Abstract
There are limited published data defining survival and treatment response in patients with mild lung disease and/or reduced gas transfer who fulfil diagnostic criteria for idiopathic pulmonary arterial hypertension (IPAH).
Patients diagnosed with IPAH between 2001 and 2019 were identified in the ASPIRE (Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre) registry. Using prespecified criteria based on computed tomography (CT) imaging and spirometry, patients with a diagnosis of IPAH and no lung disease were termed IPAHno-LD (n=303), and those with minor/mild emphysema or fibrosis were described as IPAHmild-LD (n=190).
Survival was significantly better in IPAHno-LD than in IPAHmild-LD (1- and 5-year survival 95% and 70% versus 78% and 22%, respectively; p<0.0001). In the combined group of IPAHno-LD and IPAHmild-LD, independent predictors of higher mortality were increasing age, lower diffusing capacity of the lung for carbon monoxide (DLCO), lower exercise capacity and a diagnosis of IPAHmild-LD (all p<0.05). Exercise capacity and quality of life improved (both p<0.0001) following treatment in patients with IPAHno-LD, but not IPAHmild-LD. A proportion of patients with IPAHno-LD had a DLCO <45%; these patients had poorer survival than patients with DLCO ≥45%, although they demonstrated improved exercise capacity following treatment.
The presence of even mild parenchymal lung disease in patients who would be classified as IPAH according to current recommendations has a significant adverse effect on outcomes. This phenotype can be identified using lung function testing and clinical CT reports. Patients with IPAH, no lung disease and severely reduced DLCO may represent a further distinct phenotype. These data suggest that randomised controlled trials of targeted therapies in patients with these phenotypes are required.
Short abstract
Patients with IPAH who have mild parenchymal lung disease have significantly worse outcomes, in terms of survival and treatment response, when compared to patients with IPAH who do not have evidence of parenchymal lung disease http://bit.ly/3agkYn0
Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a rare condition (estimated incidence <5 million cases per year) defined haemodynamically as mean pulmonary arterial pressure (mPAP) >20 mmHg, left atrial pressure ≤15 mmHg and pulmonary vascular resistance >3 Wood units [1, 2]. It is defined clinically as the absence of conditions or risk factors associated with the development of precapillary pulmonary hypertension, including connective tissue disease, congenital heart disease, chronic thromboembolic disease and lung disease [3]. Several medical therapies have been shown to improve haemodynamics, exercise capacity and clinical events, and survival has improved significantly over the past three decades [4–6]. Chronic lung disease-associated pulmonary hypertension (CLD-PH) is common; 90% of patients with severe COPD have a mPAP >20 mmHg [7]. Significant pulmonary hypertension in association with lung disease is less common; ≤5% of COPD patients have mPAP ≥35 mmHg [8].
Mild lung disease may be present in patients with severe precapillary haemodynamics. This can create diagnostic uncertainty as to whether a patient has group 1 (pulmonary arterial hypertension (PAH)) or group 3 (CLD-PH) disease. The recent 6th World Symposium on Pulmonary Hypertension (WSPH) suggested that patients with coexisting lung disease should be diagnosed with PAH when pulmonary hypertension is moderate–severe, when only modest spirometric or parenchymal abnormalities are present and when diffusion capacity of the lung for carbon monoxide (DLCO) is low with respect to obstructive or restrictive lung function [7].
There are few data defining survival and treatment response in patients with mild lung disease who fulfil the diagnostic criteria for IPAH suggested by the 6th WSPH. We hypothesised that even mild lung disease and/or low gas transfer have a negative effect on outcomes in patients with a diagnosis of IPAH. Therefore, we performed a study of characteristics, survival and response to therapy of patients who had been assigned a diagnosis of IPAH at a large pulmonary hypertension referral centre over an 18-year period.
Methods
Patients who had been assigned a diagnosis of IPAH or heritable PAH or CLD-PH between February 2001 and January 2019 at our centre were identified from the ASPIRE (Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Centre) registry, a database consisting of consecutive patients referred to the Sheffield Pulmonary Vascular Disease Unit (Sheffield, UK), who undergo multimodality assessment and multidisciplinary team discussion, as previously described [9]. Radiology images and reports, lung function tests, pulmonary haemodynamics and clinical correspondence were retrieved, blinded to outcomes. Computed tomography (CT) images had been reported at the time of diagnosis by experienced pulmonary vascular radiologists, blinded to haemodynamics and spirometry, using a qualitative assessment of the extent of parenchymal lung disease: none, minor, mild, moderate or severe. In the absence of moderate to severely abnormal spirometry (defined as forced expiratory volume in 1 s (FEV1) <60% and/or forced vital capacity (FVC) <70%), patients with a diagnosis of IPAH or heritable PAH who had no parenchymal lung disease were termed IPAHno-LD while those who had minor or mild emphysema or fibrosis on their original CT report were termed IPAHmild-LD. Patients with moderate to severely abnormal spirometry and/or those with moderate or severe parenchymal lung disease were defined as CLD-PH. Patients with pulmonary hypertension caused by respiratory disease other than COPD, emphysema or interstitial lung disease (ILD) were excluded. In addition, patients with two or more radiological features of possible pulmonary veno-occlusive disease (PVOD: centrilobular ground-glass opacities, mediastinal lymphadenopathy and interlobular septal lines) were excluded [10]. Smoking status and history were retrieved from clinical notes.
Quality of life was assessed by emPHasis-10 score [11] (scored out of 50; lower score represents lower symptom burden).
Mortality data
Mortality data were obtained from systems linked to the National Health Service Personal Demographics Service (PDS), which is updated when a death is registered in the UK. Patients who emigrated (n=3) were excluded, as were patients without a record on the PDS (n=2). Patients undergoing lung transplantation were censored at the time of surgery, and mortality data were collected using a census date of May 31, 2019.
Follow-up
Two follow-up time points were used to assess treatment response: first follow-up beyond 90 days of diagnosis and first follow-up between 9 and 15 months in patients receiving oral combination therapy within 6 months of diagnosis. The latter time point was used to enable comparison between patients who had received a similar therapeutic approach.
Statistics
Statistical analysis was performed using SPSS (v25; IBM, Armonk, NY, USA) and GraphPad Prism (v8; GraphPad, La Jolla, CA, USA). Unless otherwise specified, continuous data are presented as mean±sd (compared using paired/unpaired t-tests) or median (interquartile range) for nonparametric data (compared using Wilcoxon signed-rank/Mann–Whitney U-tests). Frequencies were compared using the Chi-squared test. Multivariate Cox regression was performed in a forward direction on parameters with a p-value <0.2 at univariate analysis. To allow comparison at univariate and multivariate analysis, continuous variables were scaled to the mean. For other statistical tests, a p-value of <0.05 was considered significant. Kaplan–Meier survival curves were compared using the log-rank test, truncated at 5 years. Where appropriate, 95% confidence intervals were derived for median values using a bootstrap resampling technique.
Ethics
Ethical approval was granted by Sheffield Teaching Hospitals NHS Foundation Trust (STH14169) and approved by the National Research Ethics Service (16/YH/0352).
Results
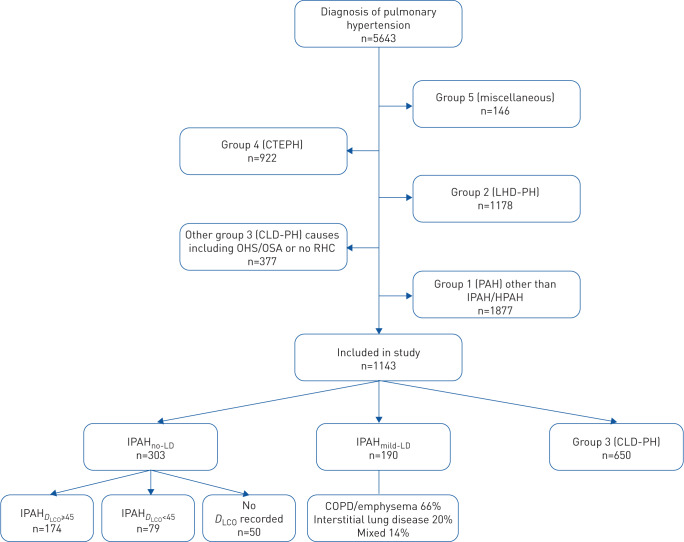
Of 5643 patients diagnosed with all forms of pulmonary hypertension, 493 incident patients were identified who had a diagnosis of either idiopathic or heritable PAH (hereafter grouped as IPAH, who formed the main study population) (figure 1). Following reassessment of patients assigned a diagnosis of IPAH, 303 had no evidence of parenchymal lung disease (IPAHno-LD) while 190 had minor or mild parenchymal lung disease (IPAHmild-LD). Baseline right heart catheterisation data were available in 98%, spirometry in 97% and DLCO in 83% of patients with IPAHno-LD and IPAHmild-LD.
FIGURE 1.
Flow chart demonstrating selection of patients for participation in study. CTEPH: chronic thromboembolic pulmonary hypertension; LHD-PH: pulmonary hypertension due to left heart disease; CLD-PH: chronic lung disease-associated pulmonary hypertension; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnoea; RHC: right heart catheterisation; PAH: pulmonary arterial hypertension; IPAH: idiopathic PAH; HPAH: heritable PAH; IPAHno-LD: IPAH with no lung disease; IPAHmild-LD: IPAH with mild lung disease; IPAHDLCO≥45: IPAH with no lung disease with DLCO ≥45% pred; IPAHDLCO<45: IPAH with no lung disease with DLCO <45% pred.
Comparison of IPAHno-LD versus IPAHmild-LD
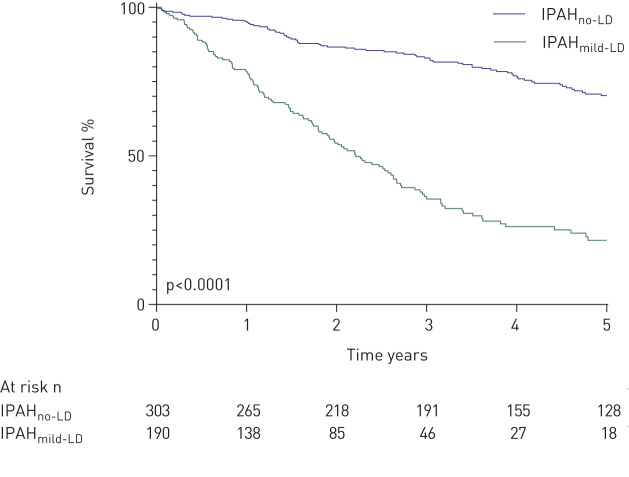
Patients with IPAHno-LD were younger (mean age 53 versus 70 years; p<0.0001), had a female predominance (73% versus 47%; p<0.0001), a higher mean mPAP and mixed venous oxygen saturations (mPAP 55 mmHg versus 50 mmHg, mixed venous oxygen saturation (SvO2) 60% versus 62%; both p<0.05) and were less likely to have a smoking history than patients with IPAHmild-LD (p<0.0001) (table 1). Spirometric volumes were well preserved in patients with IPAHno-LD and IPAHmild-LD. Patients with IPAHno-LD had significantly better survival than patients who had IPAHmild-LD (1- and 5-year survival 95% and 70% versus 78% and 22%, respectively; p<0.0001) (figure 2). When patients with IPAHno-LD and IPAHmild-LD were analysed together in a multivariate model, independent predictors of higher mortality were increasing age, lower DLCO % predicted, lower incremental shuttle walking test distance (ISWD) and a diagnosis of IPAHmild-LD (table 2). In view of the different forms of parenchymal lung disease encompassed by IPAHmild-LD, this multivariate model was reanalysed using data for the emphysema or interstitial lung disease subtypes separately. Increasing age, lower ISWD and a diagnosis of IPAHmild-LD remained as independent predictors of mortality. There was no significant difference in survival between patients with IPAHmild-LD who had emphysema or ILD (supplementary figure S1). Baseline demographics and haemodynamics of these groups were also very similar (supplementary table S1). Lung function data for subtypes of IPAHmild-LD are also shown in supplementary table S1.
TABLE 1.
Baseline demographics and maximal treatment data
| IPAHno-LD | IPAHmild-LD | p-value | |
| Subjects n | 303 | 190 | |
| Female | 73 | 47 | <0.0001 |
| Age years | 53±17 | 70±10 | <0.0001 |
| WHO FC I/II/III/IV | 0/21/60/19 | 0/9/56/35 | |
| BMI kg·m−2 | 29±6 | 28±6 | 0.15 |
| mRAP mmHg | 11±6 | 11±5 | 0.39 |
| mPAP mmHg | 55±13 | 50±9 | <0.0001 |
| PAWP mmHg | 10±3 | 11±3 | 0.10 |
| PVR WU | 11.9±5.8 | 11.1±4.5 | 0.10 |
| SvO2 % | 62±9 | 59±9 | 0.02 |
| Cardiac output L·min−1 | 4.3±1.6 | 4.0±1.4 | 0.04 |
| Cardiac index L·min−1·m−2 | 2.3±0.8 | 2.2±0.7 | 0.07 |
| FEV1 % pred | 89±15 | 89±17 | 0.64 |
| FVC % pred | 100±17 | 103±18 | <0.05 |
| FEV1/FVC | 75±9 | 68±8 | <0.0001 |
| DLCO % pred | 56±20 | 30±13 | <0.0001 |
| ISWD m | 210 (80, 360) | 80 (40, 180) | <0.0001 |
| Current smokers | 40 | 82 | <0.0001 |
| Smoking history pack-years | 25±17 | 32±18 | 0.03 |
| Maximal treatment# | |||
| None | 1 | 1 | |
| CCB | 5 | 1 | |
| Oral monotherapy | 19 | 34 | |
| Combination oral | 44 | 50 | |
| Prostanoid±oral | 31 | 14 |
Data are presented as %, mean±sd or median (interquartile range), unless otherwise stated. IPAH: idiopathic pulmonary arterial hypertension; IPAHno-LD: IPAH with no lung disease; IPAHmild-LD: IPAH with mild lung disease; WHO FC: World Health Organization functional class; BMI: body mass index; mRAP: mean right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; WU: Wood units; SvO2: mixed venous oxygen saturation; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; ISWD: incremental shuttle walking test distance; CCB: calcium-channel blockers. #: received during the study.
FIGURE 2.
Survival from diagnosis in idiopathic pulmonary arterial hypertension with no lung disease (IPAHno-LD) and with mild lung disease (IPAHmild-LD).
TABLE 2.
Univariate and multivariate Cox regression analysis of prognostic factors in patients with idiopathic pulmonary arterial hypertension with no lung disease (IPAHno-LD) or mild lung disease (IPAHmild-LD)
| Univariate | Multivariate | |||
| Scaled HR | p-value | Scaled HR | p-value | |
| IPAHmild-LD (ref. IPAHno-LD) | 4.287 | <0.0001 | 2.168 | <0.0001 |
| Age (years) | 2.320 | <0.0001 | 1.432 | 0.014 |
| Sex (ref. female) | 1.549 | 0.001 | ||
| Smoking history (ref. none) | 2.373 | <0.0001 | ||
| WHO FC III and IV (ref. I and II) | 3.246 | <0.0001 | ||
| FEV1 % pred | 0.995 | 0.945 | ||
| FVC % pred | 1.072 | 0.328 | ||
| FEV1/FVC | 0.767 | <0.0001 | ||
| DLCO % pred | 0.340 | <0.0001 | 0.739 | 0.039 |
| mRAP (mmHg) | 1.239 | <0.0005 | ||
| PVR (WU) | 0.999 | 0.986 | ||
| SvO2 (%) | 0.740 | <0.0001 | ||
| Cardiac index (L·min−1·m−2) | 0.788 | 0.003 | ||
| ISWD (m) | 0.434 | <0.0001 | 0.559 | <0.0001 |
For continuous variables, hazard ratios (HR) are scaled to the mean. ref: reference; WHO FC: World Health Organization functional class; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; mRAP: mean right atrial pressure; PVR: pulmonary vascular resistance; WU: Wood units; SvO2: mixed venous oxygen saturation; ISWD: incremental shuttle walking test distance.
Effect of DLCO on survival in IPAHno-LD
A bimodal distribution of DLCO % predicted (modes 30, 65) was observed in patients with IPAHno-LD with an optimal cut-point of 45% (supplementary figure S2). Patients with IPAHno-LD who had a DLCO <45% pred (IPAHDLCO<45) were older (mean 65 versus 48 years), had a lower mPAP (51 versus 56 mmHg) and a lower SvO2 (60% versus 63%) than patients with DLCO ≥45% predicted (IPAHDLCO≥45) (all p<0.05). Detailed demographics are shown in supplementary table S2. Those with IPAHDLCO<45 were more likely to have a history of smoking (52% versus 36%; p<0.05). There was no significant difference in lung volumes (FEV1 88% versus 91%, FVC 100% versus 100%; both p>0.05), but FEV1/FVC ratio was lower (71% versus 76%; p<0.0001) in patients with IPAHDLCO<45. 1- and 5-year survival was significantly lower in patients with IPAHDLCO<45 (86% and 45% versus 99% and 84%; p<0.0001) (figure 3); this survival difference persisted when adjusted for age.
FIGURE 3.
Survival in idiopathic pulmonary arterial hypertension patients with no lung disease stratified by diffusing capacity of the lung for carbon monoxide <45% pred (IPAHDLCO<45) versus ≥45% pred (IPAHDLCO≥45).
Response to treatment
99% of patients with IPAHno-LD and IPAHmild-LD received PAH therapy; treatment response data are shown in table 3 and figure 4. Baseline ISWD was significantly higher in IPAHno-LD than IPAHmild-LD: median 210 m versus 80 m (p<0.0001). There was no significant difference in time to follow-up between patients with IPAHno-LD and IPAHmild-LD. At both first follow-up and at 1-year assessment in patients who received combination oral therapy within 6 months of diagnosis, patients with IPAHno-LD demonstrated significant improvement with respect to ISWD and quality-of-life score (p<0.0001), whereas patients with IPAHmild-LD did not. In patients receiving oral combination therapy within 6 months of diagnosis, survival was significantly better in patients with IPAHno-LD compared to patients with IPAHmild-LD (1- and 5-year survival 98% and 74% versus 71% and 13%; p<0.0001) (figure 5). Patients with IPAHno-LD and IPAHmild-LD who received oral combination therapy within 6 months of diagnosis and had 1-year follow-up data available had more severe haemodynamics than those who did not; haemodynamic and treatment data for all patients who had follow-up assessments available are displayed in supplementary table S3.
TABLE 3.
Baseline and follow-up incremental shuttle walking test distance (ISWD) and emPHasis-10 quality-of-life score (E-10)
| Subjects | IPAHno-LD | Subjects | IPAHmild-LD | Subjects | IPAHDLCO<45 | Subjects | IPAHDLCO≥45 | |
| Baseline and first follow-up beyond 90 days | ||||||||
| ISWD | ||||||||
| Baseline m | 279 | 210 (80–360) | 159 | 80 (40–180)**** | 71 | 80 (30–210) | 170 | 260 (130–430)#### |
| ΔISWD m | 215 | 40 (−10–120) | 124 | 0 (−32–30)**** | 47 | 20 (−13–70) | 139 | 50 (−10–160)# |
| ΔISWD p-value | <0.0001 | 0.90 | <0.05 | <0.0001 | ||||
| E-10 score | ||||||||
| Baseline | 84 | 32 (20–40) | 83 | 32 (26–41) | 25 | 38 (33–44) | 55 | 27 (0–35)## |
| ΔE-10 | 64 | −4 (−12–3) | 65 | 0 (−5–8)* | 19 | −4 (−13–2) | 43 | −4 (−11–3) |
| ΔE-10 p-value | 0.005 | 0.57 | 0.08 | 0.03 | ||||
| Baseline and 1-year follow-up: patients treated with combination oral treatment within 6 months of diagnosis | ||||||||
| ISWD | ||||||||
| Baseline ISWD m | 125 | 200 (80–340) | 76 | 60 (20–140)**** | ||||
| ΔISWD m | 79 | 40 (−10–140) | 32 | −20 (−50–50)** | ||||
| ΔISWD p-value | <0.0001 | 0.83 | ||||||
| E-10 score | ||||||||
| Baseline E-10 | 57 | 34 (22–42) | 41 | 35 (28–41) | ||||
| ΔE-10 | 27 | −4 (−18–5) | 17 | −4 (−11–3) | ||||
| ΔE-10 p-value | 0.03 | 0.19 |
Data are presented as n or median (interquartile range). IPAH: idiopathic pulmonary arterial hypertension; IPAHno-LD: IPAH with no lung disease; IPAHmild-LD: IPAH with mild lung disease; IPAHDLCO<45: IPAHno-LD with DLCO <45% pred; IPAHDLCO≥45: IPAHno-LD with DLCO ≥45% pred; Δ: change. *: p<0.05; **: p<0.01; ****: p<0.0001 compared to IPAHno-LD; #: p<0.05; ##: p<0.01; ####: p<0.0001 compared to IPAHDLCO<45.
FIGURE 4.
Change at first follow-up beyond 90 days in a) incremental shuttle walking test distance (ISWD); b) emPHasis-10 quality-of-life score (E-10). Data are presented as median (95% CI). IPAH: idiopathic pulmonary arterial hypertension; IPAHno-LD: IPAH with no lung disease; IPAHmild-LD: IPAH with mild lung disease; IPAHDLCO<45: IPAHno-LD with diffusing capacity of the lung for carbon monoxide (DLCO) <45% pred; IPAHDLCO≥45: IPAHno-LD with DLCO ≥45% pred.
FIGURE 5.
Survival in patients with idiopathic pulmonary arterial hypertension with no lung disease (IPAHno-LD) and mild lung disease (IPAHmild-LD) treated with oral combination therapy within 6 months of diagnosis.
In the IPAHno-LD group, significant change (Δ) in ISWD was seen in patients with IPAHDLCO<45 (median Δ +20 m) and IPAHDLCO≥45 (Δ +50 m) who received treatment; both p<0.05. Baseline emPHasis-10 scores were significantly higher in patients with IPAHDLCO<45 than in patients with IPAHDLCO≥45 (median 38 versus 27; p<0.01). Median change in emPHasis-10 at follow-up was not significant in patients with IPAHDLCO<45 (ΔemPHasis-10 –4; p=0.08) but was significant in patients with IPAHDLCO≥45 (ΔemPHasis-10 –4; p<0.05).
Discussion
In the current study we reassessed a large number of patients who had been assigned a diagnosis of IPAH at a large pulmonary hypertension referral centre. By using radiology reports and lung function from the time of diagnosis we have identified phenotypes of IPAH with different characteristics, response to therapy and survival. Specifically, we have demonstrated that the presence of even mild parenchymal lung disease in patients who have been diagnosed with IPAH (IPAHmild-LD) is associated with a distinct clinical picture (well-preserved spirometry, low DLCO % pred and severe pulmonary hypertension) and has a large negative effect on outcomes. In addition, we have observed that a proportion of patients with IPAH with no parenchymal lung disease and unremarkable spirometry have a low DLCO. Differentiation of IPAH from CLD-PH represents a continual diagnostic challenge to all involved in the care of patients with pulmonary hypertension. Mild pulmonary hypertension in the context of severe lung disease (assessed radiologically and/or spirometrically) is common, and easily ascribed to group 3 (CLD-PH). Likewise, most would agree that patients with severe pulmonary haemodynamics and severe lung disease, where the severity of pulmonary hypertension is proportionate to the degree of lung disease, also have group 3 disease. The presence of more modest lung disease in patients who fulfil traditional criteria for IPAH presents greater diagnostic and therapeutic challenges
Survival and response to therapy in IPAHmild-LD
In the current study, survival in patients with IPAHmild-LD was significantly worse than in IPAHno-LD. In addition, although patients with IPAHno-LD experienced significant improvements in walk distance and emPHasis-10 score following initiation of PAH therapies, this same improvement was not observed in patients with IPAHmild-LD. Some retrospective studies of patients with mild-to-moderate lung disease and severe pulmonary hypertension have reported improved haemodynamics and exercise capacity following commencement of PAH therapies [12–14]. Conversely, Brewis et al. [15] failed to demonstrate improvement in 6-min walk distance (6MWD) or World Health Organization functional class in 118 patients with severe pulmonary hypertension and varying degrees of lung disease following PAH therapy, while we have previously reported treatment response to pulmonary vascular therapy in only 19% of patients with severe CLD-PH [16]. Prospective randomised controlled studies (RCTs) of PAH therapies in patients with CLD-PH due to COPD/emphysema have suffered from methodological weaknesses [17, 18] or recruited patients with mild pulmonary hypertension [19, 20], although Vitulo et al. [21] performed a RCT in 31 patients with severe CLD-PH due to COPD and demonstrated significant improvements in pulmonary haemodynamics, but no improvement in 6MWD.
The 6th WSPH task force on CLD-PH recommended that in patients with coexisting lung disease, PAH should be diagnosed when pulmonary hypertension is moderate–severe, when only modest spirometric (i.e. FEV1 >60% pred and FVC >70% pred) or parenchymal abnormalities are present and when DLCO is low with respect to obstructive or restrictive lung function [7]. Using these criteria, patients in our IPAHmild-LD group would keep their original diagnosis of IPAH. Our observations regarding the effect that mild parenchymal lung disease has on response to therapy and survival suggests that IPAHmild-LD is a distinct phenotype and that further prospective studies to assess treatment response in these patients are warranted. Recognition of an IPAHmild-LD phenotype also has implications for risk stratification, decisions regarding transplantation and PAH therapy clinical trial design.
IPAH with DLCO <45%
Trip et al. [22] observed a bimodal distribution of percentage predicted DLCO in a cohort of 166 patients diagnosed with IPAH, and demonstrated that a DLCO <45% pred conferred worse survival. While they included patients who had mild or moderate lung disease, we observed a similar distribution of percentage predicted DLCO in patients without any lung disease (IPAHno-LD, supplementary figure S2). Subsequently, Olsson et al. [23] described a subgroup of patients with IPAH with no parenchymal lung disease but severely reduced gas transfer. In keeping with these two studies, our cohort of IPAHno-LD patients with DLCO <45% pred (IPAHDLCO<45) was older, more likely to have a smoking history and had a lower exercise capacity. Although survival in patients with IPAHDLCO<45 was significantly worse than those with IPAHDLCO≥45, significant improvements in ISWD were observed following PAH therapy, unlike in patients with IPAHmild-LD. The cause of the reduced DLCO in a proportion of IPAH patients is unclear. Pulmonary veno-occlusive disease is a rare cause of low DLCO and is haemodynamically indistinguishable from PAH [10]. However, we excluded patients (n=18) where there was a possibility of PVOD based on radiological assessment. Given the increased frequency of smoking in the IPAHDLCO<45 group it is possible that the reduced DLCO may represent emphysema not visible on cross-sectional imaging [24]. However, tobacco smoke has also been shown to cause pulmonary vascular remodelling in animal models, and specifically to cause damage to the pulmonary capillaries [25]. Therefore, the data from the current study provide further support for the existence of a vanishing capillary syndrome as proposed by Hoeper et al. [26]. Further histological data are required to fully explain this phenomenon.
Limitations
This is a retrospective study and hence there were some data availability issues, including first follow-up ISWD data which was not available in 19% of patients. Patients who were unable to attempt the incremental shuttle walking test due to their pulmonary hypertension were ascribed a distance of 0 m, which would minimise any potential bias resulting from missing data. Baseline scores for emPHasis-10 were only available for 34% of patients, since it was only introduced in our centre in 2014. A small number of patients (3%) with IPAHno-LD and IPAHmild-LD did not have spirometry available and were categorised based on CT data alone. Parenchymal lung disease assessment was based on qualitative clinical reports provided by radiologists at the time of initial diagnosis in our unit, and not on fully quantitative assessments. However, our data demonstrate that “real-world” clinical radiological assessments of the presence and extent of parenchymal lung disease can be used to identify patient groups with different outcomes. While our patients now routinely undergo high-resolution CT (HRCT) imaging and CT pulmonary angiography, some patients did not have specific HRCT imaging and so assessment of lung parenchyma in these patients may have been more limited. As there was no control group in this study, we cannot rule out a treatment effect of PAH therapies on patients with IPAHmild-LD.
Conclusion
The presence of even mild parenchymal lung disease in patients who, based on current recommendations, would be classified as having IPAH has a significant adverse effect on survival and, in this patient cohort, was associated with a lack of significant improvement in exercise capacity following treatment. Patients with the phenotype of IPAHmild-LD can be identified using lung function testing and qualitative clinical description of the presence and extent of parenchymal lung disease on routine radiological reporting. In addition, a proportion of patients with IPAH and no evidence of lung disease or PVOD have a severely reduced diffusion capacity. Our data support the need for prospective RCTs in patients with these phenotypes to assess the effects of PAH therapies on short- and long-term outcomes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00041-2020.SUPPLEMENT (287.2KB, pdf)
Shareable PDF
Supplementary Material
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00606-2020
This article has supplementary material available from erj.ersjournals.com
Support statement: A.A.R. Thompson is supported by a British Heart Foundation Intermediate Clinical Fellowship (FS/18/13/3328). A.J. Swift is supported by a Wellcome Trust Clinical Research Career Development Fellowship (205188/Z/16/Z). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: R.A. Lewis reports non-financial support from Actelion Pharmaceuticals, outside the submitted work.
Conflict of interest: A.A.R. Thompson reports grants from British Heart Foundation, during the conduct of the study; support for meeting attendance from Actelion Pharmaceuticals Ltd, outside the submitted work.
Conflict of interest: C.G. Billings has nothing to disclose.
Conflict of interest: A. Charalampopoulos reports grants, personal fees and non-financial support from Actelion Pharmaceuticals, personal fees and non-financial support from Novartis, grants from Bayer and GSK, outside the submitted work.
Conflict of interest: C.A. Elliot reports personal fees for advisory board work and lectures from Actelion Pharmaceuticals, GlaxoSmithKline and Bayer, grants from Pfizer, Actelion Pharmaceuticals and Bayer, support for meeting attendance from Bayer and Actelion Pharmaceuticals, outside the submitted work.
Conflict of interest: N. Hamilton reports personal fees from MSD and Actelion, grants and personal fees from Bayer, outside the submitted work.
Conflict of interest: C. Hill has nothing to disclose.
Conflict of interest: J. Hurdman has nothing to disclose.
Conflict of interest: S. Rajaram has nothing to disclose.
Conflict of interest: I. Sabroe reports grants and personal fees for advisory board from AstraZeneca, grants from GSK, outside the submitted work.
Conflict of interest: A.J. Swift has nothing to disclose.
Conflict of interest: D.G. Kiely reports grants, personal fees and non-financial support from Actelion, Bayer and GSK, personal fees and non-financial support from MSD, outside the submitted work.
Conflict of interest: R. Condliffe reports grants, personal fees and non-financial support from Actelion Pharmaceuticals and Bayer, grants from GSK, outside the submitted work.
References
- 1.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. doi: 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. doi: 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. doi: 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- 7.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 9.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. doi: 10.1183/09031936.00078411 [DOI] [PubMed] [Google Scholar]
- 10.Montani D, O'Callaghan DS, Savale L, et al. Pulmonary veno-occlusive disease: recent progress and current challenges. Respir Med 2010; 104: Suppl. 1, S23–S32. doi: 10.1016/j.rmed.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Yorke J, Corris P, Gaine S, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J 2014; 43: 1106–1113. doi: 10.1183/09031936.00127113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fossati L, Müller-Mottet S, Hasler E, et al. Long-term effect of vasodilator therapy in pulmonary hypertension due to COPD: a retrospective analysis. Lung 2014; 192: 987–995. doi: 10.1007/s00408-014-9650-1 [DOI] [PubMed] [Google Scholar]
- 13.Girard A, Jouneau S, Chabanne C, et al. Severe pulmonary hypertension associated with COPD: hemodynamic improvement with specific therapy. Respiration 2015; 90: 220–228. doi: 10.1159/000431380 [DOI] [PubMed] [Google Scholar]
- 14.Calcaianu G, Canuet M, Schuller A, et al. Pulmonary arterial hypertension-specific drug therapy in COPD patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration 2016; 91: 9–17. doi: 10.1159/000441304 [DOI] [PubMed] [Google Scholar]
- 15.Brewis MJ, Church AC, Johnson MK, et al. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46: 1378–1389. doi: 10.1183/13993003.02307-2014 [DOI] [PubMed] [Google Scholar]
- 16.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 17.Valerio G, Bracciale P, Grazia D'Agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3: 15–21. doi: 10.1177/1753465808103499 [DOI] [PubMed] [Google Scholar]
- 18.Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53: 81–85. [PubMed] [Google Scholar]
- 19.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010; 181: 270–278. doi: 10.1164/rccm.200907-0988OC [DOI] [PubMed] [Google Scholar]
- 20.Goudie AR, Lipworth BJ, Hopkinson PJ, et al. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2: 293–300. doi: 10.1016/S2213-2600(14)70013-X [DOI] [PubMed] [Google Scholar]
- 21.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. doi: 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 22.Trip P, Nossent EJ, de Man FS, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J 2013; 42: 1575–1585. doi: 10.1183/09031936.00184412 [DOI] [PubMed] [Google Scholar]
- 23.Olsson KM, Fuge J, Meyer K, et al. More on idiopathic pulmonary arterial hypertension with a low diffusing capacity. Eur Respir J 2017; 50: 1700354. doi: 10.1183/13993003.00354-2017 [DOI] [PubMed] [Google Scholar]
- 24.Kirby M, Owrangi A, Svenningsen S, et al. On the role of abnormal DLCO in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax 2013; 68: 752–759. doi: 10.1136/thoraxjnl-2012-203108 [DOI] [PubMed] [Google Scholar]
- 25.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011; 147: 293–305. doi: 10.1016/j.cell.2011.08.035 [DOI] [PubMed] [Google Scholar]
- 26.Hoeper MM, Vonk-Noordegraaf A. Is there a vanishing pulmonary capillary syndrome? Lancet Respir Med 2017; 5: 676–678. doi: 10.1016/S2213-2600(17)30291-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00041-2020.SUPPLEMENT (287.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00041-2020.Shareable (277.5KB, pdf)