Abstract
Coronavirus disease 2019 (COVID-19) is a novel and lethal infectious disease, posing a threat to global health security. The number of cases has increased rapidly, but no data concerning kidney transplant (KTx) recipients infected with COVID-19 are available. To present the epidemiological, clinical, and therapeutic characteristics of KTx recipients infected with COVID-19, we report on a case series of five patients who were confirmed as having COVID-19 through nucleic acid testing (NAT) from January 1, 2020 to February 28, 2020. The most common symptoms on admission to hospital were fever (five patients, 100%), cough (five patients, 100%), myalgia or fatigue (three patients, 60%), and sputum production (three patients, 60%); serum creatinine or urea nitrogen levels were slightly higher than those before symptom onset. Four patients received a reduced dose of maintenance immunosuppressive therapy during hospitalization. As of March 4, 2020 NAT was negative for COVID-19 in three patients twice in succession, and their computed tomography scans showed improved images. Although greater patient numbers and long-term follow-up data are needed, our series demonstrates that mild COVID-19 infection in KTx recipients can be managed using symptomatic support therapy combined with adjusted maintenance immunosuppressive therapy.
Keywords: Clinical feature, Coronavirus disease 2019, Kidney transplant recipient
1. Case series
1.1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that causes coronavirus disease 2019 (COVID-19), which emerged in Wuhan, China, in December 2019 and has become a growing threat to global health security [1]. Clinically, the disease is characterized with fever, cough, lymphopenia, dyspnea, and eventually respiratory failure and multiple organ damage in severe cases [2].
Immunosuppression after transplantation renders kidney transplant (KTx) recipients susceptible to a variety of viral pathogens. Several cases of transplant recipients with SARS-CoV or Middle East respiratory syndrome coronavirus (MERS-CoV) infection have been reported [3], [4], [5]. However, epidemiological and clinical information characterizing SARS-CoV-2 infections in KTx recipients remains unknown. Here, we aimed to comprehensively describe epidemiological and clinical features in five KTx recipients infected with COVID-19. Our study findings are likely to be of considerable value for the diagnosis and treatment of these patients.
1.2. Cases
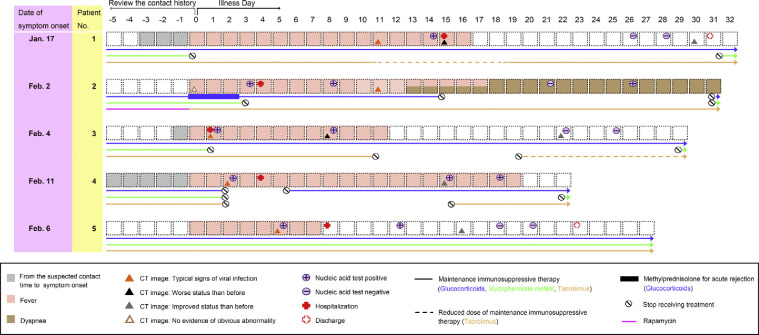
From January 2015 to December 2019, 803 cases of kidney transplantation were performed in our hospital and 743 KTx recipients were followed up; six cases had symptoms, of whom five were diagnosed to have COVID-19 with confirmed SARS-CoV-2 infection using nucleic acid testing (NAT) from January 1, 2020 to February 28, 2020 (Fig. 1 ) [6]. After the National Health Commission of China gave approval for data collection (written informed consent was waived as part of a public health outbreak investigation) and oral consent was obtained from patients, we followed up the five KTx recipients until March 4, 2020.
Fig. 1.
Timeline of epidemiological and clinical characteristics of kidney transplant recipients infected with COVID-19. Date of symptom onset was defined as origin point, and the contact history was reviewed. Symptoms, CT image, nucleic acid test, hospital stay, and adjustment of immunosuppressive therapy were listed according to the day of illness.
COVID-19 = coronavirus disease 2019; CT = computed tomography.
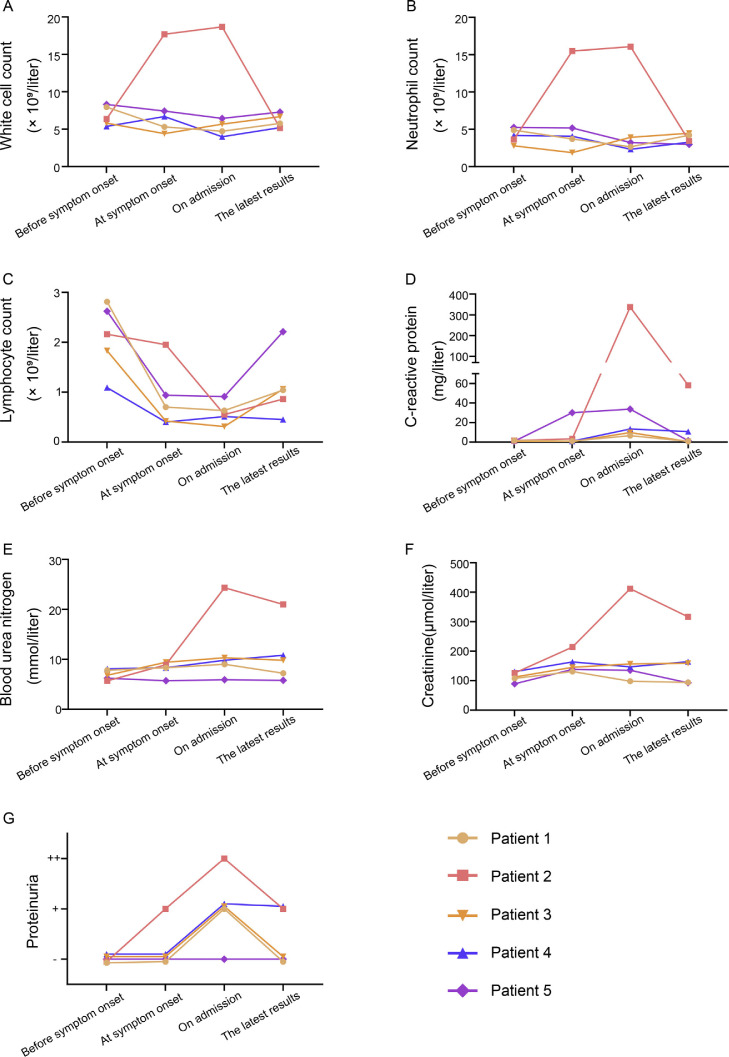
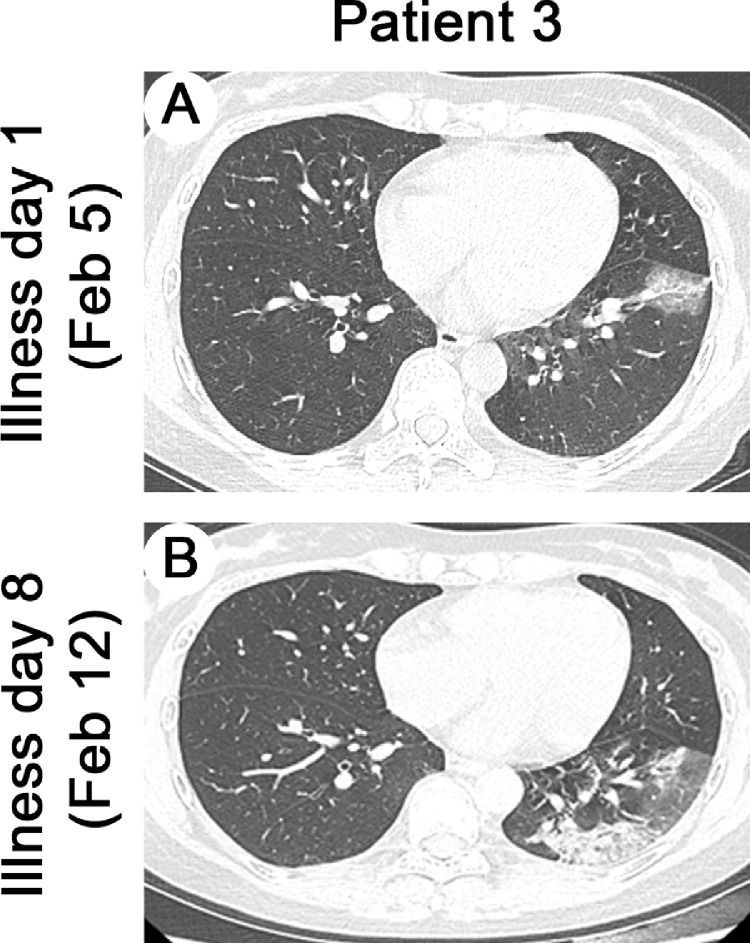
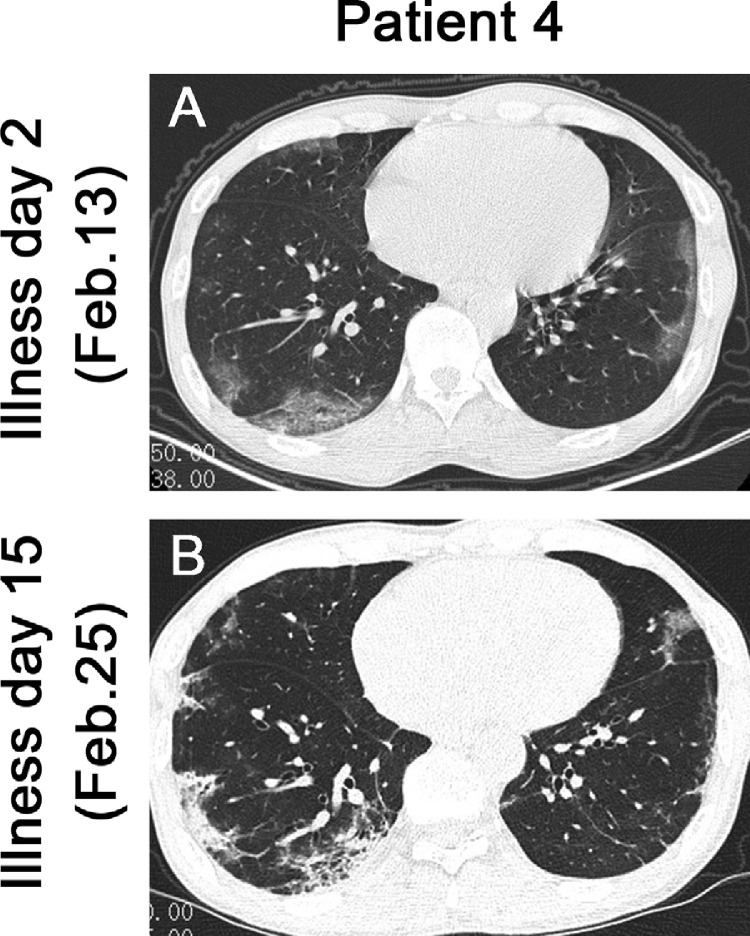
The mean age of these five patients was 45 yr (standard deviation, 11 yr), and four were male. All patients had undergone donation after cardiac death renal transplants between January 2016 and November 2019. Comorbidities included hypertension (n = 2), diabetes (n = 1), or bladder cancer (n = 1); the most common symptoms were fever (five patients, 100%), cough (five patients, 100%), myalgia or fatigue (three patients, 60%), and sputum production (three patients, 60%; Table 1 ). At symptom onset, white blood cell and neutrophil levels of all patients were within normal range except in patient 2, but serum creatinine or urea nitrogen levels were slightly higher than those before symptom onset (Fig. 2 A, 2B, 2E, and 2 F). On admission, five patients developed lymphopenia and an elevated C-reactive protein (CRP) level, and proteinuria appeared in four patients (Fig. 2C, 2D, and 2 G and Table 2 ). During hospitalization, all patients received antiviral therapy (oseltamivir or arbidol); patients 2 and 3 also received antibacterial therapy (cefixime) and intravenous immunoglobulin, respectively. More importantly, triple immunosuppression with glucocorticoids, mycophenolate mofetil (MMF), and calcineurin inhibitors (CNIs) had been used in four recipients prior to symptom onset; however, after the onset of illness, the immunosuppressant was reduced or stopped in these four patients (Fig. 1). In patients 1 and 3, the second chest computed tomography (CT) scans showed deterioration, but this resolved later, as shown in the third CT scans (Fig. 1, Fig. 3 ). As of March 4, 2020 four patients had improved chest CT findings, with three having negative NAT results twice in succession (Fig. 1, Fig. 4 ). Symptoms of all patients resolved gradually, except in patient 2. None of these five patients required mechanical ventilation or admission to intensive care units, and two were discharged while three remained hospitalized (Fig. 1).
Table 1.
Clinical characteristic of the five kidney transplant recipients infected with COVID-19 on admission to hospitala
| Patient no. |
Summary | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Sex | Male | Male | Female | Male | Male | – |
| Age (yr) | 38 | 64 | 37 | 47 | 38 | 45 ± 11 |
| Time of kidney transplant surgery | Oct 23, 2019 | Jan 16, 2016 | Aug 19,2019 | Feb 26, 2019 | Jul 27, 2017 | – |
| Sources of donor kidneys | DCD | DCD | DCD | DCD | DCD | – |
| Comorbidities other than kidney diseases | ||||||
| Hypertension | – | – | + | – | + | 2+ |
| Diabetes | – | – | – | – | + | 1+ |
| Bladder cancer | – | + | – | – | – | 1+ |
| Fever | + | + | + | + | + | 5+ |
| Cough | + | + | + | + | + | 5+ |
| Sputum production | – | + | – | + | + | 3+ |
| Myalgia or fatigue | – | + | – | + | + | 3+ |
| Dyspnea | – | – | – | – | – | 0+ |
| Gastrointestinal symptoms | – | – | – | – | – | 0+ |
| Body temperature (°C) | 38.9 | 38.3 | 39 | 39.8 | 39.1 | 39.2 ± 0.5 |
| Oximetry saturation on room air (%) | 99 | 96 | 99 | 98 | 97 | 97.8 ± 1.3 |
COVID-19 = coronavirus disease 2019; DCD = donation after cardiac death.
Plus-minus values are means ± standard deviation. A plus sign indicates that the sign or symptom was present, and a minus sign that it was absent.
Fig. 2.
Dynamic profiles of clinical laboratory findings. We chose four time points to record the dynamic profiles of laboratory findings: (1) 1 mo before the symptom onset, (2) when the patient developed symptoms, (3) when the patient was admitted to hospital, and (4) the patient's latest laboratory test.
Table 2.
Laboratory findings of the five kidney transplant recipients infected with COVID-19 on admission to hospitala
| Patient no. |
Summary | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| White blood cell count (×109/l) | 4.73 | 17.67 | 5.67 | 3.99 | 6.44 | 7.70 ± 5.65 |
| Neutrophil count (×109/L) | 2.66 | 16.07 | 3.93 | 2.33 | 3.22 | 5.64 ± 5.86 |
| Lymphocyte count (×109/l) | 0.63 | 0.55 | 0.31 | 0.51 | 0.91 | 0.58 ± 0.22 |
| Platelet count (×109/l) | 222 | 136 | 158 | 186 | 228 | 186 ± 40 |
| Hemoglobin (g/l) | 99 | 139 | 107 | 85 | 148 | 116 ± 27 |
| PT (s) | 12.3 | 12.7 | 13.7 | 14.0 | 12.6 | 13.0 ± 0.7 |
| APTT (s) | 32.4 | 37.8 | 38.2 | 43.2 | 36.9 | 37.7 ± 3.8 |
| D-dimer (mg/l) | 0.37 | 1.26 | 2.03 | 0.45 | 0.39 | 0.90 ± 0.73 |
| CRP (mg/l) | 6.68 | 337.11 | 9.77 | 13.38 | 33.72 | 80.13 ± 144.04 |
| ESR (mm/h) | 7 | >100 | 17 | 12 | 44 | >36 ± 39 |
| Albumin (g/l) | 34.2 | 29.3 | 33.6 | 37.7 | 45.2 | 36.0 ± 5.9 |
| Total bilirubin (μmol/l) | 9.2 | 14.7 | 4.6 | 12.8 | 10.4 | 10.3 ± 3.9 |
| Direct bilirubin (μmol/l) | 3.1 | 2.0 | 1.8 | 3.9 | 4.9 | 3.1 ± 1.3 |
| ALT (U/l) | 66 | 21 | 70 | 7 | 20 | 37 ± 29 |
| AST (U/l) | 41 | 31 | 49 | 26 | 21 | 34 ± 11 |
| LDH (U/l) | 193 | 180 | 160 | 235 | 248 | 203 ± 37 |
| Urea (mmol/l) | 9.02 | 24.34 | 10.30 | 9.82 | 5.92 | 11.88 ± 7.17 |
| Creatinine (μmol/l) | 98.0 | 411.7 | 137.0 | 146.9 | 135.4 | 185.8 ± 127.6 |
| Proteinuria | + | ++ | + | + | – | – |
ALT = alanine aminotransferase; APTT = activated partial thromboplastin time; AST = aspartate aminotransferase; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; LDH = lactate dehydrogenase; PT = prothrombin time.
Plus-minus values are means ± SD. Normal ranges of laboratory findings are as follows: for white blood cell count, (3.5–9.5) × 109/l; for neutrophil count, (1.8–6.3) × 109/l; for lymphocyte count, (1.1–3.2) × 109/l; for platelet count, (125–350) × 109/l; for hemoglobin, (130–175) g/l; for PT, 11.0–16.0 s; for APTT, 28.0–43.5 s; for D-dimer, 0–0.5 mg/l; for CRP, 0–8 mg/l; for ESR, 0–15 mm/h; for albumin, 35–55 g/l; for total bilirubin, 5.1–19.0 μmol/l; for direct bilirubin, 1.7–6.8 μmol/l; for ALT, 5–40 U/l; for AST, 8–40 U/l; for LDH, 109–245 U/l; for urea, 2.9–8.2 mmol/l; for creatinine, 44.0–133.0 μmol/l; for proteinuria, “–” indicates that protein excretion is <10 mg/dl, “+” indicates that protein excretion is between 30 and 100 mg/dl, and “++” indicates that protein excretion is between 100 and 300 mg/dl.
Fig. 3.
Chest CT images of patient 3. (A) Transverse chest CT image of patient 3 showed unilateral ground-glass opacity with sparing of subpleural regions on illness day 1. (B) On illness day 8, CT image from patient 3 showed larger lesions in the lower lobe of the left lung with partial consolidation. Figure showed dynamic profiles of white cell count (A), neutrophil count (B), lymphocyte count (C), C-reactive protein (D), blood urea nitrogen (E), creatinine (F), proteinuria (G).
CT = computed tomography.
Fig. 4.
Chest CT images of patient 4. (A) Transverse chest CT image from patient 4 shows bilateral ground-glass opacity with sparing of subpleural regions on illness day 2. (B) CT image on illness day 15 shows multiple consolidations and fibrous stripes in both lungs.
CT = computed tomography.
The maintenance immunosuppressive therapy of patient 2 was glucocorticoid, MMF, and rapamycin before symptom onset. On illness day 0, the patient complained of fever and anuria, with increased creatinine, white blood cell, and neutrophil levels. Considering the normal chest CT scan, fever, anuria, hypercreatinemia, and leukocytosis at that time (Fig. 1), we regarded patient 2 as having acute transplantation rejection, in line with authoritative European Association of Urology guidelines on renal transplantation [7]. Thus, patient 2 was treated with methylprednisolone pulse therapy (500, 250, and 250 mg) for 3 d, after which the patient’s fever abated, and urine output and serum creatinine levels returned to normal. However, a high fever resumed the next day, with a positive NAT for SARS-CoV-2 confirming that patient 2 had COVID-19. On admission, the urea (24.34 mmol/l) and creatinine (411.7 μmol/l) levels of patient 2 were the highest among these five patients, and the patient also had elevated white blood cell, neutrophil, and CRP levels (Fig. 2 and Table 2). After a week of hospitalization, the second CT scan for patient 2 showed a typical presentation of viral pneumonia. During hospitalization, despite the gradual disappearance of fever, patient 2 complained of dyspnea on exertion with normal oxygen saturation and refused to leave the isolation ward for a third CT scan (Fig. 1).
2. Discussion
Although recent studies have reported epidemiological and clinical features of COVID-19 [1], this is the first study to characterize KTx recipients with COVID-19. After the outbreak of COVID-19, we identified five infected KTx recipients. While three patients had a suspected history of exposure, the incubation period concerning the other two patients was unclear (Fig. 1). However, these two patients were residents of Wuhan, the center of the outbreak, and their infection may have been due to exposure to undiagnosed, asymptomatic carriers of SARS-CoV-2. Furthermore, specifically concerning patient 2, the patient was treated with methylprednisolone pulse therapy, which involves greater immunosuppression and could be a predisposing factor for SARS-CoV-2 infection.
Although SARS-CoV-2 nucleic acid reverse transcription polymerase chain reaction analysis using throat swab specimens can confirm COVID-19 infection, false negatives are possible due to the sampling techniques, viral load of the upper respiratory tract, and mutations of the virus gene. For example, on illness day 21, the NAT result of patient 2 was not definitive, which suggests that trained technicians should conduct standard multisampling in strict accordance with protocols to improve the sensitivity of specimens. Apart from lower respiratory tract sampling, whole genome sequencing and detection of serum antibodies should be considered to optimize diagnostic methods.
Concerning laboratory findings, lymphocytopenia developed in the five patients on admission. Although we could not explain this, lymphocytes have been identified as primary targets of SARS-CoV injury [8]. Notably, the absolute leukocyte and neutrophil value was increased in patient 2. However, because bacterial culture results were not available, the presence of bacterial coinfections could not be confirmed. It should also be noted that glucocorticoids, such as methylprednisolone, and acute rejection can contribute to increasing leukocytes and neutrophils on initiation [9]. Thus, the use of the leukocyte differential could be helpful in determining whether an abnormal number of leukocytes and neutrophils are due to bacterial coinfections, acute rejection, or initiation of methylprednisolone.
Apart from symptomatic support therapy, no specific treatment for COVID-19 has been confirmed, although several ongoing clinical trials may identify some options [10]. Even for KTx recipients infected with SARS and MERS, the treatment options are limited [3], [4], [5]. Therefore, enhancing personal protection precautions, early identification, and timely management of affected cases are of crucial importance. Despite the lack of evidence on clinical efficacy, each patient in this study received antiviral therapy, and patients 2 and 3 also received antibacterial agent and intravenous immunoglobulin, respectively.
Given the extent of lymphocytes shown to be consumed by SARS-CoV-2 [10], MMF treatment was withdrawn in four patients. Moreover, glucocorticoids and tacrolimus dosages were also adjusted. There is no evidence to confirm the favorable effects of glucocorticoids in COVID-19 treatment [11]. Furthermore, whether SARS-CoV-2 can be inhibited by tacrolimus is unclear, although replication of SARS-CoV was diminished after tacrolimus treatment [12]. Notably, cyclosporine, another CNI drug, may be used as an option of host-directed therapies for COVID-19 [13]. Strikingly, no significant kidney impairment was detected except in patient 2, implying that reducing immunosuppressive therapy for a short period would not lead to acute rejection. However, the long-term effect is uncertain. For patient 5, immunosuppressant use was not changed because of the relatively mild symptoms. The satisfactory clinical outcome of patient 5 suggested that an immunosuppressive maintenance dosage might not compromise the antiviral immune effect in relatively mild COVID-19 cases. In addition, aggressive reduction or withdrawal of the immunosuppressant should be considered cautiously only for recipients with severe pneumonia or acute respiratory distress syndrome [14]. More importantly, renal function should be monitored frequently because renal failure resulting from acute rejection could make treatment more difficult.
During hospitalization, symptoms of four patients were gradually controlled, whereas those of patient 2 got worse, which, in this case, might be related in part to former acute rejection. Additionally, as COVID-19 progresses, kidney impairment in patients infected with SARS-CoV-2 might contribute to poor outcomes, similar to SARS-CoV where pathological findings concerning kidney specimens from patients with SARS-CoV shows acute tubular necrosis [15], [16]. Moreover, based on recent studies [1], old age and malignancy could be further risk factors for patient 2. However, whether other factors may affect prognosis could not be determined due to the limited data available.
In conclusion, although the five KTx recipients were immunocompromised, severe COVID-19 was not found. Mild COVID-19 in KTx recipients can be managed using symptomatic support therapy combined with adjusted maintenance immunosuppressive therapy. Meanwhile, physicians should pay attention to the influence of comorbidities and the possibility of coinfections. More data are needed to gain better understanding of KTx recipients infected with COVID-19.
Funding support: This work was supported by a clinical research trainee grant from Elite program of China Organ Transplant Development Foundation (no. 2019JYJH09) and the National Natural Science Foundation of China (nos. 81874091, 81974396, 81772724, and 81672529).
Conflicts of interest: The authors have nothing to disclose.
Acknowledgments: We thank all the patients and their families involved in the study. Special thanks to Dr. Xiao-Shan Wei for her dedication to data entry and verification.
Associate Editor: James Catto
References
- 1.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. In press. https://doi.org/10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed]
- 3.Chiu M.C. Suggested management of immunocompromised kidney patients suffering from SARS. Pediatr Nephrol. 2003;18:1204–1205. doi: 10.1007/s00467-003-1325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AlGhamdi M., Mushtaq F., Awn N., Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15:1101–1104. doi: 10.1111/ajt.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mailles A., Blanckaert K., Chaud P. First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18:20502. [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez Faba O., Boissier R., Budde K. European Association of Urology guidelines on renal transplantation: update 2018. Eur Urol Focus. 2018;4:208–215. doi: 10.1016/j.euf.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Gu J., Gong E., Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa M., Terashima T., D’Yachkova Y., Bondy G.P., Hogg J.C., van Eeden S.F. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–2313. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. In press. https://doi.org/10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 11.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbajo-Lozoya J., Muller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–6. doi: 10.1016/S0140-6736(20)30305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. World Health Organization Web site. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 15.Cheng Y., Luo R., Wang K. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv. 2020 doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]