Abstract
This study was designed to investigate the changes of physicochemical, microbiological and sensory properties of oyster tissues during chilled storage at 4 °C, including digestive gland (DG), the gonad and surrounding mantle area (GM), adductor muscle (AM). Sensory evaluation showed that the decrease of sensory scores of the three oyster tissues was more rapid than the whole oyster (WO). The drip loss of DG was more than other tissues and the WO. Moreover, the GM showed higher extent of lipid oxidation than other tissues and WO, while the AM showed higher TVB-N value and microbial counts than other tissues and WO. It is concluded that the spoilage of oyster during chilled storage greatly depended on the composition of oyster tissues. Overall, the findings may provide new insights to control the spoilage of oyster based on the changes of oyster tissues during storage.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04280-1) contains supplementary material, which is available to authorized users.
Keywords: Oyster, Chilled storage, Oyster tissues, Spoilage
Introduction
Oyster is an important marine resource due to its higher nutritional value and production (Tran et al. 2015; Lee et al. 2013). The production of oyster is about 483 million tons in China in 2016 (CSY 2017), and Pacific oyster (Crassostrea gigas) accounts for one-third of the total production of oyster in China (Zhang et al. 2012).
Fresh aquatic food is highly perishable and generally rapidly deteriorates over other muscle foods due to its high water activity, neutral pH and autolytic enzymes (Chouhan et al. 2015). The drip loss, pH, total volatile basic nitrogen (TVB-N), thiobarbituric acid reactive substances (TBARS) and microbial counts of seafood are regarded as some important physical indicators to evaluate the quality of seafood. The drip loss is related to the denaturation of myosin with the decrease in water-holding capacity (Mohan et al. 2012; Songsaeng et al. 2010). The pH value might be affected by autolysis and bacterial attack during the decomposition process of fish (Ghaly et al. 2010). The pH and total volatile basic nitrogen (TVB-N) detection are traditional chemical approaches widely used in evaluating the degree of protein degradation of meat products (Gao et al. 2014). The degree of lipid oxidation of oyster is also represented by the value of thiobarbituric acid reactive substances (TBARS), while the microbial growth status in oyster depends on the nutritional content and living environment (Liu et al. 2016).
Oysters, as filter-feeding shellfish, can be easily enriched with microorganisms and therefore have a shorter shelf-life (Chen et al. 2013; Wang et al. 2014a, b). The parts of edible oyster include digestive gland (DG), the gonad and surrounding mantle area (GM) and adductor muscle (AM). Particularly, GM showed the highest bacterial diversity and initial aerobic plate counts (Chen et al. 2013; Wang et al. 2014a, b). It was mentioned by a few previous studies (Azandégbé et al. 2012; King et al. 2012; Wang et al. 2014a, b) that different bacterial communities were breeding on different tissues of oyster, such as glands, gut, stomach and gills.
There are a lot of studies for prolonging shelf-life of oyster using different processing methods, including heated treatment, irradiation, high density carbon dioxide sterilization and high pressure processing (HPP) (Chouhan et al. 2015; Cruz-Romero et al. 2004, 2007, 2008). To the best of our knowledge, there is little information regarding the deterioration of oysters during chilled storage. We hypothesized that different tissues of oyster, including DG, GM and AM, affected the changes of physicochemical, microbiological and sensory properties during storage. The purpose of the present study was to firstly investigate the TVB-N, TBARS value and microbiological, quality changes of these three tissues from oyster to better control the quality of oysters during storage.
Materials and methods
Oyster supply and handling
Pacific oysters (C. gigas) of commercial size, i.e., measuring 12–14 cm in shell length, were harvested in May 2017 and stored in foam boxes with ice and transported to the laboratory within 30 min.
Preparation of the oyster tissues
The oysters were manually shucked with a sterile knife and divided into three parts, including digestive gland (DG), gonad and surrounding mantle area (GM), adductor muscle (AM) (Supplementary Fig. S1) according to the method reported by Berthelin et al. (2000). The obtained DG, GM, AM tissues were placed on 30 white plastic plates, respectively, and each plate contained 6 samples. All plates were completely wrapped by polyethylene plastic wrap. The oyster without any separation was as the control. All samples were stored at 4 ± 1 °C for a 10-day storage trial and detected every 2 days.
The moisture, ash, protein, glycogen and lipids content analysis
The composition contents of fresh oyster samples were analyzed according to standard methods (AOAC 2005). Moreover, nitrogen was determined to measure the protein content by using the micro-base nitrogen method and the nitrogen content was calculated using a transformation factor (nitrogen-protein) of 6.25. The lipid content of oyster meat was quantitatively determined by Soxhlet apparatus. The glycogen was determined by phenol–sulfuric acid method (Li et al. 2000).
Sensory evaluation
The sensory quality of oysters was carried out using the method of ISO 8586-1. Eight panelists trained participated in this evaluation. The panelists recorded their scores from 1 to 10 for color (from white to grayish, discolored), odor (from very fresh, fruity to sour, stench), mucous state (from very bright to turbid) and overall acceptability (from acceptable to unacceptable), as prescribed by Cao et al. (2009).
Drip loss
Drip loss (%) was measured by comparing the difference in weights of the oyster sample with and without exudate
and where WA and WB denote the weight of the oyster sample dried out with the filter paper before and after t days of storage, respectively.
pH measurement
Minced oyster samples (5.0 g) were homogenized with 45 mL of distilled water and kept the solution still for 30 min. The pH of this mixture was measured using a pH meter (PHS-3C, Shanghai, China).
Determination of total volatile basic nitrogen (TVB-N)
The TVB-N of oyster were detected according to the method of Goulas and Kontominas (2005) with minor modifications. Briefly, five gram of minced oyster samples were added to 45 mL 5% perchloric acid solution and homogenized for 10 min. After filtration, the extract was used for TVB-N value analysis using the micro-base nitrogen method. Results were expressed as mg TVB-N per 100 g oyster sample.
Determination of thiobarbituric acid reactive substances (TBARS)
The TBARS were measured as described by Kirk and Sawyer (1991) with slight modifications. Briefly, five grams of minced oyster samples were mixed with 50 mL trichloroacetic acid (TCA) solution (75 g TCA/L, 1 g propyl gallate/L, 1 g EDTA/L) and homogenized for 2 min. The mixture was put in constant temperature shaker for 30 min, at 50 °C and then cooled with the mixture of ice and water. After filtering, five milliliters of the extract was mixed with 5 mL of 0.02 M thiobarbituric acid solution, followed by reacting for 30 min in a boiling water bath. The absorbance of the resulting solution was measured at 532 nm. 1,1,3,3-tetraethoxypro- pane (TEP) was used to prepare the standard curve. TBARS was expressed as mg malonaldehyde/100 g of sample, as described by Wu et al. (2017).
Microbiological analysis
The total bacteria counts (TBC) in oyster samples were measured according to the method of Liu et al. (2016) with slight modifications. Briefly, five grams of minced oyster sample were weighed accurately under sterile conditions and homogenized with 45 mL of sterile 0.9% saline water in a sterile stomacher bag for 2 min. After homogenizing, a decimal dilution series was prepared for microbiological analysis. The resulting solution (1 mL) were poured into petri dishes and mixed with plate count agar (PCA). The plates were inverted and incubated at 28 °C for 24–48 h to determine total plate count.
Statistical analysis
The mean values and standard deviations were determined using EXCEL (2010, Microsoft office, New York, NY). Data from the different quality parameters were performed using SPSS 19.0 software, and significant differences between mean values were identified at a level of P < 0.05 by one-way analysis of variance (ANOVA) and the Duncan test.
Results and discussion
Composition of different tissues of oyster meat
The proportion and composition of three tissues from the whole oyster (WO) are shown in Table 1. The proportion of the digestive gland (DG), gonad and surrounding mantle area (GM), and adductor muscle (AM) weighed were 47.13%, 44.01% and 8.86%, respectively. Compared to the WO, the DG contained higher glycogen levels. In particular, the GM contained much higher fat contents (4.74%) than the other tissues (P < 0.05). Conversely, protein (18.99%) was the dominant composition of AM, which was twice as high as that of WO (P < 0.05). And the AM contained a low concentration of fat (1.17%) and glycogen (0.59%).
Table 1.
The proportion and composition of the different tissues from fresh oyster
| Item | Proportion of tissues (%) | Moisture | Ash | Protein | Glycogen | Fat |
|---|---|---|---|---|---|---|
| WO | 82.07c | 1.73b | 9.83b | 2.88c | 3.34b | |
| DG | 47.13 | 79.86ab | 1.98c | 9.36b | 3.84d | 3.44b |
| GM | 44.01 | 81.16c | 1.93c | 8.49a | 2.61b | 4.74c |
| AM | 8.86 | 77.12a | 1.51a | 18.99c | 0.59a | 1.17a |
| SEM | ND | 0.71 | 0.05 | 1.26 | 0.36 | 0.39 |
WO the whole oyster, DG the digestive gland, GM the gonad and surrounding mantle area, AM the adductor muscle, SEM Pooled standard error of the mean for predetermined, ND not detected
abcThe same letter in the same column indicates that the samples were not significantly different at 5%
Drip loss and pH value
The changes of drip loss and pH values of different tissues of oyster are shown in Table 2. During the first 4 days of storage, the drip loss of WO, GM and AM increased dramatically (P < 0.05); on the 10th day, the drip loss of WO, DG, GM, and AM reached to 26.35%, 28.94%, 22.33% and 17.66%, respectively. Especially, the DG sample among all oyster samples showed the higher drip loss (25.39%) on the 6th day, and then reached to 28.94% on the 10th day.
Table 2.
The drip loss (%) and pH value of oyster samples during storage for 10 days
| Indicator | Storage time (d) | WO | DG | GM | AM |
|---|---|---|---|---|---|
| Drip loss (%) | 2 | 10.63a | 15.95a | 16.44a | 10.17a |
| 4 | 23.70b | 22.60b | 20.75b | 17.25b | |
| 6 | 24.67b | 25.39c | 21.72 cd | 17.50bc | |
| 8 | 25.06bc | 27.90d | 22.02 cd | 17.76c | |
| 10 | 26.35c | 28.94d | 22.33d | 17.66c | |
| SEM | 2.37 | 2.42 | 1.93 | 1.59 | |
| pH | 0 | 6.55e | 6.49e | 6.62c | 6.46a |
| 2 | 6.52d | 6.40d | 6.49d | 6.44a | |
| 4 | 6.29c | 6.37c | 6.18a | 6.49b | |
| 6 | 6.41b | 6.28a | 6.39c | 6.54bc | |
| 8 | 6.39a | 6.31b | 6.30b | 6.69c | |
| 10 | 6.41b | 6.34b | 6.29b | 6.80d | |
| SEM | 0.02 | 0.02 | 0.04 | 0.03 |
WO the whole oyster, DG the digestive gland, GM the gonad and surrounding mantle area, AM the adductor muscle, SEM Pooled standard error of the mean for predetermined
abcThe same letter in the same column indicates that the samples were not significantly different at 5%
To the best of our knowledge, there were few studies on the drip loss of oyster tissues during chilled storage. Myosin, which can prevent moisture leakage before it degenerated, may influence the drip loss of oyster (Mohan et al. 2012). In addition to the influence of protein on the drop loss (Mohan et al. 2012; Wattanachant et al. 2005) in oyster meats, the possible reason for increase of the drip loss of DG was that it contained bubble membrane tissue, which could wrap a lot of water (Cruz-Romero et al. 2004). Our present results have showed that the DG and GM played an important role on the quality deterioration of oyster during storage (Table 1).
The initial pH value of the whole oyster was about 6.55, which was similar with some previous results (Cruz-Romero et al. 2004). The pH value of WO declined sharply within the first 4 days of storage, which was related to the comprehensive effect of lactic acid accumulation during anaerobic glycolysis (Abdollahi et al. 2014; Feng et al. 2012). Subsequently, the increase of pH value showed that proteins and other nitrogenous substances were broken down into volatile bases, the amines and trimethylamine through the action of microorganisms and enzymes, and then led to an increase in pH value (Mohan et al. 2012).
The similar changes in pH value for the DG and GM were represented. With the extension of storage time, the pH value of DG and GM decreased at the beginning of storage and then increased after the 4th and 6th day, respectively. At the end of storage, the pH value of DG and GM was significantly lower than that of WO (P < 0.05). The reason for the lower pH in the DG and GM value was possibly due to the lower content of protein (Table 1) (Mohan et al. 2012). Inversely, the pH value of AM showed a slight decrease within the first 2 days and then increased significantly (P < 0.05) (from 6.44 to 6.80). From the present results, the higher pH of oyster in AM was associated with the lower sensory attributes (He et al. 2002).
Sensory evaluation
Sensory evaluation is an important indicator for seafood to evaluate the changes of freshness during storage (Cao et al. 2009; He et al. 2002; Liu et al. 2016). Based on the analysis of the color, odor, mucous state and overall acceptability and elasticity, the total sensory scores of oyster tissues and the whole oyster during storage (Fig. 1) were further investigated. The photos of oyster samples during storage are also shown in Supplementary Table S1.
Fig. 1.

Sensory scores of oyster tissues during storage at 4 °C for 10 days. Values were expressed as the mean ± SD (n = 3). WO—the whole oyster; DG—the digestive gland; GM—the gonad and surrounding mantle area; AM—the adductor muscle. Different letters in the same line indicate the significant differences among different samples during storage (P < 0.05)
The fresh whole oyster was physically intact, almost mucous-free, and indicated a distinctive oyster scent, which could be given to a ten-point sensory evaluation. Subsequently, sensory scores of WO declined rapidly (from 10 to 4.7) during the whole storage and showed a significantly quality deterioration (P < 0.05) on the 10th day. Compared to the WO, the decrease of the sensory scores of the three oyster tissues was more rapid. Particularly, the DG and GM on the 8th day began to emit stench. Meanwhile, the AM began to grow reddish-brown plaque on the 8th day (Supplementary Table S1).
In my present study, the 6.0-score sensory evaluation was regarded as the minimal permissible limit. On the 8th day, the sensory scores of WO, DG, GM, and AM reached to 6.75, 6.70, 5.95 and 5.20, respectively. Therefore, the gonad and surrounding mantle area and the adductor muscle were more prone to spoilage when compared to the WO. Chen et al. (2019) also found that the spoilage of gill in oyster during refrigerated storage occurred earlier than that of other tissues, based on the anlysis of sensory assessment.
Total volatile basic nitrogen (TVB-N)
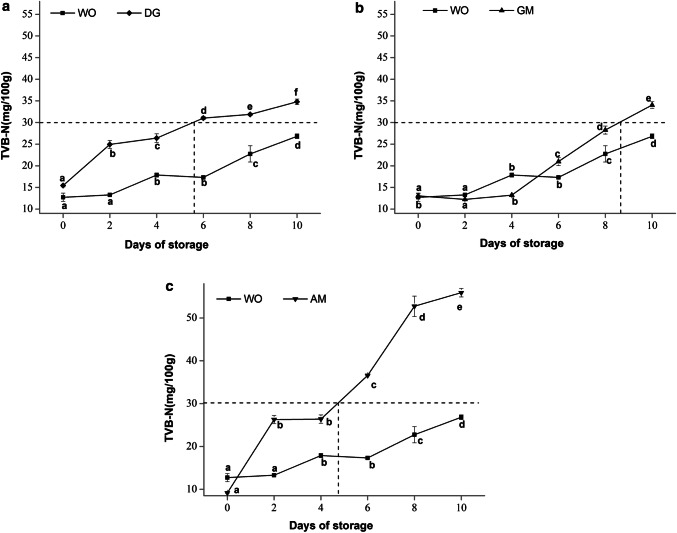
Generally, oysters are considered to be the end of their shelf life when their TVB-N concentration reaches 30 mg/100 g (Cao et al. 2009). In this study, the changes of TVB-N of oyster tissues during storage for 10 days are shown in Fig. 2 . The TVB-N of WO increased from the initial 12.73 mg/100 g to 26.83 mg/100 g on the 10th day. However, the TVB-N level, more than 30 mg/100 g, of DG, GM and AM was on 6th, 8th and 6th day, respectively. Particularly, at the end of storage, the TVB-N level (55.90 mg/100 g) of AM was much higher than that of GM (34.8 mg/100 g) and DG (34.05 mg/100 g).
Fig. 2.
TVB-N values of oyster tissues during storage at 4 °C for 10 days. Values were expressed as the mean ± SD (n = 3). WO—the whole oyster; DG—the digestive gland; GM—the gonad and surrounding mantle area; AM—the adductor muscle. Different letters in the same line indicate the significant differences among different samples during storage (P < 0.05)
The TVB-N level meats or aquatic food may be related to their protein content. De Lacey et al. (2014) reported that the TVB-N produced was a result of bacterial spoilage and endogenous enzymes, which in turn decomposed protein and non-protein nitrogenous compounds. Therefore, we hypothesized that the higher protein content of AM and DG than WO and GM was the main reason for the sharply increase in TVB-N value during storage period. Taking into account the low proportion of AM in the whole oysters (Table 1), the DG played the important role in the formation of TVB-N during chilled storage of oyster.
Thiobarbituric acid reactive substances (TBARS)
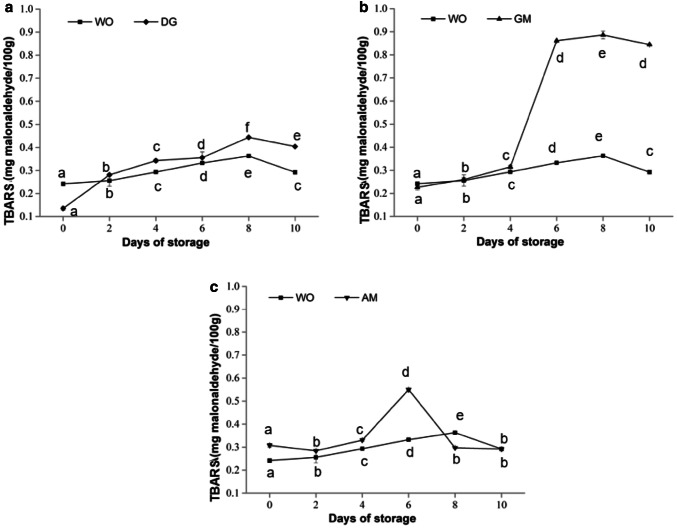
The changes of TBARS value of oyster tissues during storage for 10 days are shown in Fig. 3. The TBARS value of all samples showed a similar tendency that increased initially and then decreased.
Fig. 3.
TBARS values of oyster tissues during storage at 4 °C for 10 days. Values were expressed as the mean ± SD (n = 3). WO—the whole oyster; DG—the digestive gland; GM—the gonad and surrounding mantle area; AM—the adductor muscle. Different letters in the same line indicate the significant differences among different samples during storage (P < 0.05)
The TBARS value of the WO showed a significant increase from 0.24/100 to 0.36 mg/100 g on 8th day and then declined sharply. The initial TBARS values of DG, GM and AM were 0.14, 0.23, 0.27 mg/100 g, respectively, and then changes of TBARS values in the three tissues followed the similar trends as the WO. Particularly, the peak values of TBARS of DG and GM, far more than the value of AM and WO, were 0.44 mg/100 g and 0.69 mg/100 g, respectively.
Mohan et al. (2012) have reported that the content and composition of fat affected the extent of lipid degeneration, and greatly affected the TBARS level. In the present study, the higher fat content in GM considerably contributed to the formation of TBARS during storage; however, the changes of TBARS in DG with higher fat content did not have the trend, and the reasons behind this behavior need to be further investigated. We also found that the emerge of the peak of TBARS value of AM was earlier than that of DG and WO, which possibly attributed to the recovery of microorganism growing in AM by the later stage of storage. From the present results, the GM may be the major contributor to the changes in the TBARS value of whole oysters during chilled storage, which may be due to its high weight ratio (Table 1).
Microbiological analysis
The changes of TBC of oyster tissues during storage for 10 days are shown in Fig. 4. The initial TBC of WO were proximately 3.38 log CFU/g. Compared to the WO, the initial TBC values of DG 3.48 log CFU/g, GM 3.84 log CFU/g and AM 3.45 log CFU/g were slightly higher.
Fig. 4.
Total bacteria count (TBC) of oyster tissues during storage at 4 °C for 10 days. Values were expressed as the mean ± SD (n = 3). WO—the whole oyster; DG—the digestive gland; GM—the gonad and surrounding mantle area; AM—the adductor muscle. Different letters in the same line indicate the significant differences among different samples during storage (P < 0.05)
With the extension of storage time, the TBC of WO increased significantly (P < 0.05) and reached to 7.54 log10 CFU/g on the 10th day, which exceeded the maximum acceptable limit (7.00 log10 CFU/g) according to Kim et al. (2002). The more rapid increase for the TBC value of AM was observed when compared to other tissues and the WO. On the 4th day, the TBC of AM than DG and GM were just a little higher (0.86 and 1.4 log10 CFU/g, respectively) and sharply increased to 10.45 log10 CFU/g at the end of storage. Meanwhile, the TBC for DG and GM increased to 5.65 and 7.23 log10 CFU/g, respectively. In contrast, the TBC of DG increased slowly, and reached to 5.72 at the end of storage, which was considered acceptable. Meanwhile, the total TBC in GM and AM reached to the maximal permissible limit (7.00 log10 CFU/g) on the 10th and 6th day, respectively.
From the present result, the changes of the TBC value in different tissues of oyster during chilled storage showed the different patterns, and the reasons behind this behavior were still not known. We hypothesized that the difference between compositions of different tissues of oyster could play a part in the growth of microorganism. The TBC of AM was relatively higher than GM at the later stage of storage due to its higher pH value (Mohan et al. 2012), which were consistent with its sensory attributes. In addition, the separated process from the whole oyster possibly enhanced the spoilage of AM.
Conclusion
The changes of physicochemical, microbiological and sensory quality of oyster during chilled storage were greatly affected by its tissues. The decrease of sensory quality of three oyster tissues was more rapid than the whole oyster; the more drip loss of the digestive gland than other tissues was observed; the gonad and surrounding mantle area showed higher extent of lipid oxidation than other tissues, while the adductor muscle showed higher TVB-N level and microbial counts. This study suggested that more attention should be paid to changes of oyster tissues during storage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by National Key R&D Program of China (2018YFD0901005), Key Research & Development Project of Shandong Province (2018GHY115012) and Project 31771919 of the National Natural Science Foundation of China (NSFC).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shiyuan Dong, Email: dongshiyuan@ouc.edu.cn.
Mingyong Zeng, Email: Mingyz@ouc.edu.cn.
References
- Abdollahi M, Rezaei M, Farzi G. Influence of chitosan/clay functional bionanocomposite activated with rosemary essential oil on the shelf life of fresh silver carp. Int J Food Sci Technol. 2014;49:811–818. doi: 10.1111/ijfs.12369. [DOI] [Google Scholar]
- AOAC Association of Offcial Analytical Chemists (2005) AOAC offcial methods of analysis (18th ed). Gaithersburg, AOAC 935
- Azandégbé A, Poly F, Andrieux-Loyer F, Kérouel R, Philippon X, Nicolas JL. Influence of oyster culture on biogeochemistry and bacterial community structure at the sediment–water interface. FEMS Microbiol Ecol. 2012;82:102–117. doi: 10.1111/j.1574-6941.2012.01410.x. [DOI] [PubMed] [Google Scholar]
- Berthelin C, Kellner K, Mathieu M. Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France) Comp Biochem Physiol. 2000;125:359–369. doi: 10.1111/j.1574-6941.2012.01410.x. [DOI] [PubMed] [Google Scholar]
- Cao R, Xue CH, Liu Q, Xue Y. Microbiological, chemical, and sensory assessment of Pacific oysters (Crassostrea gigas) stored at different temperatures. Czech J Food Sci. 2009;27:102–108. doi: 10.17221/166/2008-CJFS. [DOI] [Google Scholar]
- Chen H, Liu Z, Wang M, Chen S, Chen T. Characterisation of the spoilage bacterial microbiota in oyster gills during storage at different temperatures. J Sci Food Agric. 2013;93:3748–3754. doi: 10.1002/jsfa.6237. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang M, Yang C, Wan X, Ding HH, Shi Y, Zhao C. Bacterial spoilage profiles in the gills of Pacific oysters (Crassostrea gigas) and Eastern oysters (C. virginica) during refrigerated storage. Food Microbiol. 2019;82:209–217. doi: 10.1016/j.fm.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Chouhan A, Kaur BP, Rao PS. Effect of high pressure processing and thermal treatment on quality of hilsa (Tenualosa ilisha) fillets during refrigerated storage. Innov Food Sci Emerg. 2015;29:151–160. doi: 10.1016/j.ifset.2015.03.016. [DOI] [Google Scholar]
- Cruz-Romero M, Smiddy M, Hill C, Kerry JP, Kelly AL. Effects of high pressure treatment on physicochemical characteristics of fresh oysters (Crassostrea gigas) Innov Food Sci Emerg Technol. 2004;5:161–169. doi: 10.1016/j.ifset.2004.01.002. [DOI] [Google Scholar]
- Cruz-Romero M, Kelly AL, Kerry JP. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas) Innov Food Sci Emerg Technol. 2007;8:30–38. doi: 10.1016/j.ifset.2006.05.002. [DOI] [Google Scholar]
- Cruz-Romero M, Kerry JP, Kelly AL. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control. 2008;19:1139–1147. doi: 10.1016/j.foodcont.2007.12.004. [DOI] [Google Scholar]
- CSY . China fishery statistical yearbook. Beijing: China Agriculture Press; 2017. [Google Scholar]
- De Lacey AL, López-Caballero ME, Montero P. Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT Food Sci Technol. 2014;55:559–564. doi: 10.1016/j.lwt.2013.09.028. [DOI] [Google Scholar]
- Feng L, Jiang T, Wang Y, Li J. Effects of tea polyphenol coating combined with ozone water washing on the storage quality of black sea bream (Sparus macrocephalus) Food Chem. 2012;135:2915–2921. doi: 10.1016/j.foodchem.2012.07.078. [DOI] [PubMed] [Google Scholar]
- Gao M, Feng L, Jiang T, Zhu J, Fu L, Yuan D, Li J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control. 2014;37:1–8. doi: 10.1016/j.foodcont.2013.09.010. [DOI] [Google Scholar]
- Ghaly AE, Dave D, Budge S, Brooks MS. Fish spoilage mechanisms and preservation techniques: review. Am J Appl Sci. 2010;7:859–877. doi: 10.3844/ajassp.2010.859.877. [DOI] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93:511–520. doi: 10.1016/j.foodchem.2004.09.040. [DOI] [Google Scholar]
- He H, Adams RM, Farkas DF, Morrissey MT. Use of high-pressure processing for oyster shucking and shelf-life extension. J Food Sci. 2002;67:640–645. doi: 10.1111/j.1365-2621.2002.tb10652.x. [DOI] [Google Scholar]
- Kim YM, Paik HD, Lee DS. Shelf-life characteristics of fresh oysters and ground beef as affected by bacteriocin-coated plastic packaging film. J Sci Food Agric. 2002;82:998–1002. doi: 10.1002/jsfa.1125. [DOI] [Google Scholar]
- King GM, Judd C, Kuske CR, Smith C. Analysis of stomach and gut microbiomes of the eastern oyster (Crassostrea virginica) from coastal Louisiana, USA. PLoS One. 2012;7:e51475. doi: 10.1371/journal.pone.0051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RS, Sawyer R. Pearson’s compostion and analysis of foods. 9. Harlow: Addison-Wesley Longmam Inc; 1991. p. 285. [Google Scholar]
- Lee SY, Kim HJ, Han JS. Anti-inflammatory effect of oyster shell extract in LPS-stimulated Raw 264.7 cells. Prev Nutr Food Sci. 2013;18:23. doi: 10.3746/pnf.2013.18.1.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Osada M, Mori K. Seasonal biochemical variations in Pacific oyster gonadal tissue during sexual maturation. Fish Sci. 2000;66:502–508. doi: 10.1046/j.1444-2906.2000.00067.x. [DOI] [Google Scholar]
- Liu F, Li Z, Cao B, Wu J, Wang Y, Xue Y. The effect of a novel photodynamic activation method mediated by curcumin on oyster shelf life and quality. Food Res Int. 2016;87:204–210. doi: 10.1016/j.foodres.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Mohan CO, Ravishankar CN, Lalitha KV, Gopal TS. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012;26:167–174. doi: 10.1016/j.foodhyd.2011.05.005. [DOI] [Google Scholar]
- Songsaeng S, Sophanodora P, Kaewsrithong J, Ohshima T. Quality changes in oyster (Crassostrea belcheri) during frozen storage as affected by freezing and antioxidant. Food Chem. 2010;123:286–290. doi: 10.1016/j.foodchem.2010.04.033. [DOI] [Google Scholar]
- Tran NK, Kwon JE, Kang SC, Shim SM, Park TS. Crassaostrea gigas oyster shell extract inhibits lipogenesis via suppression of serine palmitoyltransferase. Nat Prod. 2015;10:349–352. [PubMed] [Google Scholar]
- Wang D, Zhang Q, Cui Y, Shi X. Seasonal dynamics and diversity of bacteria in retail oyster tissues. Int J Food Microbiol. 2014;173:14–20. doi: 10.1016/j.ijfoodmicro.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Z, Dong S, Zhao Y, Zeng M. Effects of vacuum and modified atmosphere packaging on microbial flora and shelf-life of Pacific white shrimp (Litopenaeus vannamei) during controlled freezing-point storage at − 0.8 °C. Food Sci Tec Res. 2014;20:1141–1152. doi: 10.3136/fstr.20.1141. [DOI] [Google Scholar]
- Wattanachant S, Benjakul S, Ledward DA. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005;93:337–348. doi: 10.1016/j.foodchem.2004.09.032. [DOI] [Google Scholar]
- Wu Y, Chang S, Nannapaneni R, Zhang Y, Coker R, Mahmoud BS. The effects of X-ray treatments on bioaccumulated murine norovirus-1 (MNV-1) and survivability, inherent microbiota, color, and firmness of Atlantic oysters (Crassostrea virginica) during storage at 5 °C for 20 days. Food Control. 2017;73:1189–1194. doi: 10.1016/j.foodcont.2016.10.036. [DOI] [Google Scholar]
- Zhang YH, Wang ZP, Yan XW, Yu ZF, Huo ZM, Yao T, Yu RI. Phenotypic traits of both larvae and juvenile Crasstrea hongkongensis and C. gigas. Acta Ecol Sin. 2012;32:1105–1114. doi: 10.5846/stxb201101020001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.