Abstract
Purpose
Methylglyoxal (MGO) and MGO related advance end product (AGE) are thought to contribute to the development of diabetes and its complications. The present study was intended to determine plasma MGO and sRAGE levels in T2DM patients and to assess the relationship between MGO and other parameters, such as sRAGE and oxidative markers.
Methods
The study was carried out in 100 control and T2DM subjects. Methylglyoxal, sRAGE, HbA1c, and other markers were measured by using a standard protocol and the relationship between variables was analyzed using Spearman’s correlation analysis.
Results
Plasma MGO levels in patients with T2DM (221.1 ± 9.50 ng/mL) were significantly higher than in control subjects (121.1 ± 6.52 ng/mL, P < 0.001). The plasma level of MGO was positively correlated with glycosylated hemoglobin (HbA1c, r = 0.50, P < 0.001). Plasma soluble form of RAGE (sRAGE) was significantly decreased in T2DM subjects (5.3 ± 0.64 ng/mL) as compared to the control group (7.7 ± 0.86 ng/mL, p < 0.05). However, at increased level of glycation (HbA1c > 10%), the sRAGE level was 6.2 ± 0.42 ng/mL and was not statistically significant as compared to control healthy group (> 0.05). Moreover, we have not found any correlation between MGO and other markers (p > 0.05).
Conclusions
The findings of the present study showed that increased plasma MGO level is significantly associated with the HbA1c levels in T2DM patients. Moreover, the study shows that plasma sRAGE level is significantly augmented at increased level of glycation (HbA1c > 10%) in T2DM patients.
Keywords: Type 2 diabetes mellitus, Methylglyoxal, sRAGE, Oxidative stress
Introduction
Diabetes mellitus is a metabolic disorder that arises from an abnormality in insulin secretion, insulin action, or both that contributes to chronic hyperglycemia in the patients due to the disturbances in the metabolism of carbohydrate, fat, and protein [1]. According to the International Diabetes Federation, diabetes mellitus is estimated to impact more than 424 million people worldwide in 2017. The widespread prevalence of the disease is escalating and it is postulated that more than 628 million people will have diabetes by 2045 [2]. To prevent diabetic complications, early diagnosis and aggressive management of hyperglycemia are necessary. However, the high prevalence of diabetes mellitus and its related morbidity and mortality rates are at least partly since most people only get aware of this disease in a state when symptoms and complications already occur.
Hyperglycemia in which an excessive amount of glucose circulates in the blood is considered to be the main cause of diabetic complications [3]. A variety of toxic α-oxoaldehydes are produced under hyperglycemia in diabetes mellitus and methylglyoxal (MGO) is the key oxoaldehydes. Moreover, it can modify amino acids in proteins by reacting with arginine, cysteine, and lysine residues of proteins to form advanced glycation end products (AGEs) [4, 5]. In normoglycemic conditions, cells are protected against MGO toxicity by the glyoxalase system and are the most important pathway for the detoxification of MGO. Glyceraldehyde-3-phosphate (Glyceraldehyde-3P) and dihydroxyacetone phosphate (DHAP), the intermediates of glycolysis are the main precursors of the MGO. MGO detoxification requires a glyoxalase system that consists of the glyoxalase-1 and glyoxalase-2 enzymes. These enzymes react with MGO in the presence of reduced glutathione (GSH) to form a lactate [6].
MGO in hyperglycemia plays a key role in endothelial dysfunction that leads to insulin resistance, hypertension, and nephropathy in diabetic patients. It is significantly more reactive than glucose in glycation processes. Moreover, AGEs are scientifically validated well-known factors for the onset of diabetes and its complications [5]. Glycation of several biologically important proteins which leads to their impairment of its normal functioning [7]. There is growing evidence that interaction of AGEs and their specific receptor stimulates oxidative stress and inflammation that play a critical role in type 2 diabetes mellitus (T2DM) [8]. Besides, we have evaluated the levels of oxidative markers in T2DM patients and found the oxidative markers were significantly changed in T2DM patients [9]. Receptor for advanced glycation end product (RAGE) was discovered as a receptor for AGEs and several truncated forms of RAGE have been discovered [10]. The soluble form of RAGE (sRAGE) is either part of full-length RAGE or flaking/cleavage product of membrane-bound RAGE. It has been known that it is in the circulation of humans and can act as a decoy to avoid interaction of RAGE with its pro-inflammatory ligands (AGEs) [11].
Plasma glucose and HbA1c levels are key biochemical targets for the management of diabetes mellitus. However, many studies have established that the limitation of these biomarkers [12, 13]. Therefore, it is pertinent to explore the levels of MGO (a potent intermediary biomolecules) and sRAGE in T2DM. Therefore, the present study is undertaken to determine the levels of MGO and sRAGE in T2DM patients and evaluate its relation with oxidative markers.
Methods
The study was carried out in 100 subjects, in which 50 subjects were suffering from T2DM in the age group of 30–60 years, and on the other hand 50 healthy subjects were taken in the control group. We have further categorized the T2DM patients in three groups as per their HbA1c level (Group 1- HbA1c: 6.5- 8%, Group 2- 8.1-10%, Group 3- >10%) to find out the levels of sRAGE and MGO with progression to glycation level in patients. The pregnant females, the subjects having anemia, patients having kidney, liver, thyroid disease, and having an autoimmune disorder were excluded. The study was approved by the Institutional ethics committee for human study. Informed written consent was signed by patients before participating in the study. Sociodemographic data including, age, gender, height, weight, diabetes duration, and lifestyle were recorded. A blood sample was drawn after an overnight fast into plain and EDTA mix vacutainers (VACU- ETTE®, Greiner Bio-One). The plasma glucose level was measured using Siemens Dimension Xpand Plus automated biochemistry analyzer using the kit purchased from M/S Siemens, Mumbai, India. HbA1c level was analyzed in NGSP certified Bio-Rad D-10™ high-performance liquid chromatography system using the kit supplied by M/S Bio-Rad, Gurugram, India. The oxidative markers; plasma GSH, catalase, SOD and MDA levels were measured by spectrophotometric methods using standard protocols [14]. Plasma MGO level was measured using the photometric standard method [15]. Further, plasma sRAGE level was measured by ELISA method using the kits supplied by Bioassay Technology Laboratory, Shanghai, China.
Statistical analysis
All the analyses were performed in duplicate and data have been expressed as mean ± SEM or otherwise indicated. Descriptive statistics were applied to present demographic data. The correlation study was carried out by Spearman’s correlation analysis. The comparison of study groups was investigated using unpaired t-test. If the p-value was < 0.05, it was considered the statistical significant difference.
Results
The study was carried out in 100 subjects, out of this, 50 in the control group and 50 in the T2DM group. Out of 50 healthy subjects, 42% were male and 58% were female. Moreover, 46% and 54% of males and females, respectively were recruited in the diabetic group. There were 18% and 22% of smokers in control and T2DM groups, respectively (Table 1). The average plasma glucose level and HbA1c in diabetic subjects were 195.8 ± 13.19 mg/dL and 9.1 ± 0.29%, respectively and the levels were significantly higher in diabetic subjects as compared to the control group (p < 0.001). The level of malondialdehyde (MDA) was significantly higher in T2DM subjects (2.0 ± 0.1 µmol) as compared to control group (0.29 ± 0.02 µmol) and the p-value was < 0.001. The level of antioxidant superoxide dismutase (SOD) was significantly lower (p < 0.05) in T2DM subjects (14.4 ± 1.25 U/ml) than control group (18.3 ± 1.43 U/ml). The level of antioxidant catalase was significantly reduced in T2DM subjects (105.6 ± 9.00 KU) as compared to the control group (147.4 ± 11.28 KU) [p < 0.01]. Moreover, reduced glutathione level was decreased in T2DM subjects (71.9 ± 1.62 µmol) than control group (117.7 ± 2.05 µmol) and the p-value was < 0.001. Moreover, the levels of oxidative markers in T2DM subjects is similar to the data reported in our previous study [9]. The level of MGO was significantly higher in T2DM subjects (221.1 ± 9.50 nmol) than in control group (121.1 ± 6.52 nmol) [p < 0.001]. The level of soluble form of RAGE (sRAGE) was significantly lower in T2DM subjects (5.3 ± 0.64 ng/mL) as compared to control group (7.7 ± 0.86 ng/mL) [p < 0.05] (Table 2).
Table 1.
Demographic characteristics of the study subjects
| Control group (n = 50) mean ± SEM |
T2DM group (n = 50) mean ± SEM |
|
|---|---|---|
| Age (years) | 46.02 ± 1.38 | 48.78 ± 1.26 |
| Gender distribution (n) |
Male = 21 (42%) Female = 29 (58%) |
Male = 23 (46%) Female = 27 (54%) |
| Body weight (kg) | 58.02 ± 0.98 | 67.56 ± 1.63 |
| Duration of Diabetes (years) | - | 5.69 ± 0.94 |
| Smoker (n) | 09 (18%) | 11 (22%) |
p value > 0.05 (statistically not significant)
Table 2.
Biochemical parameters in control and T2DM subjects
| Biochemical parameter | Control (n = 50) mean ± SEM |
T2DM group (n = 50) mean ± SEM |
p value |
|---|---|---|---|
| Plasma glucose (mg/dL) | 100.9 ± 1.02 | 195.8 ± 13.19 | < 0.001 |
| HbA1c (%) | 5.2 ± 0.04 | 9.1 ± 0.29 | < 0.001 |
| MDA (µmol) | 0.29 ± 0.02 | 2.0 ± 0.10 | < 0.001 |
| SOD (U/ml) | 18.3 ± 1.43 | 14.4 ± 1.25 | < 0.05 |
| Catalase (KU) | 147.4 ± 11.28 | 105.6 ± 9.00 | < 0.01 |
| GSH (µMol) | 117.7 ± 2.05 | 71.9 ± 1.62 | < 0.001 |
| MGO (nmol) | 121.1 ± 6.52 | 221.1 ± 9.50 | < 0.001 |
| sRAGE (ng/ml) | 7.7 ± 0.86 | 5.3 ± 0.64 | < 0.05 |
Data are expressed as mean ± SEM. Data of patient group compared with control group. P < 0.05 considered as statistical significant difference. HbA1c-glycated hemoglobin; MDA- malondialdehyde; SOD-superoxide dismutase; GSH- reduced glutathione; MGO-methylglyoxal; sRAGE; soluble form of receptor for advanced glycation end product
Further, we have carried out the correlation study to establish any association between the biomarkers. MGO was significantly increased in diabetic patients compared to the control group. Moreover, MGO revealed a significant positive correlation with HbA1c (p = 0.0003, r = 0.4980) (Fig. 1). The oxidative markers were significantly increased in T2DM patients. However, no statistically significant correlation was found between sRAGE, MGO, and these markers (p > 0.05). Moreover, no statistically significant correlation was found between HbA1c and sRAGE (p = 0.54, r= -0.09) (Table 3). sRAGE was significantly decreased in diabetic subjects compared to the control group. Besides, we have further categorized the T2DM patients in three groups as per their HbA1c level (Group 1- HbA1c: 6.5- 8%, Group 2- 8.1-10%, Group 3- >10%) to find out the levels of sRAGE and MGO with progression to glycation level in patients (Fig. 2). We found that sRAGE level was significantly decreased in controlled diabetes (Group 1; HbA1c 6.5-8%) and interestingly, sRAGE level was augmented with increasing glycation in T2DM patients. Moreover, the levels of sRAGE in uncontrolled diabetes (group 3; HbA1c > 10%) is significantly increased as compared to group 1 (p < 0.001). The levels of sRAGE in group 3 are comparable to control subjects (p > 0.05). Further, the level of MGO was significantly increased at different levels of glycation as compared to the control group (p < 0.05).
Fig. 1.
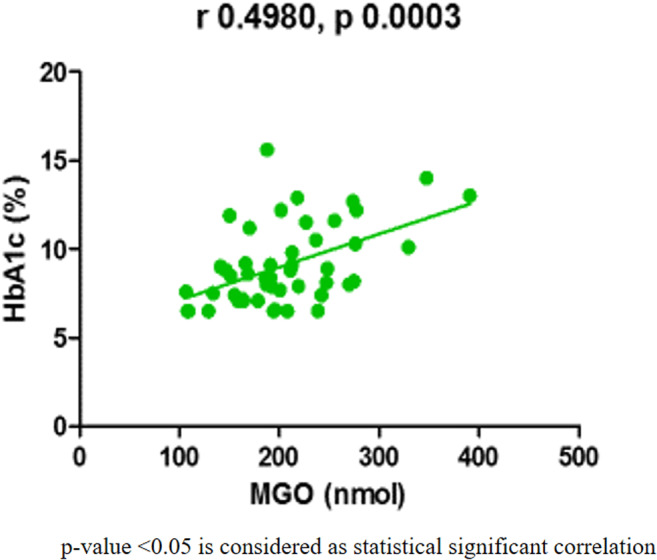
Linear regression curves between the MGO and HbA1c that showed significant correlations on Pearson’s correlation analysis in T2DM subjects (n = 50)
Table 3.
Correlation study (spearman correlation coefficient) of biomarkers in T2DM subjects
| Correlation study group | r-value | p-value |
|---|---|---|
| MGO vs. MDA | -0.098 | 0.5 |
| MGO vs. SOD | 0.11 | 0.47 |
| MGO vs. GSH | 0.034 | 0.81 |
| MGO vs. catalase | -0.097 | 0.51 |
| sRAGE vs. HbA1c | -0.09 | 0.54 |
| sRAGE vs. MGO | -0.004 | 0.98 |
| sRAGE vs. MDA | -0.20 | 0.18 |
| sRAGE vs. SOD | -0.2069 | 0.13 |
| sRAGE vs. GSH | -0.088 | 0.55 |
| sRAGE vs. catalase | -0.1744 | 0.23 |
HbA1c-glycated hemoglobin; MDA- malondialdehyde; SOD-superoxide dismutase; GSH- reduced glutathione; MGO-methylglyoxal; sRAGE; soluble form of receptor for advanced glycation end product. + r value showed positive correlation and – r value showed negative correlation. p > 0.05 (not significant)
Fig. 2.
Levels of sRAGE and methylglyoxal (MGO) in T2DM patients with progression to glycation level (HbA1c)
Discussion
Over the past decades, there has been a widespread increase in the incidence of diabetes mellitus. Diabetes is a risk factor for various micro and macrovascular complications such as retinopathy, neuropathy, cardiovascular disease, and nephropathy [16]. The present study has investigated the levels of MGO, sRAGEs and explored its association with oxidative markers in T2DM patients. Plasma MGO level is significantly augmented in diabetic patients compared to healthy humans. Moreover, MGO-related advanced end products (AGEs) are accountable for many diabetes-related complications in the patients [17, 4] [18]. MGO is the intermediary compound of many metabolic pathways. However, most of the MGO formed through glycolysis. The levels of Reactive Oxygen Species (ROS) are significantly increased in diabetes mellitus that lead to activation of nuclear enzyme, poly ADP ribose polymerase-1 (PARP) [19]. PARP activation depletes its substrate (NAD+), slows the rate of glycolysis and electron transport chain as a result accumulation of glycolytic intermediates such as MGO, consequently activating protein kinase C, polyol, and hexosamine pathways. The activation of these metabolic pathways disturb the cellular functions by decreased nitric oxide levels, altered gene expression and formation of AGEs [19]. In present study, we found that MGO level is significantly augmented compared to control group and shows a statistically significant correlation with HbA1c. Moreover, level of oxidative stress is significantly increased in T2DM patients. An organism has a compensatory mechanism against oxidative stress by the synthesis of several antioxidant enzymes. However, the synthesis of these enzymes is impaired or overused in T2DM patients as compared to healthy control subjects [1]. Superoxide dismutase (SOD) is the antioxidant enzyme that catalyzes the conversion of superoxide anion (O2−) into hydrogen peroxide and molecular oxygen. It plays very significant protective roles against cellular and histological damages that are produced by ROS [20]. The effect of MGO in Cu, Zn-SOD activity was studied by in vitro incubation and found progressive increased covalent crosslinking of the protein (enzyme), led to the loss of enzymatic activity [21]. MGO mediated inactivation of Cu, Zn-SOD may perturb the antioxidant system. It is also reported that MGO mediated oxidative stress in pancreatic β-cells leads to the β-cell dysfunction and impairment of insulin production [22]. In present study, we found that SOD level is significantly decreased in T2DM patients as compared to control group (< 0.05). However, there is no direct correlation found between MGO and SOD (p > 0.05). In present study, plasma MDA level was significantly increased in T2DM patients, however, it is not showing any correlation with MGO. A study was conducted in T2DM showed a positive correlation of MDA with hyperglycemia. However, there was no association was found between levels of MDA and duration of diabetes [23]. A study conducted in newly diagnosed T2DM patients showed a positive correlation between MGO and MDA levels [24]. It is observed that the level of lipid peroxide escalates as per the increase in the concentration of blood glucose and its reactive compound (MGO) [24]. There is an increase in lipid peroxidation as reflected by the increase in plasma levels of MDA. Moreover, similar observation was found in our previous study in which MDA level is significantly increased in microalbuminuria group of T2DM [9]. It has been also proved that reduced glutathione has a role in detoxification of MGO [6]. In present study, plasma GSH level was significantly decreased in T2DM patients as compared to control group (p < 0.001). It is well established that hyperglycemia leads to oxidative stress in T2DM patients [14]. However, in present study, the by-product of glucose, i.e. MGO is not showing any correlation with oxidative markers.
MGO is a highly formidable glycating agent with significant higher reactivity than glucose and generates MGO-adducts such as 8-OH-dG (8-hydroxy-deoxyguanosine); CEdG (N2(1-carboxyethyl)-deoxyguanosine); Nδ-(5-hydro-4-imidazolon-2-yl) ornithine (G-H1); fructosyllysine (fl.); Nε-carboxymethyl-lysine (CML); and bis(lysyl) crosslinks –MOLD (methylglyoxal lysine dimer). The generation of these adducts causes terrible effects on cellular functions and induction of AGE-RAGE interactions resulting in detrimental conditions [25]. The binding of AGEs with receptors for advanced glycation end-products (RAGEs) leads to oxidative stress and inflammation in cells by the activation of nuclear factor κB pathway [26]. The soluble form of RAGE (sRAGE) is present in the plasma and acts as a ‘decoy’ by binding pro-inflammatory ligands such as AGEs and averting them from reaching membrane RAGE [11]. In present study, we found that the plasma sRAGE level is considerably decreased in diabetic patients as compared to control group. However, sRAGE level is increased in T2DM patients with increasing glycation levels in patients (HbA1c > 10%). The reason for this augmentation is not very clear but may have some role of other mystifying factors like the duration of diabetes, inflammation, and diabetic complications [27, 28]. Besides, a study showed that the levels of sRAGE was significantly increased in type 1 diabetes mellitus and was considerably associated with the incident of cardiovascular disease. Moreover, the HbA1c level in the study group was 9.5 ± 1.5% [29]. Further, sRAGE has not shown any correlation with HbA1c and other oxidative markers. Very limited studies are available in the association of MGO and sRAGE in T2DM patients. The putative role of MGO and sRAGE in T2DM is summarized in Fig. 3. Hyperglycemia leads to increased production of MGO and reactive oxygen species in the T2DM patients. Moreover, hyperglycemia along with MGO increased the synthesis of AGEs as a result of dysregulation of plasma sRAGE levels and that may have a role in the onset of T2DM and its complications. The sample size is the limitation of the present study. Moreover, future studies are further required to establish the relation of MGO with sRAGE and its role in the onset of diabetes and its complications.
Fig. 3.
Putative role of MGO and sRAGE in T2DM
Conclusions
MGO is a reactive glucose metabolite and a major precursor for AGEs. In summary, we concluded that MGO level was significantly increased in T2DM patients and do not show any relationship with oxidative markers in T2DM patients. The sRAGE level was significantly decreased in T2DM patients. However, at increased level of glycation (HbA1c > 10%), the level of sRAGE was significantly increased and value is comparable to the healthy control group. These findings suggest that plasma soluble form of RAGE (sRAGE) and MGO may serve as a potential biochemical target (s) for the onset of diabetes, its progression, and complications.
Acknowledgements
The authors are grateful for financial support from the Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi. This research did not receive any specific grant from funding agencies.
Abbreviations
- T2DM
type 2 diabetes mellitus
- MGO
methylglyoxal
- RAGE
Receptor for advanced glycation end product
- sRAGE
soluble form of receptor for advanced glycation end product
- AGEs
Advanced glycation end products
- HbA1c
glycated hemoglobin
- MDA
malondialdehyde
- SOD
superoxide dismutase
- GSH
reduced glutathione
- CML
Nε-carboxymethyl-lysine
- MOLD
methylglyoxal lysine dimer
Author contributions
AR, KC, S Alam, SK, and S Agarwal designed the study. AR, S Alam, and SK participated in subjects recruitment. AR, KC, S Alam conducted the assays. AR, KC, SK, S Agarwal analyze and interpreted the data. AR and KC prepared the manuscript. All authors critically read and edited several drafts before submission and approved the final manuscript.
Compliance with ethical standards
Conflicts of interest
We confirmed that there are no known conflicts of interest associated with this publication.
Source(s) of support
Hamdard Institute of Medical Sciences and Research, Jamia Hamdard.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandra K, Dwivedi SP, Jain S. Diabetes mellitus and oxidative stress: A co-relative and therapeutic approach. J Clin Diagn Res. 2019;13:7–12. [Google Scholar]
- 2.IDF Diabetes Atlas. 8th ed. 2017.
- 3.Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes – second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47:39–48. doi: 10.3109/10715762.2013.789136. [DOI] [PubMed] [Google Scholar]
- 4.Rabbani N, Thornalley PJ. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes. 2014;63:50–2. doi: 10.2337/db13-1606. [DOI] [PubMed] [Google Scholar]
- 5.Thornalley PJ. Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci. 2005;1043:111–7. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 6.Allaman I, Bélanger M, Magistretti PJ, Sims N, Bolanos JP. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9:23–35. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh Jaggi A, Parkash Singh V, Bali A, Singh N. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kay AM, Simpson CL, Stewart JA., Jr The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res. 2016;2016:6809703. doi: 10.1155/2016/6809703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balyan K, Sharma P, Chandra K, Agarwal S, Jain SK. Oxidative stress is independent factor for end-stage renal disease in type 2 diabetes mellitus patients. Ann Natl Acad Med Sci. 2018;54:147–52. [Google Scholar]
- 10.Ramasamy R, Yan SF, Herold K, Clynes R, Schmidt AM. Receptor for advanced glycation end products: fundamental roles in the inflammatory response: winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann N Y Acad Sci. 2008;1126:7–13. doi: 10.1196/annals.1433.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: Potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–35. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller SR. A Summary of the ADVANCE Trial on Behalf of the ADVANCE Collaborative group. Diabetes Care. 2009;32:357–61. doi: 10.2337/dc09-S339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra K, Khan W, Jetley S, Ahmad S, Jain S. Antidiabetic, toxicological, and metabolomic profiling of aqueous extract of Cichorium intybus seeds. Pharmacogn Mag. 2018;14:377. doi: 10.4103/pm.pm_583_17. [DOI] [Google Scholar]
- 15.Gilbert RP, Brandt RB. Spectrophotometric determination of methyl glyoxal with 2,4-dinitrophenylhydrazine. Anal Chem. 1975;47:2418–22. doi: 10.1021/ac60364a003. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Randell E, Han Y, Adeli K, Krahn J, Meng QH. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem. 2011;44:307–11. doi: 10.1016/j.clinbiochem.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care. 2013;36:3234–9. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacher P, Szabó C. Role of poly(ADP-Ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–80. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, et al. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N Am J Med Sci. 2013;5:213–9. doi: 10.4103/1947-2714.109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JH. Modification and inactivation of human Cu,Zn-superoxide dismutase by methylglyoxal. Mol Cells. 2003;15:194–9. [PubMed] [Google Scholar]
- 22.Bo J, Xie S, Guo Y, Zhang C, Guan Y, Li C, et al. Methylglyoxal impairs insulin secretion of pancreatic β-cells through increased production of ROS and mitochondrial dysfunction mediated by upregulation of UCP2 and MAPKs. J Diabetes Res. 2016;2016:1–14. doi: 10.1155/2016/2029854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal M, Varghese A, Gaviraju VK, Talwar SN, Malini SS. Impact of hyperglycaemia on molecular markers of oxidative stress and antioxidants in type 2 diabetes mellitus. Clin Diabetol. 2019;8:215–22. doi: 10.5603/DK.2019.0015. [DOI] [Google Scholar]
- 24.Kong X, Ma M, Huang K, Qin L, Zhang H, Yang Z, et al. Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes. J Diabetes. 2014;6:535–40. doi: 10.1111/1753-0407.12160. [DOI] [PubMed] [Google Scholar]
- 25.Stitt AW. AGEs and diabetic retinopathy. Investig Ophthalmol Vis Sci. 2010;51:4867–74. doi: 10.1167/iovs.10-5881. [DOI] [PubMed] [Google Scholar]
- 26.Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, et al. RAGE Axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–9. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 27.Biswas SK, Mohtarin S, Mudi SR, Anwar T, Banu LA, Alam SMK, et al. Relationship of soluble RAGE with insulin resistance and beta cell function during development of type 2 diabetes mellitus. J Diabetes Res. 2015;2015:1–6. doi: 10.1155/2015/150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas MC, Woodward M, Neal B, Li Q, Pickering R, Marre M, et al. Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care. 2015;38:1891–7. doi: 10.2337/dc15-0925. [DOI] [PubMed] [Google Scholar]
- 29.Nin JWM, Jorsal A, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, et al. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12-year follow-up study. Diabetes. 2010;59:2027–32. doi: 10.2337/db09-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]




