Abstract
The co-crystalized powder prepared from Securigera securidaca seed extract was used for the formulation of a functional beverage in the study. In doing so, various ratios of co-crystalized powder from the plant extract, water, citric acid, mint essential oil, honey, apple extract, and stevia were mixed together. Three beverage formulas along with the control sample were prepared and evaluated by panelists using six-point hedonic scale. The formula containing 2 g of the extract powder was introduced as the best beverage from the panelists’ perspective and received an average score. The samples were stored at 4 °C for 3 months and examined for pH, acidity, brix, turbidity, vitamin C, phenolic compounds, radical scavenging capacity, total microorganism count, molds and yeasts to evaluate shelf life. The pH and acidity changes were almost constant until day 60; but pH and acidity were decreased and increased in the last month, respectively. The brix changes were slight and had a slight decrease in the last month of storage. Although turbidity changes were small, they were associated with an increase over the storage time. Moreover, the beverage could well retain the phenolic compounds and the radical scavenging capacity and the antioxidant activity had a little change during storage. The changes trend in vitamin C was declining and equal to 16.85 mg/ml in the last month. No mold and yeast contamination were observed in any of the treatments according to ISO 21527 and mesophilic aerobic bacterial counts were in the range based on the ISO 4833. Hence, the herbal beverages produced with 2 g of the extract powder can replace industrial beverages given their proper chemical and microbial properties.
Keywords: Functional beverage, Co-crystalized, Herbal extract, Natural antioxidant, Shelf life
Introduction
Herbal drinks have been in the public interest for thousands of years, and today, their use as a popular beverage and natural and healthy product is rising (Pyrzynska and Sentkowska 2019). Medicinal herbs possess unique and valuable properties and thus have been of great significance so far. The extracts obtained from the plants have long been used as fragrant or flavoring in foods, beverages, and as herbal medicines (Van Vuuren et al. 2009). Natural plant sources have a high variety of naturally occurring active compounds such as flavonoids, terpenes, vitamins, terpenoids, carotenoids, coumarins, alkaloids, polyacetylene and ferulic acids (Chandrasekara and Shahidi 2018). Phenolic compounds, as one of the main constituent groups of medicinal herbs, are responsible for preventing diseases such as cancer and cardiovascular disease. Natural bioactive compounds found in medicinal plants have antimicrobial, antioxidant, anti-allergic, anti-inflammatory, vasodilator, as well as anti-irritant and antiviral activities (Pyrzynska and Sentkowska 2019; Chandrasekara and Shahidi 2018). In this regard, the dietary pattern of developed and developing societies has guided towards the production of functional and healthful food with higher quality and nutritional value (Contor 2001). Securigera securidaca (L.) is an annual herbaceous plant with a height of 10–40 cm and no fluff widely spread on the ground with many branches. It has a light brown fruit with each fruit having several quadrilateral and flat reddish-brown seeds. This plant is found all over the world and mostly grows along the gardens, streams, and wheat fields (Ghahraman 1993). The most important compounds reported in the plant seed include the triterpenoid saponins, cardenolides, flavonoids, coumarins, and sterols. In addition to the mentioned compounds, sterol compounds have also been reported in this plant (Sameni et al. 2016; BehnamNik et al. 2019). Seeds of S. securidaca are used in traditional medicine as lowering blood pressure and sugar; treatment of diabetes and hyperglycemia; and anticonvulsants drug (Ali et al. 1998). The consumption of functional beverages with low sugar and calorie has been focused by public in recent years. Thus, although sugar is an essential element in human diet, the use of natural sugars instead of synthetic sweeteners has been increased (Sylvetsky et al. 2019; Corbo et al. 2014). Among these sweeteners, stevia with sugar more than sucrose, fewer calories, and lower glycemic index, obtained from stevia leaves, is a suitable herbal sweetener (Gasmalla et al. 2014). Antioxidants and phenolic compounds are sensitive to the environmental conditions and undergo oxidation and degradation reactions during food processing and storage, resulting in reduced and loss of antioxidant property (Kaushik and Roos 2007). Microencapsulation can be an approach to overcome these problems. The process of microcapsulation is a strategy to protect certain food properties such as flavor, color, texture, sensitive compounds, etc. that leads to desirable nutritional, industrial or therapeutic properties. (Kaushik and Roos 2007). Microencapsulation by co-crystallization method includes spontaneous crystallization of sucrose at temperatures above 120 °C that leads to accumulation of very fine sucrose crystals in the range from 3 to 30 μm (Bhandari et al. 1998). This process can be detected by observing turbidity in the syrup that results from formation of irregular agglomerates. The latent heat of crystallization during this process is high enough for evaporation of moisture so that a dry final product can be obtained. The products obtained during this process can be used as sugar-based raw materials to coat the bitter taste of many active compounds (Awad and Chen 1993). Securigera securidaca has a bitter taste and the purpose of microencapsulation on the sucrose matrix is to reduce the bitter taste of the extract by mixing it with sucrose besides preserving antioxidant compounds. The purpose of this study was to determine the effect of herbal beverage formulation on the sensory and qualitative characteristics of the beverage during the storage time.
Materials and methods
Extracting
In this study, S. securidaca seed was obtained from Razavi Khorasan province, Iran as needed and its extra parts were cleaned and dried naturally after washing. For extraction by maceration, the cleaned S. securidaca seeds were crushed with a mill, and after sifting, the seeds were mixed with 1:10 wt% Ethanol solvent. Then the sample, in a semi-continuous form, was placed in an ultrasound (Euronda 4D) device for 30 min at 50 Hz and temperature of 30 °C. Then, it was placed in hot plate (Velp) at 300 rpm for 24 h at ambient temperature and filtered using Whitman No. 1 filter paper. It was concentrated at 40 °C for 40 min, dried under a hood (Lab Tech), and kept in a closed, non-permeable to air container at 4 °C until use to remove the solvent and carry out the extraction by rotary evaporator (Stuart RE300B) (Rakić et al. 2006).
Extract microencapsulation
Sucrose (Merck) was used to make the powder. In doing so, 3 mg of S. securidaca seed extract was mixed with 50 g sucrose. The obtained mixture was stirred continuously in a metal container on a heater until it reached 132 °C. Heating was stopped upon seeing slight turbidity in the syrup; whereas, stirring was continued until the mixture reached ambient temperature. Then, the co-crystalized product was dried for 40 h by oven (Memmert) at 40 °C for 15 h, then milled, sieved and kept in air and moisture-proof plastic packages for the next examinations (López-Córdoba et al. 2014).
Optimizing the formulation of the functional beverage
Beverage formulation
Three types of beverages were formulated from different amounts of extracts, peppermint essential oil, apple extract, natural stevia honey and citric acid (Table 1). The raw materials were thoroughly mixed after mixing with formulation. The beverage was then poured into a PET bottle and sealed. Then, information related to each bottle, including formulation number and date of manufacture was recorded on the bottle label. For pasteurizing the beverages, the bottles were pasteurized by water bath for 10 min at 75 °C and then cooled up to 35 °C (Mishra and Hati 2016).
Table 1.
Formulation of the manufactured beverages
| No. | Co-crystalized powder (g) | Mint (ml) | Stevia (g) | Apple extract (ml) | Water (ml) | Citric acid (g) | Honey (ml) |
|---|---|---|---|---|---|---|---|
| A | 1 | 0.1 | 10 | 1 | 150 | 1 | 1 |
| B | 2 | 0.1 | 11 | 1.5 | 150 | 1 | 2 |
| C | 4 | 0.1 | 15 | 2 | 150 | 1 | 3 |
| Control | – | 0.1 | 10 | 1 | 150 | 1 | 1 |
The samples were stored for 90 days at 4 °C (fridge) for 90 days to examine their shelf life. During the storage, the chemical analysis including pH, brix, acidity, turbidity, vitamin C, phenolic compounds, antioxidant compounds, total count of microorganisms, molds and yeasts were measured in three iterations during the storage at 0, 30, 60, and 90 days.
Evaluation of sensory properties
Sensory evaluation of the samples was done by 10 panelists who has been selected from students. The samples were evaluated for color, taste, turbidity, odor, deposition, and general acceptance (General acceptance means the final opinion of the panelists on the beverage). The scores were assigned based on a 6-point hedonic scale (5 for the best and 1 for the worst) (AOAC 2005).
Physicochemical analysis
Determining pH
A digital pH meter (EDT GP 353) was used to determine pH, which was calibrated by buffers 4 and 7 (Merck) and the pH of the samples was measured at 20 °C (AOAC 2005).
Measuring brix
Beverages brix was measured using a refractometer (AKRUSS HR) at 20 °C (AOAC 2005).
Measuring acidity
To measure the total acidity, 20 ml of beverage was poured into beaker and some drops of phenolphthalein reagent (Merck) were added to it and stabilized with 0.01 N sodium hydroxide solution (Merck) until a light pink color was created (titrated for about 30 s by burette 25 ml TGI). Then, the acidity was calculated using the following formula (Silva et al. 1999).
Measuring turbidity
Turbidity change during storage was measured by spectrophotometer (Jenway 6305) by measuring the amount of light passing at 800 nm (Penders et al. 1998).
Measuring vitamin C
HPLC machine (Knauer model) was used. Isolation was done with c18 column, Eurospher, with dimensions (5 × 4/6 × 250) and injection volume of 20 µl and column temperature was constant at 25 °C. Phosphate buffer 1(pH 2.8) was used as the solvent at a flow rate of 0.7 ml/min (Meléndez et al. 2004).
Measuring phenolic compounds
The amount of phenolic compounds was measured by Folin–Ciocalteu colorimetric method. 0.5 ml of beverage samples formulated with 2.5 ml of Folin–Ciocalteu reagent 1 N (Merck) was mixed in the test tube and after 10 min, 2 ml of 7.5% sodium carbonate solution (Merck) was added and incubated in the dark. The absorbance was read using a spectrophotometer (Jenway 6305) at 760 nm. The gallic acid standard curve was drawn in the concentration range of 0.04–0.4 mg/ml and the amount of phenolic compounds based on gallic acid was calculated based on the equation. (Stoilova et al. 2007).
Measurement of percentage of free radical scavenging (DPPH)
Firstly, 0.5 ml of the beverage samples were added to 3.5 ml of 0.004% DPPH (Sigma) solution in ethanol. After 40 min of darkening at room temperature, the sample light absorbance was read at 517 nm against blank. The percentage of free radical scavenging (DPPH) was calculated using the following formula (Wojdyło et al. 2007).
Microbial count, mold, and yeast
Pour Plate method was used to count the total microorganisms according to ISO 4833 standard and ISO 21527 standard was used to count molds and yeasts (ISO 4833-1 2013; ISO 21527-1 2008).
Statistical analysis
Data was analyzed using completely randomized design in SPSS, version 24, software. The means were compared using Duncan’s test at 5% confidence level and the graphs were plotted in Excel 2019. All experiments were performed in three replications.
Results and discussion
Examining sensory and qualitative properties
Table 2 shows the results of sensory evaluation of various beverage treatments. Statistical analysis of data obtained from questionnaires completed by panelists indicated that the samples had no significant differences in terms of color. Moreover, there were no significant differences between the two groups in terms of odor (P > 0.05). The results of statistical analysis showed that B treatment was significantly different in taste and as compared with other treatments and with 3.86 points, it received the best score in taste according to the panelists (P < 0.05). Examining deposit and turbidity of treatments showed no significant differences between different beverage samples. Statistical analysis showed no significant differences between treatment A and control sample (P > 0.05). The best treatment in terms of sensory and qualitative properties as selected by the evaluator panelists was treatment B and the worst score was given to treatment C, which can be related to the bitter extract of the plant, which was increased with its increase in treatment C. The bitter taste overcame the sweetness of the drink and was neither favored by the panelists, nor could the bitter taste of stevia be overlooked at higher concentrations. S. Securidaca has a bitter taste and one of the reasons for the selection of the microcapsulation based on co-crystallization method was the reduction of bitter taste due to the use of sucrose in this method. According to Table 1, in the treatment C, more extract of the plant and Stevia was used than the other treatments. The greater the amount of Stevia, the more bitter the taste in the mouth, and using more amount of the plant extract, due to its bitter taste, leads to the dominance of bitter taste in the beverage. Therefore, the panelists attributed the lower score of the treatment C to the bitter taste. Hashemi et al. (2015) examined the effect of stevia sugar substitution with sucrose on the physicochemical and sensory properties of saffron diet syrup and reported only significant differences in taste and overall acceptance between the treatments (P < 0.05). The rest of the traits were statistically identical. They reported that when stevia sugar is added, the taste will be slightly bitter and not favorable to the consumer. Thus, this effect was observed less in the samples with sucrose content of more than 50%.
Table 2.
The sensory and qualitative characteristics of the beverages made with various formulations
| Treatment | Color | Odor | Taste | Deposit | Turbidity | General acceptance |
|---|---|---|---|---|---|---|
| Control | 3.80 ± 0.67a | 3.00 ± 0.65a | 3.13 ± 0.35b | 3.73 ± 0.45a | 3.60 ± 0.63a | 3.26 ± 0.45b |
| A | 3.80 ± 0.67a | 3.06 ± 0.59a | 3.13 ± 0.91b | 3.40 ± 0.50a | 3.66 ± .061a | 3.26 ± 0.59b |
| B | 3.76 ± 0.48a | 3.40 ± 0.50a | 3.86 ± 0.35a | 3.33 ± 0.89a | 3.60 ± 0.63a | 3.86 ± 0.35a |
| C | 3.66 ± 0.48a | 3.13 ± 0.63a | 1.93 ± 0.59c | 3.33 ± 0.61a | 3.26 ± 0.45a | 2.40 ± 0.63c |
*Values are means of three determinations ± SD. Similar letters within a column indicate no significant differences (P > 0.05)
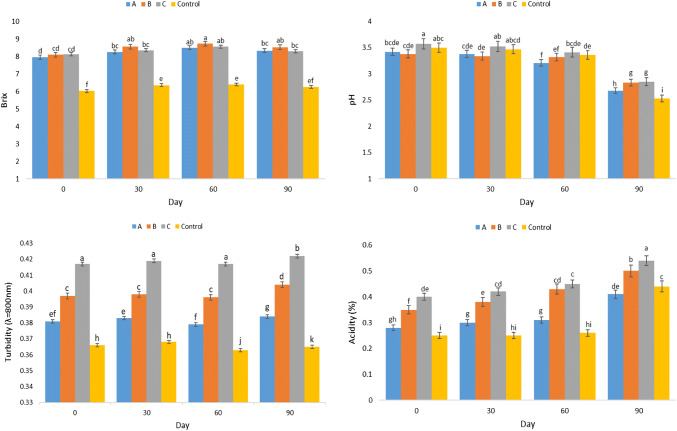
Examining pH changes
According to Fig. 1, pH changes of the formulated beverages indicated a decreasing trend over 90-day storage at 4 °C. Nonetheless, statistical analysis showed no significant differences in pH of the formulated beverages during the first 60-day storage and only treatments A and B showed significant differences at 5% level compared to the previous days of storage. However, at day 90, all treatments and control samples indicated a significant difference compared to the previous days. Changes in pH are often caused by chemical and biological reactions. Beverages and juices low pH, and the presence of fermentable sugars make their environment selective for the growth of certain groups of acidophilic bacteria like lactic acid bacteria, which decrease environment’s pH given the production of metabolites and they become acidic (Varnam and Sutherland 1994). Ardali et al. (2014) stated that by an increase in the percentage of stevia sugar, pH was decreased in the orange juice samples. However, Alizadeh et al. (2014) reported that replacing stevia sugar with sugars consumed in fruit milk production does not decrease or increase the pH of the samples. Perhaps the difference in pH changes of the nutrient besides the bacterial activity depends on the type, nutrient system and other soluble compounds. There is no universal standard regarding pH value of non-carbonated extracted herbal extracts, yet according to the Iranian Standard, non-carbonated herbal beverages (INSO 11077) the standard pH value is between 2.5 and 5.5 and all beverages formulated in this range remain during storage (INSO 11077 2016).
Fig. 1.
Changes in pH, brix, acidity and turbidity of formulated beverages during storage
Examining brix changes
The brix changes in the formulated beverage and the control sample is shown in Fig. 1. The statistical results showed a significant increase in brix at day 30 between the beverage and control samples compared to the production day (P < 0.05). However, on the day 60, the treatments showed no significant differences (P > 0.05) with each other and other treatments and only the control sample had significant difference with other treatments. At day 90 of storage, a slight decrease in brix of all samples was observed but no statistically significant differences were observed between the treatments at day 60 of storage (P > 0.05). The cause of the change in brix value of the product is sugars consumption by microorganisms (Varnam and Sutherland 1994). Brix include water-soluble solids and sugars in the beverage are part of it. Microorganisms need to consume sugars in the beverage to grow, thus over time, the sugars are consumed by the microorganisms and the amount of brix is reduced by the reduction of the sugar amount (Krasaekoopt et al. 2008; Salari et al. 2014). Lack of change in beverage brix during the storage showed that the outcome of effective parameters for product storage like temperature and pasteurization time, product composition and type of packaging was such that prevented effective reactions in brix change. According to ISO 2173 standard, brix value should not be less than 5 (ISO 2173 2003). In addition, according to Iranian Standard, non-carbonated herbal beverages, brix value should be 8.5 at maximum showing that the formulated beverages were within the standard range (INSO 11077 2016).
Examining acidity changes
Figure 1 shows the acidity changes in the formulated and control samples of S. securidaca plant. Statistical analyses showed that the acidity of the treatments showed no significant changes after the storage time from production until 60 day of storage (P > 0.05). Only treatment B at day 60 of storage had a statistically significant difference, compared to the previous days, at 5% level. From day 60 on, there was a sudden and significant increase in all treatments compared to each other and the month before the storage. The highest increase was for the control sample, so that acidity was increased from 0.25% at day 0 to 0.44% at day 90 (P < 0.05). The reason for the increase in acidity could be the increased production of acid by microorganisms, which reduces the pH of the beverage and makes the acidic environment (Zakipour Rhomabadi et al. 2018). Total acidity of non-carbonated herbal beverages according to standard (INSO 11077) should be 0.5 g per 100 ml, with the produced beverage at the standard range (INSO 11077 2016).
Examining turbidity changes
Figure 1 shows the turbidity changes of beverages formulated during the storage. There were no statistically significant differences between the treatments until day 30 of the storage (P > 0.05). There were no significant differences between samples B and C on day 60 of the storage, yet the difference between control and A samples was statistically significant (P < 0.05). The changes in turbidity at day 90 of storage was statistically significant in all treatments (P < 0.05). The general flow of changes in turbidity of the different beverage treatments during storage was increasing, but these changes were slight and gradual until day 60 as shown in the graph. However, the changes were almost constant but increased in the final month of the storage which was statistically significant. The highest turbidity during storage was in treatment C, and then treatment B and A, respectively and the least turbidity was in the control. Phenolic compounds like tannins, pectic compounds, proteins and polysaccharides like cellulose and starch are among the most significant reasons of turbidity, each of which alone or together (by shaping complexes between phenolic substances and proteins, phenolic and pectin substances, phenolic substances together, and so on) play a role in turbidity that add to turbidity with the pass of time (Varnam and Sutherland 1994; Hassani 2005). Given the lack of inadequate information on the presence of the above compounds in S. securidaca or other ingredients used in the formulation of the beverage, these factors cannot be assumed definitively effective. Other substances used in beverage formulation may contribute to transmission of turbidity agents. Elhami Rad and Mohammadi (2006) reported a significant increase in the rate of change in the duration of 6 months storage of carbonated beverage syrup and reported a statistically significant effect of time on turbidity in all treatments at 0.05% confidence level.
Examining the changes in vitamin C
Vitamin C has a significant role in the body in preventing oxidative processes and preventing illnesses like cancer and scorbut (McCall and Frei 1999). The trend of vitamin C changes was declining in the best beverage of choice in terms of sensory properties (treatment B) during 90-day storage, which was statistically significant (P < 0.05). The reason for this decrease can be the extremely high sensitivity of vitamin C to environmental conditions. Vitamin C is extremely sensitive to storage conditions and is rapidly affected and loses its properties. Keeping beverage in the fridge (at 4 °C) has not been able to fully protect the vitamin C during storage, and the trend of the changes has been decreasing. The value of vitamin C on day 0 of production was increased from 28.46 to 16.85 mg/ml on day 90, which was statistically significant (P < 0.05). Singh (2018) reported a declining trend in the changes of vitamin C of carbonated beverage made from hemidesmus indicus root during 28 days of storage which was statistically significant at 5% level.
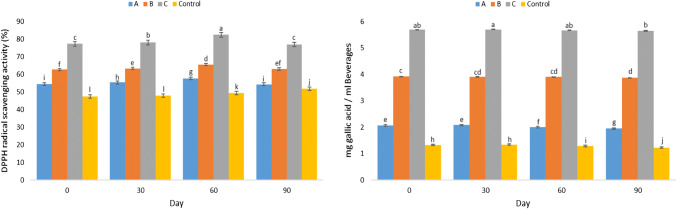
Examining the changes in phenolic compounds and radical scavenging capacity of the beverages
Phenolic compounds exist in vegetables, fruits, herbs, teas and juices; thus, they are essential components of the human diet that reduce the risk of cardiovascular disease, stroke, and certain cancer types (Klimczak et al. 2007; Kaur and Kapoor 2001). Phenolic compounds prevent free radical activity by donating hydrogen atoms (Hajimahmoodi et al. 2012). Figure 2 shows the changes in phenolic compounds and antioxidant activity of the beverages. The highest value of phenolic compounds immediately after the production was in treatment C equal to 5.69 mg/ml and the lowest in the control sample was 2.07 mg/ml. The results of mean comparison showed that the effect of various concentrations of co-crystalized seed powder on phenolic compounds in various days at 4 °C was significant at 5% probability level, so that with increase in co-crystalized powder in the beverage increased phenolic compounds as well (P < 0.05). The effects of other ingredients used in the formulation of beverages such as honey, apple extract, mint essential oil, and stevia cannot be ignored, which may have an effect on the increase of phenolic compounds. The change in phenolic compounds during 90-day storage was almost constant and the decreasing trend in the last month was statistically significant (P < 0.05). Examining the effect of packing and time on phenolic compounds and antioxidant activity of Centella asiatica (Siah et al. 2011) and examining the phenolic compounds and antioxidant activity of two commercial orange juice during the storage (Klimczak et al. 2007) reported a decrease in phenolic and antioxidant compounds during the final days of storage.
Fig. 2.
The changes in phenolic compounds and free radical scavenging activity (DDPH) of beverages formulated during storage
With an increase in concentration and or degree of hydroxylation of phenolic compounds, free radical scavenging activity (DPPH) increases, defined as antioxidant activity. This activity is carried out at very low concentrations as well, which is due to the high sensitivity of free DPPH radicals in the presence of hydrogen atom donors (reductive compounds like phenolic compounds of the extract), which leads to their conversion to non-radical form and the decrease in the absorbance of DPPH solution at 512 nm (Iqbal et al. 2006). According to Fig. 2, the percentage of free radical scavenging changes of various beverage treatments up to day 60 of storage was partially incremental compared to each other and the other treatments, which was statistically significant (P < 0.05). There were only no significant differences between days 0 and 30 of storage between the produced control samples (P > 0.05). However, in the third month of storage, the percentage of free radical scavenging in all treatments except the control sample was significantly reduced and only the control sample was increased. As this percentage reduction was not observed in the control sample, but in the treatments containing co-crystalized powder of S. securidaca seed, it is possible to consider the reduction of the percentage of free radical scavenging effect due to the chemical reaction between herbal extracts and other ingredients in the beverage such as stevia, apple puree, mint, or honey. Moreover, the compounds used in this beverage like stevia, honey and apple extract have various compounds including sugar and chelating agents such as citric acid that, together with the pasteurization process, can affect the complex reactions and mechanisms of antioxidant compounds. Changes in the structure of phenolic antioxidants and their interaction with other nutritional compounds may justify the reduction of antioxidant activity in beverages (Siah et al. 2011; Nicoli et al. 1999). Overall, the reduction in antioxidant activity can be because of oxidative changes due to dissolved oxygen in the beverage or oxygen in the bottle head or oxygen transmitted from the packaging material that speed up the degradation of the polyphenols responsible for the antioxidant activity. As is seen, the antioxidant activity has reduced more relative to phenolic compounds. The more decrease in antioxidant capacity was probably because of the lower antioxidant components being less significant (Kim et al. 2011).
Examining the microbial quality of the beverage
Regarding the number of mold and yeast in the samples stored in the fridge at 4 °C, no mold and yeast contamination was observed immediately after pasteurization and after 3 months. Figure 3 shows the changes in overall count of mesophilic aerobic bacteria. Comparison of the mean total count of mesophilic aerobic microorganisms in all storage months was statistically significant (P < 0.05). The lowest value of bacteria was at the time of production and with time, the bacteria increased up to 60 days of the storage. However, in the final days of the storage, the bacteria growth was decreased. One of the reasons for this decrease is the decrease in the nutrient supply for the bacteria and a decrease in cellular capacity to proliferate during storage. Moreover, due to changes in acidity, pH and also the antioxidant compounds in the beverage, alterations in the pyruvate cycle interfere in metabolic process of bacteria and ultimately, they won’t be able to metabolize the carbohydrates which will lead to the cell death (Ghorbani et al. 2018).
Fig. 3.
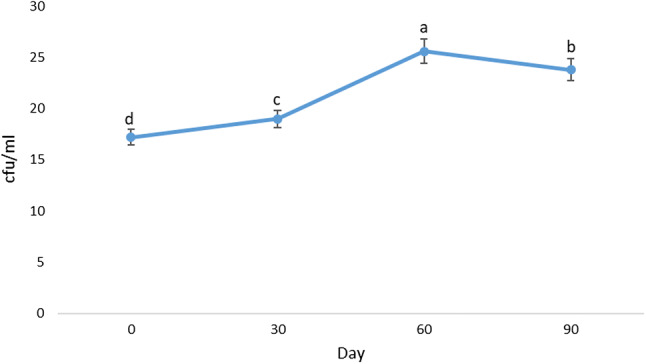
The changes in total mesophilic aerobic bacteria of the beverages formulated during storage
Conclusion
The results of sensory examination showed that the beverages containing co-crystalized powder of S. securidaca seeds received an average score in overall acceptance, with more than 80% of the panelists stating a slightly bitter taste, especially in the formulations with higher values of co-crystalized products and reported inappropriate products. Examining the physicochemical and microbial changes showed that the beverage had good stability. Thus, the result of the factors affecting the quality of the product including ingredients and packaging, packaging type, pasteurization conditions and pH were such that during the storage (3 months), the product quality parameters—pH, brix, acidity, turbidity, vitamin C and the microbial level—had not been changed significantly. Moreover, examining phenolic compounds and radical scavenging capacity indicated that the antioxidant compounds did not change significantly during the storage and were preserved well. Ultimately, one can conclude that among the examined various beverage samples, the sample with 2 g of co-crystalized powder can be used as a functional product instead of non-alcoholic beverages given its physicochemical and microbial stability, the value of phenolic compounds, proper radical scavenging capacity, and gaining higher sensory evaluation score.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali AA, Mohamed MH, Kamel MS, et al. Studies on Securigera securidacea (L.) Deg. et Dörfl. (Fabaceae) seeds, an antidiabetic Egyptian folk medicine. Die Pharmazie. 1998;53:710–715. doi: 10.1002/chin.199904190. [DOI] [PubMed] [Google Scholar]
- Alizadeh M, Azizi-lalabadi M, Hojatansari H, et al. Effect of stevia as a substitute for sugar on physicochemical and sensory properties of fruit based milk shake. J Sci Re Rep. 2014;3:1421–1429. doi: 10.9734/JSRR/2014/8623. [DOI] [Google Scholar]
- AOAC Association of Official Analytical Chemist . Official methods of analysis. 18. Washington: AOAC International; 2005. [Google Scholar]
- Ardali FR, Alipour M, Shariati MA, et al. Replacing sugar by Rebaudioside A in orange drink and produce a new drink. Indian J Res Pharm Biotechnol. 2014;2:1131–1135. [Google Scholar]
- Awad A, Chen AC. A new generation of sucrose products made by cocrystallization. Food Technol (USA) 1993;47:146–148. [Google Scholar]
- BehnamNik A, Vazifedoost M, Didar Z, et al. The antioxidant and physicochemical properties of microencapsulated bioactive compounds in Securigera securidaca (L.) seed extract by co-crystallization. Food Qual Saf. 2019;3:243–250. doi: 10.1093/fqsafe/fyz022. [DOI] [Google Scholar]
- Bhandari BR, Datta N, D’Arcy BR, et al. Co-crystallization of honey with sucrose. LWT Food Sci Technol. 1998;31:138–142. doi: 10.1006/fstl.1997.0316. [DOI] [Google Scholar]
- Chandrasekara A, Shahidi F. Herbal beverages: bioactive compounds and their role in disease risk reduction—a review. J Tradit Complement Med. 2018;8:451–458. doi: 10.1016/j.jtcme.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contor L. Functional food science in Europe. Nutr Metab Cardiovasc Dis NMCD. 2001;11:20–23. [PubMed] [Google Scholar]
- Corbo MR, Bevilacqua A, Petruzzi L, et al. Functional beverages: the emerging side of functional foods: commercial trends, research, and health implications. Compr Rev Food Sci Food Saf. 2014;13:1192–1206. doi: 10.1111/1541-4337.12109. [DOI] [Google Scholar]
- Elhami Rad AH, Mohammadi AS. Formulation of salix aegyptiaca hydrolat base carbonated drink and evaluation of its physicochemical and microbial changes during storage. Iran Food Sci Technol Res J. 2006;2:27–39. [Google Scholar]
- Gasmalla MAA, Yang R, Musa A, et al. Physico-chemical assessment and rebauidioside A. Productively of natural sweeteners (Stevia rebaudiana Bertoni) J Food Nutr Res. 2014;2:209–214. doi: 10.12691/jfnr-2-5-1. [DOI] [Google Scholar]
- Ghahraman A. Flore de Iranien Couleurs Naturelle. Tehran: Tehran University Press; 1993. p. 1478. [Google Scholar]
- Ghorbani N, Nateghi L, Tajabadi N. Investigating the possibility of using Lactobacillus plantarum and Lactobacillus kunkeei isolated from honey in the preparation of probiotic pomegranate juice. J Food Sci Technol. 2018;15:13–23. [Google Scholar]
- Hajimahmoodi M, Aliabadipoor M, Moghaddam G, et al. Evaluation of in vitro antioxidant activities of lemon juice for safety assessment. Am J Food Technol. 2012;7:708–714. doi: 10.3923/ajft.2012.708.714. [DOI] [Google Scholar]
- Hashemi N, Rabiee H, Tavakolipour H, et al. Effect of stevia (stevia rebaudiana) as a substitute for sugar on physicochemical, rheological and sensory properties of dietary saffron syrup. Saffron Agron Technol. 2015;2:303–310. doi: 10.22048/jsat.2015.8623. [DOI] [Google Scholar]
- Hassani M (2005) Formulation and production of barberry-based soft drinks. M.Sc Dissertation. Islamic azad university, Sabzever Branch, Iran
- INSO 11077. Iranian National Standardization Organization (2016) Non-carbonated herbal extract drink- specifications and test methods
- Iqbal S, Bhanger MI, Akhtar M, et al. Antioxidant properties of methanolic extracts from leaves of Rhazya stricta. J Med Food. 2006;9:270–275. doi: 10.1089/jmf.2006.9.270. [DOI] [PubMed] [Google Scholar]
- ISO 21527-1 (2008) Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of yeasts and molds. Part 1: colony count technique in products with water activity greater than 0, 95
- ISO 2173 (2003) Fruit and vegetable products, determination of soluble solids, Refractometric method
- ISO 4833-1: 2013 (2013) Microbiology of the food chain–horizontal method for the enumeration of microorganisms
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables—the millennium’s health. Int J Food Sci Technol. 2001;36:703–725. doi: 10.1111/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- Kaushik V, Roos YH. Limonene encapsulation in freeze-drying of gum Arabic–sucrose–gelatin systems. LWT Food Sci Technol. 2007;40:1381–1391. doi: 10.1016/j.lwt.2006.10.008. [DOI] [Google Scholar]
- Kim Y, Welt BA, Talcott ST. The impact of packaging materials on the antioxidant phytochemical stability of aqueous infusions of green tea (Camellia sinensis) and yaupon holly (Ilex vomitoria) during cold storage. J Agric Food Chem. 2011;59:4676–4683. doi: 10.1021/jf104799y. [DOI] [PubMed] [Google Scholar]
- Klimczak I, Małecka M, Szlachta M, et al. Effect of storage on the content of polyphenols, vitamin C and the antioxidant activity of orange juices. J Food Compos Anal. 2007;20:313–322. doi: 10.1016/j.jfca.2006.02.012. [DOI] [Google Scholar]
- Krasaekoopt W, Pianjareonlap R, Kittisuriyanont K. Survival of probiotics in fruit juices during refrigerated storage. Thai J Biotechnol. 2008;8:129–133. [Google Scholar]
- López-Córdoba A, Deladino L, Agudelo-Mesa L, et al. Yerba mate antioxidant powders obtained by co-crystallization: stability during storage. J Food Eng. 2014;124:158–165. doi: 10.1016/j.jfoodeng.2013.10.010. [DOI] [Google Scholar]
- McCall MR, Frei B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic Biol Med. 1999;26:1034–1053. doi: 10.1016/S0891-5849(98)00302-5. [DOI] [PubMed] [Google Scholar]
- Meléndez AJ, Bejines E, Vicario IM, et al. Vitamin C in orange juices determined by HPLC: influence of the wavelength of detection. Ital J Food Sci. 2004;16:79–85. [Google Scholar]
- Mishra BK, Hati S. Dairy and Food Product Technology. New Delhi: Biotech Books; 2016. pp. 265–280. [Google Scholar]
- Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Penders MH, Scollard DJ, Needham D, et al. Some molecular and colloidal aspects of tea cream formation. Food Hydrocoll. 1998;12:443–450. doi: 10.1016/S0268-005X(98)00060-5. [DOI] [Google Scholar]
- Pyrzynska K, Sentkowska A. Herbal beverages as a source of antioxidant phenolics. Nat Beverages. 2019;13:125–142. doi: 10.1016/B978-0-12-816689-5.00005-5. [DOI] [Google Scholar]
- Rakić S, Povrenović D, Tešević V, et al. Oak acorn, polyphenols and antioxidant activity in functional food. J Food Eng. 2006;74:416–423. doi: 10.1016/j.jfoodeng.2005.03.057. [DOI] [Google Scholar]
- Salari M, Razavi SH, Gharibzahedi SMT. Characterising the synbiotic beverages based on barley and malt flours fermented by Lactobacillus delbrueckii and paracasei strains. Qual Assur Saf Crops Foods. 2014;7:355–361. doi: 10.3920/QAS2013.0390. [DOI] [Google Scholar]
- Sameni HR, Jadidi M, Sadeghi S, et al. Effects of hydroalchoholic extract of Securigera securidaca L. Seed on acute, chronic and visceral pain in male albino mice. Koomesh. 2016;17:288–296. [Google Scholar]
- Siah WM, Faridah H, Rahimah MZ, et al. Effects of packaging materials and storage on total phenolic content and antioxidant activity of Centella asiatica drinks. J Trop Agric Fd Sc. 2011;39:1–7. [Google Scholar]
- Silva CR, Simoni JA, Collins CH, et al. Ascorbic acid as a standard for iodometric titrations. An analytical experiment for general chemistry. J Chem Educ. 1999;76:1421. doi: 10.1021/ed076p1421. [DOI] [Google Scholar]
- Singh RR. Formulation of a carbonated herbal health drink with hemidesmus indicus root extract. Int J Adv Ind Eng. 2018;6:100–104. doi: 10.14741/ijaie/v.6.2.1. [DOI] [Google Scholar]
- Stoilova I, Krastanov A, Stoyanova A, et al. Antioxidant activity of a ginger extract (Zingiber officinale) Food Chem. 2007;102:764–770. doi: 10.1016/j.foodchem.2006.06.023. [DOI] [Google Scholar]
- Sylvetsky AC, Hiedacavage A, Shah N, et al. From biology to behavior: a cross-disciplinary seminar series surrounding added sugar and low-calorie sweetener consumption. Obes Sci Pract. 2019;5:203–219. doi: 10.1002/osp4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren SF, Suliman S, Viljoen AM. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett Appl Microbiol. 2009;48:440–446. doi: 10.1111/j.1472-765X.2008.02548.x. [DOI] [PubMed] [Google Scholar]
- Varnam A, Sutherland JM. Beverages: technology, chemistry and microbiology. Berlin: Springer; 1994. [Google Scholar]
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Zakipour Rhomabadi N, Sohrabvandi S, Ruzbeh Nasiraie L. Production of synbiotic malt beverage using inulin and different probiotic strains of lactobacillus bacteria. Iran J Nutr Sci Food Technol. 2018;13:39–46. [Google Scholar]