. Thus, the results showed that MSWE inhibited the activation of NF-κB via TAK1/IκBα pathway.
Fig. 2.
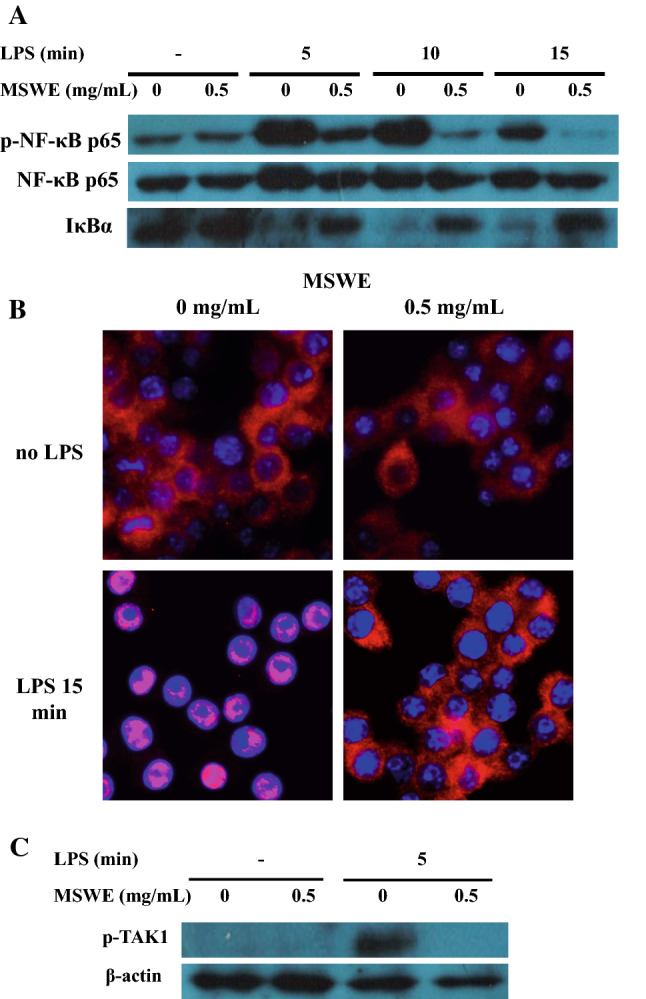
MSWE pretreatment inhibited NF-κB pathway through IκBα and TAK1 in LPS-stimulated RAW 246.7 cells. a Protein expressions of p-NF-κB p65 subunit, NF-κB p65 subunit, and IκBα were determined by western blot. b Translocation of NF-κB p65 subunit was determined by immunofluorescent staining. c Protein expression of p-TAK1 was determined by western blot
Effects of MSWE on mitogen activated protein kinase (MAPK) pathway in RAW 264.7 macrophages
Binding of LPS to TLR4 receptor activates multiple intracellular pathways which also includes MAPK signaling (Veres et al. 2004). Consequently, we investigated the effects of MSWE pretreatment on the activation of ERK1/2, p-38 subunit, and JNK. RAW 264.7 cells were pretreated with MSWE at 0.5 mg/mL for 1 h and then stimulated with 1 μg/mL of LPS for 5, 10 and 15 min. The results showed that MSWE pretreatment inhibited the phosphorylation of ERK1/2 without affecting the minimum expression level of ERK1/2 (Fig. 3). However, there was no effect of MSWE pretreatment on phosphorylation of p38 and JNK. Thus, it indicated that MSWE had an effect on MAPK pathway specifically via ERK1/2.
Fig. 3.
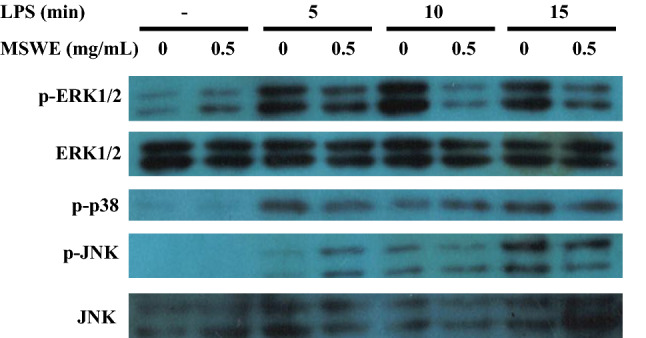
Effects of MSWE pretreatment on ERK1/2, p38 and JNK pathways in LPS-stimulated RAW 246.7 cells. Protein expressions of p-ERK1/2, ERK1/2, p-p38, p-JNK were determined by western blot
Identification of major compounds in MSWE
To identify the major compounds present in MSWE, HPLC analysis was performed. Chromatographic spectra were recorded at 280 nm and 337 nm. The spectra clearly showed the presence of various flavonoids with two major peaks at 12.6 and 13.6 min (data not shown). On comparing the spectral data of the two major peaks with the standard peaks of vitexin and isovitexin, we observed that the two peaks corresponded to vitexin and isovitexin (Rt 12.6 min and 13.6 min) which was consistent with previous studies (Li et al. 2012; Luo et al. 2016). The concentration of vitexin and isovitexin in MSWE was found to be 38.56 ± 3.3 mg/g and 28.96 ± 1.5 mg/g dry weight.
Identical effects of vitexin on protein levels of iNOS and COX2 in LPS-induced RAW 264.7 macrophages
Since vitexin was one of the major flavonoids present in MSWE, we confirmed the anti-inflammatory effects of vitexin by determining the expression levels of iNOS and COX2 in LPS-induced RAW 246.7 macrophages. RAW 246.7 cells were pretreated with vitexin at 200 μM and 400 μM and then challenged with LPS for 8 h. The results showed that both the concentrations of vitexin inhibited the expression of iNOS and COX2 in a concentration-dependent manner (Fig. 4a). Thus, the results revealed that vitexin had identical effects as MSWE.
Fig. 4.

Vitexin pretreatment inhibited inflammation in LPS-stimulated RAW 246.7 cells. a Protein expressions of iNOS and COX2 were determined by western blot. b Translocation of NF-κB p65 subunit was determined by immunofluorescent staining
Identical effects of vitexin on nuclear translocation of p65 subunit in LPS-induced RAW 264.7 macrophages
As the effects of vitexin on iNOS and COX2 were similar to the effects of MSWE, we further investigated the underlying mechanism of vitexin to validate with MSWE. We used immunofluorescence staining to examine the nuclear translocation pattern of NF-κB p65 subunit from cytoplasm after being released from IκBα on vitexin pretreatment. As shown in Fig. 4b, vitexin pretreatment inhibited the translocation of NF-κB p65 subunit from cytoplasm to nucleus compared to the basal level of nuclear translocation in LPS-treated cells without vitexin pretreatment. Thus, the results clearly suggested that vitexin is one of the bioactive compounds in MSWE that inhibits inflammation through NF-κB regulation.
Protective effects of MSWE in LPS-induced ALI mice
The therapeutic potential of MSWE was investigated using LPS-induced ALI mice. The expression of iNOS in the liver of ALI mice was found to be higher compared to iNOS expression in control mice. Oral administration with MSWE prior to LPS injection was found to lower iNOS expression in mice (Fig. 5). To confirm the effects at transcriptional levels, mRNA expression of inflammatory markers in the liver was investigated. TLR4 is reported to be the primary signaling receptor for LPS in mammals (Leon et al. 2008). Subsequently, TLR4 expression was found to be upregulated in liver granulomas of LPS-induced ALI mice. A previous study suggests that LPS-induced stimulation of TLR4 activates reactive oxygen species (ROS) which results in acute hepatocyte injury and death in the liver (Leon et al. 2008). In our study, TLR4 mRNA expression was found to be significantly increased in the liver of LPS mice compared to that in control mice, while the expression was observed to be significantly decreased in MSWE treated mice (Fig. 6a). Further, we found that LPS injection markedly increased the expression of iNOS, COX2, TNFα, IL-1β, and IL-6 in ALI mice compared to the respective expression levels in control mice. Oral administration with MSWE was found to significantly inhibit the elevated expression of iNOS and IL-1β (Fig. 6b, e). Contrastingly, the reduction in COX2, TNFα and IL-6 expressions on MSWE administration was not significant compared to the reduction in ALI mice (Fig. 6c, d, f). Macrophage infiltration contributes to ALI on stimulation with GAL/LPS. An increased monocyte chemoattractant protein-1 (Mcp1) and Emr1 mRNA expression has been reported in liver injury (Sachithanandan et al. 2011). Additionally, mRNA expression of macrophage markers has been observed in this study. We found that LPS injection dramatically increased Mcp1 and Emr1 levels in ALI mice compared to that in control mice. However, only Emr1 was significantly decreased in the liver of MSWE-treated mice while the reduction in Mcp1 was not significant (Fig. 6g, h). All results in vivo indicated that oral administration of MSWE prior to LPS injection had protective effects on LPS-induced ALI in mice.
Fig. 5.
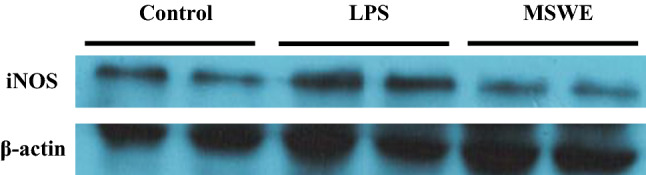
Oral MSWE pretreatment lowered iNOS expression in LPS-induced acute liver injury in mice. iNOS expression from liver extract was determined by western blot
Fig. 6.
MSWE pretreatment reduced inflammatory and macrophage markers in LPS-induced acute liver injury in mice. Relative mRNA expressions of aTLR4, biNOS, cCOX2, dTNFα, eIL-1β, fIL-6, gMCP1, hEmr1 were determined by real-time PCR. Columns represent mean ± SD. Different letters are statistically differences (p < 0.05)
Discussion
Our study reported anti-inflammatory role of MSWE in in vitro macrophages (RAW 246.7 cells) as well as in vivo LPS-induced ALI mice model. We reveal that MSWE inhibits NF-κB activation through TAK1/IκBα. Additionally, we identified vitexin, the major flavonoid in MSWE and investigated its anti-inflammatory activity.
Inflammation has been well reported to be associated with various human diseases which include diabetes, neurodegenerative diseases, and cancer (Liu et al. 2017). NF-κB and activating protein-1 (AP-1) are major regulators of stress response, cancer, and inflammation. There are diverse stimuli that induce pro-inflammatory responses through cytokine signaling such as TNFα, IL-1, and TLR signaling (Sakurai 2012). On LPS stimulation, activated TLR4 forms a dimer to recruit MyD88 and/or TRIF and then activates TAK1. Subsequently, TAK1 activates NF-κB and/or MAPK pathway that leads to translocation, synthesis, and release of pro-inflammatory mediators (Sakurai 2012; Takaesu et al. 2003). TAK1 is a part of the MAPK family that is proven to be a crucial component for NF-κB and AP-1 activation (Sakurai 2012). Thus, TAK1 is a key upstream kinase for inflammatory responses. TLR signaling activates TAK1 signaling pathway through feedback loop of TAK1 phosphorylation and polyubiquitination of TAK-binding protein 1 (TAB 1), TAB 2, and TAB 3 (Sakurai 2012). Consequently, TAK1 is considered as an essential kinase that plays significant role in cellular homeostasis by positively regulating cell survival and proinflammatory signaling pathways.
The objective of our study was to understand the molecular mechanism of natural extract and support its efficacy as a herbal medicine. In this study, we observed that MSWE inhibited the transcription of pro-inflammatory mediators (COX2, iNOS), pro-inflammatory cytokines (TNFα and IL-1β), and pro-inflammatory chemokine (CCL5) on LPS stimulation (Fig. 1a–f) which further resulted in the reduction of iNOS and COX2 expression levels (Fig. 1g). Although previous studies have reported that ethanolic mungbean extract and acetone–water mungbean extract inhibit the inflammatory cytokine expressions in J774 and RAW 246.7 macrophages, respectively (Lee et al. 2011; Zhang et al. 2013), the underlying mechanisms of these effects remain unclear.
To the best of our knowledge, this is the first study to reveal that MSWE pretreatment blocks NF-κB activation (Fig. 2a, b) which led us to investigate the upstream of this inhibition. In response to LPS treatment, TLR4 signaling is activated which results in increased phosphorylation of TAK1. Our results show that the anti-inflammatory effects of MSWE were due to the suppression of LPS-induced TAK1 phosphorylation without affecting the total TAK1 in RAW 246.7 cells (Fig. 2c). TAK1 is considered as an essential molecule for inhibition of inflammatory pathway by using a chemical inhibitor (5Z)-7-oxozeaenol or siRNA (Takaesu et al. 2003). Our results demonstrated that in response to LPS treatment, MSWE inhibits phosphorylation of ERK1/2 however does not effect the phosphorylation of p38 and JNK (Fig. 3). Collectively, the results indicated that MSWE inhibited LPS-induced inflammation in RAW 246.7 cells by inhibiting NF-κB activation through TAK1 and IκBα, and partially inhibiting ERK1/2 of MAPK pathway. Further, there have been reports on vitexin and isovitexin, major flavonoids present in the MSWE to be active compounds in mungbean (Li et al. 2012; Luo et al. 2016). In our study, we found that vitexin and isovitexin were the major flavonoids in the MSWE, which supported the previous findings. Moreover, we found that vitexin suppressed iNOS and COX2 protein expression (Fig. 4a) and inhibited the translocation of NF-κB (Fig. 4b) which indicated that the activation of NF-κB was suppressed by vitexin. TAK1 is considered as a key mediator of NF-κB that regulates TLR4 activation and an upstream signaling molecule required for IKKα/β, JNK, and p38 activation. However, the involvement of TAK1 in MAPK pathway has not been discussed yet. In fact, an earlier study in RAW 264.7 macrophages showed that on LPS stimulation, a squamosamide derivative inhibited the phosphorylation of TAK1; however it did not affect the phosphorylation of ERK1/2 (Pang et al. 2009). Previous study has also shown that vitexin from the Chinese hawthorn leaves inhibited ER-stress-induced NF-κB activation and expression of inflammatory cytokines in chondrocytes (Xie et al. 2018). The data suggested a possibility that MSWE might be inhibiting NF-κB activation partially through the reduction of endoplasmic reticulum stress induced by LPS in RAW 264.7 macrophages.
Macrophages are the immune cells against invading pathogens which play a crucial role in innate immune and inflammatory response. Furthermore, inflammatory response is the vital pathology of the various chronic diseases such as diabetes, neurodegenerative diseases, atherosclerosis, and some cancers (Ko et al. 2017). Thus, a study to understand the inhibition of inflammatory responses in macrophages will have a great impact on many chronic diseases. Our findings revealed that MSWE inhibited inflammatory responses in LPS-induced macrophages. Other studies have showed the preventive effects of black soybean seed coat extract on radiation-induced skin fibrosis and diabetes (Park et al. 2018) as well as vitexin assisted preventive effects on degeneration of cartilage in rats (Xie et al. 2018). As a result, we further investigated the anti-inflammatory effects of MSWE in LPS-induced ALI mice.
The intraperitoneal (IP) administration of lipopolysaccharide (LPS) alone or in combination with other hepatotoxic agents is widely used to induce systemic and hepatic inflammation in rodents (Hamesch et al. 2015). The present study showed that IP-injected LPS induced iNOS expression in the liver whereas oral administration of MSWE prior to LPS injection down-regulated the iNOS expression (Fig. 5). The mRNA expression of pro-inflammatory mediators and inflammatory markers was also reduced on oral administration of MSWE (Fig. 6). Moreover a crucial signaling receptor for LPS, TLR4 was reduced in MSWE-treated mice. Oral administration of MSWE also reduced the mRNA expression of macrophage markers (Fig. 6f). Overall, we found that oral administration of MSWE can prevent LPS-induced ALI in mice.
Our findings reveal the underlying mechanism of the anti-inflammatory role of mungbean seed coat extract and will further encourage the use of the mungbean seed coat as functional food and to increase the value.
Conclusion
MSWE exerted anti-inflammatory effects in both in vitro and in vivo. Its mechanism might be associated with the inhibition of IκBα degradation and NF-κB translocation by inhibiting phosphorylation of TAK1. TAK1 inhibition is reviewed as a major target for many kinds of inflammatory disorders. Interestingly, suppression of TAK1 has been shown to prevent renal inflammation and fibrosis (Ma et al. 2011) and neuronal death in cerebral ischemia (Neubert et al. 2011). This suggests that MSWE is beneficial to combat various inflammatory diseases. Further investigations are needed to show that the mungbean seed coat also has immense potential as a functional food.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Grant-in-Aid for International Cooperative Research, Graduate School of Integrated Sciences for Life, Hiroshima University, Japan.
Compliance with ethical standards
Conflict of interest
The other authors have no interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sudathip Sae-tan, Email: fagists@ku.ac.th.
Noriyuki Yanaka, Email: yanaka@hiroshima-u.ac.jp.
References
- Çalışkantürk Karataş S, Günay D, Sayar S. In vitro evaluation of whole faba bean and its seed coat as a potential source of functional food components. Food Chem. 2017;230:182–188. doi: 10.1016/j.foodchem.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Hamesch K, Borkham-Kamphorst E, Strnad P, Weiskirchen R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 2015;49:37–46. doi: 10.1177/0023677215570087. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- Jang YH, Kang MJ, Choe EO, Shin M, Kim JI. Mung bean coat ameliorates hyperglycemia and the antioxidant status in type 2 diabetic db/db mice. Food Sci Biotechnol. 2014;23:247–252. doi: 10.1007/s10068-014-0034-3. [DOI] [Google Scholar]
- Kang I, Choi S, Ha TJ, et al. Effects of Mung Bean (Vigna radiata L.) Ethanol extracts decrease proinflammatory cytokine-induced lipogenesis in the KK-Ay diabese mouse model. J Med Food. 2015;18:841–849. doi: 10.1089/jmf.2014.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko WK, Lee SH, Kim SJ, et al. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 2647 macrophages. PLoS ONE. 2017;12:e0180673. doi: 10.1371/journal.pone.0180673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Lee JH, Lee HH, et al. Effect of mung bean ethanol extract on pro-inflammtory cytokines in LPS stimulated macrophages. Food Sci Biotechnol. 2011 doi: 10.1007/s10068-011-0072-z. [DOI] [Google Scholar]
- Leon CG, Tory R, Jia J, Sivak O, Wasan KM. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. Pharm Res. 2008;25:1751–1761. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GH, Wan JZ, Le GW, Shi YH. Novel angiotensin I-converting enzyme inhibitory peptides isolated from Alcalase hydrolysate of mung bean protein. J Pept Sci. 2006;12:509–514. doi: 10.1002/psc.758. [DOI] [PubMed] [Google Scholar]
- Li H, Cao D, Yi J, Cao J, Jiang W. Identification of the flavonoids in mungbean (Phaseolus radiatus L.) soup and their antioxidant activities. Food Chem. 2012;135:2942–2946. doi: 10.1016/j.foodchem.2012.07.048. [DOI] [PubMed] [Google Scholar]
- Li AP, Li ZY, Jia JP, Qin XM. Chemical comparison of coat and kernel of mung bean by nuclear magnetic resonance-based metabolic fingerprinting approach. Spectrosc Lett. 2016;49:217–224. doi: 10.1080/00387010.2015.1133648. [DOI] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JQ, Cai WX, Wu T, Xu BJ. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016;201:350–360. doi: 10.1016/j.foodchem.2016.01.101. [DOI] [PubMed] [Google Scholar]
- Ma FY, Tesch GH, Ozols E, Xie M, Scheneider MD, Nikolic-Paterson DJ. TGF-beta1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am J Physiol Renal Physiol. 2011;300:F1410–F1421. doi: 10.1152/ajprenal.00018.2011. [DOI] [PubMed] [Google Scholar]
- Nair RM, Yang RY, Easdown WJ, Thavarajah D, Thavarajah P, Hughes JD, Keatinge JDH. Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J Sci Food Agric. 2013;93:1805–1813. doi: 10.1002/jsfa.6110. [DOI] [PubMed] [Google Scholar]
- Neubert M, Ridder DA, Bargiotas P, Akira S, Schwaninger M. Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 2011;18:1521. doi: 10.1038/cdd.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang HY, Liu G, Liu GT. Compound FLZ inhibits lipopolysaccharide-induced inflammatory effects via down-regulation of the TAK-IKK and TAK-JNK/p38MAPK pathways in RAW264.7 macrophages. Acta Pharmacol Sin. 2009;30:209–218. doi: 10.1038/aps.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Choi J, Kim J, et al. Anthocyanins from black soybean seed coat prevent radiation-induced skin fibrosis by downregulating TGF-beta and Smad3 expression. Arch Dermatol Res. 2018;310:401–412. doi: 10.1007/s00403-018-1827-7. [DOI] [PubMed] [Google Scholar]
- Peng X, Zheng Z, Cheng KW, et al. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106:475–481. doi: 10.1016/j.foodchem.2007.06.016. [DOI] [Google Scholar]
- Randhir R, Shetty K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov Food Sci Emerg Technol. 2007;8:197–204. doi: 10.1016/j.ifset.2006.10.003. [DOI] [Google Scholar]
- Sachithanandan N, Graham KL, Galic S, et al. Macrophage deletion of SOCS1 increases sensitivity to LPS and palmitic acid and results in systemic inflammation and hepatic insulin resistance. Diabetes. 2011;60:2023–2031. doi: 10.2337/db11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Sanada Y, Yamamoto T, Satake R, et al. Serum amyloid A3 gene expression in adipocytes is an indicator of the interaction with macrophages. Sci Rep. 2016 doi: 10.1038/srep38697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol. 2017;54:858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Burbach BJ, Shimizu Y. NF-kappaB activation in T cells requires discrete control of IkappaB kinase alpha/beta (IKKalpha/beta) phosphorylation and IKKgamma ubiquitination by the ADAP adapter protein. J Biol Chem. 2010;285:11100–11105. doi: 10.1074/jbc.M109.068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB. TAK1 is Critical for IκB Kinase-mediated Activation of the NF-κB Pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/S0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Veres B, Radnai B, Gallyas F, et al. Regulation of kinase cascades and transcription factors by a poly(ADP-Ribose) polymerase-1 inhibitor, 4-Hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2004;310:247–255. doi: 10.1124/jpet.104.065151. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Inaba Y, Kimura K, et al. Dietary mung bean protein reduces hepatic steatosis, fibrosis, and inflammation in male mice with diet-induced, nonalcoholic fatty liver disease. J Nutr. 2016;147:52–60. doi: 10.3945/jn.116.231662. [DOI] [PubMed] [Google Scholar]
- Xie CL, Li JL, Xue EX, et al. Vitexin alleviates ER-stress-activated apoptosis and the related inflammation in chondrocytes and inhibits the degeneration of cartilage in rats. Food Funct. 2018;9:5740–5749. doi: 10.1039/C8FO01509K. [DOI] [PubMed] [Google Scholar]
- Yanaka N, Koyama TA, Komatsu SI, Nakamura E, Kanda M, Kato N. Vitamin b6 suppresses NF-kappa B activation in LPS-stimulated mouse macrophages. Int J Mol Med. 2005;16:1071–1075. [PubMed] [Google Scholar]
- Yao Y, Chen F, Wang M, Wang J, Ren G. Antidiabetic activity of Mung bean extracts in diabetic KK-Ay mice. J Agric Food Chem. 2008;56:8869–8873. doi: 10.1021/jf8009238. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Li X, Zhang T, et al. Black soybean seed coat polyphenols prevent AAPH-induced oxidative DNA-damage in HepG2 cells. J Clin Biochem Nutr. 2017;60:108–114. doi: 10.3164/jcbn.16-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XW, Shang PP, Qin F, et al. Chemical composition and antioxidative and anti-inflammatory properties of ten commercial mung bean samples. Lwt Food Sci Technol. 2013;54:171–178. doi: 10.1016/j.lwt.2013.05.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.