Abstract
Purpose
The purpose of the study was to evaluate the possible protective effects of low dose sodium nitrate preconditioning on the peripheral neuropathy in streptozotocin (STZ)-induced diabetic model.
Methods
Male Wistar rats were randomly divided into five groups: control (no intervention), control treated sodium nitrate (100 mg/L in drinking water), diabetic (no intervention), diabetic treated NPH insulin (2-4 U), and diabetic treated sodium nitrate (100 mg/L in drinking water). Diabetes was induced by intraperitoneal injection of STZ (60 mg/kg). All interventions were done for 60 days immediately following diabetes confirmation. Thermal and mechanical algesia thresholds were measured by means of hot-plate test, von Frey test, and tail-withdrawal test before the diabetic induction and after diabetes confirmation. At the end of the experiment, serum NOx level and serum insulin level were assessed. Blood glucose concentration and body weight have recorded at the base and duration of the experiment.
Results
Both hypoalgesia, hyperalgesia along with allodynia developed in diabetic rats. Significant alterations including, decrease in tail withdrawal latency (30th day), decreased mechanical threshold (60th day), and an increase in hot plate latency (61st day) were displayed in diabetic rats compared to control rats. Nitrate and insulin preconditioning produced protective effects against diabetes-induced peripheral neuropathy. Data analysis also showed a significant increase in glucose level as well as a considerable reduction in serum insulin and body weight of diabetic rats, which restored by both insulin and nitrate preconditioning.
Conclusion
Sodium nitrate preconditioning produces a protective effect in diabetic neuropathy, which may be mediated by its antihyperglycemic effects and increased serum insulin level.
Keywords: Nitrate preconditioning, Diabetes, Peripheral nerve, Thermal algesia, Mechanical algesia
Introduction
Diabetes mellitus is considered as a chronic progressive metabolic problem with increasing global prevalence, and predict that approximately 640 million will be influenced by 2040 [1]. Diabetic neuropathy is the frequently silent and complex diabetes-associated microvascular complication, described by the progressive loss of autonomic and peripheral nerve fibers [2]. Diabetes-induced peripheral neuropathy is the most prevalent form of diabetic neuropathy observed in 7–50% of diabetic patients, within 1 year to more than 25 years of diagnosis, respectively [3]. Patients with diabetic peripheral neuropathy (DPN) experience functional and structural alterations in peripheral nerves, characterized by lowered nerve conductive velocity, axonal degeneration, and paranodal demyelination [4]. These alterations are a major cause of sensory loss, reduced touch and vibration sensation, limb numbness, pain, tingling, prickling sensations, numbness, and muscular imbalance, fall-related injury, and foot ulceration [5]. The destruction of peripheral nerve fibers in the epidermal layer of skin is reported to be clinical diagnostic criteria for DPN detection [6].
It has been shown that vascular factors have an important role in the incidence and development of DPN [7]. In diabetic models, the thickened basement membrane of endoneurial microvessel is suggesting a close relationship between DPN and microvessel integrity [8]. Microcirculatory disturbances reported in the early stage of diabetes are associated with hyperglycemia [9]. Prolonged hyperglycemia play a vital role in the development of diabetic neuropathy by activation of the hexosamine and polyol pathways, production of advanced glycation end products (AGE), activation of PKC isoforms, and increasing the intracellular ratio of NADH/NAD+ [10–14]. Clinical studies from the past decades indicate that strict control of glucose level reduces the occurrence of DPN, suggesting a key role for hyperglycemia [15]. Therefore, diabetic-induced neuropathy is thought to occur as a result of two important mechanisms including hyperglycemia-induced injury to nerve cells and neuronal ischemia arising from reduction of neurovascular flow owing to hyperglycemia [16].
There is currently no symptomatic and effective treatment for DPN, it is thereby vital to develop therapeutic ways for this condition. It has been shown that strict glycemic manage and therapies targeting neurovascular function, slow the progression and development of DPN in diabetes [17, 18]. It is recognized that nitric oxide (NO) plays a significant role in the mediation of close communication between neurons and vessels, biological processes of the blood vessels and neurotransmission, and neurovascular coupling [19, 20]. Moreover, nNOS derived NO emerges essentially for neurovascular coupling [21]. In addition to the L-arginine pathway, NO is synthesized from nitrate and nitrite [22]. Previous findings provide evidence showing that both nitrite and nitrate have beneficial effects on various organs in healthy and diabetic cases including the cardiovascular and reproductive system [23, 24]. However, the preconditioning effects of sodium nitrate on diabetes-induced peripheral neuropathy have not been studied yet.
If DPN recognized and treated early, the severe fiber damage and even amputation may be avoided. It is of crucial importance to detect DPN early to prevent its progression and development. Manifestations of diabetic neuropathy such as hyperalgesia (exaggerated pain resulting from a painful stimulus) and allodynia (pain resulting from a non-painful stimulus) have been documented [25]. Therefore, the present study was designed to evaluate the preconditioning effects of sodium nitrate against streptozotocin (STZ)-induced DPN in male Wistar rats.
Materials and methods
Animals
The experiments were carried out according to regulations specified by the National Institutes of Health “Principles of Laboratory Animal Care and protocols of Tabriz University of Medical Sciences for animal studies. Forty male Wistar rats, 85–115 g body weight, were fed standard rat chow and had free access to water. Animals were randomly allocated into 5 groups: C; control group with no intervention, CN; controls treated with 100 mg/L sodium nitrate, D; diabetics with no intervention, DI; diabetics treated with 2-4 U of NPH-insulin and DN; diabetics treated with 100 mg/L sodium nitrate. Animals in nitrate groups were housed individually while animals in other groups were kept two or three rats in standard cages.
Diabetes induction and experimental protocols
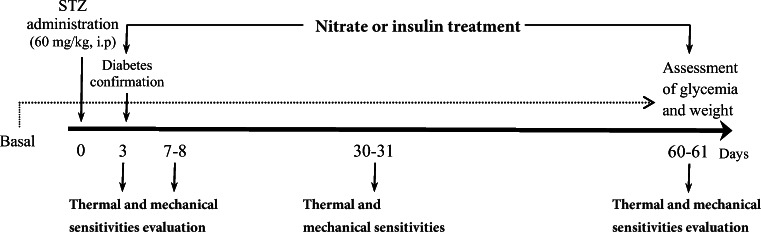
Diabetes was induced by 60 mg/kg STZ (Sigma-Aldrich, Germany) as described [26]. Blood samples for glucose measurements were taken from the tail vein 72 h following the STZ injection. The rats with blood glucose more than 250 mg/dl were considered diabetic [27]. All treatments were started immediately after confirmation of diabetes for 60 days. NPH insulin (Pharmaceutical Mfg. Co., Iran) was administered subcutaneously at fixed times, 2-4 U daily adjusted according to the blood glucose level [28]. The dose of 100 mg/L sodium nitrate (Merck KGaA, 64,271 Darmstadt, Germany) administration was started immediately after the confirmation of diabetes in both CN and DN groups [29]. Blood glucose and body weight were assessed at the beginning of the study and during the experiment. After 60 days, blood samples were collected to assess serum insulin and NOx levels. Design of the experimental protocols of the study has depicted in Fig. 1.
Fig. 1.
Experimental design
Serum insulin and NOx assessment
Serum insulin concentration was evaluated using ELISA kits (Shanghaicrystal Day Biotech Co., LTD, China) according to the manufacturer’s instruction. Nitric oxide metabolites (NOx) level was measured using the Griess method in accordance with a protocol explained by previous studies [29].
Behavioral tests
The behavioral tests have been done in the following order: mechanical algesia was assessed using flexible von Frey filaments and thermal algesia was evaluated by both tail withdrawal test and hot plate test. Von Frey and tail withdrawal tests were done at 0th, 7th, 30th, and 60th days of the experiment. The hot plate test was performed 1 day later, at 0th, 8th, 31st, and 61st days. All three behavioral tests were performed in a quiet room between 8:00 a.m. and 2:00 p.m. The von Frey and tail immersion tests were performed with an interval of 1–2 h [30]. All behavioral tests were performed in a blinded fashion.
Mechanical allodynia
Mechanical allodynia was assessed the mechanical touch sensitivity with flexible von Frey filaments (Touch Test™ Sensory Evaluator Kit, USA) ranged from 0.04 to 300 g on the mid-plantar part of both hind paws. Von Frey monofilaments are nylon or metal hairs of different diameter that exert exact levels of force against the skin. Their use allows assessment of mechanical stimulation. The test was done by placing an animal on an elevated wire mesh platform 30 min before the examination for habituation. The Von Frey hair was inserted perpendicular to the plantar surface of both right and left hind paw. The test was started with the thinnest hair filament; the pressure increased slowly for 2 s. The typical withdrawal responses were considered as a shaking of the hind paw or quickly flicking the paw away from the stimulation. Lifting the hind paw and licking it were also reported as a positive response. When a withdrawal response was observed to the stimulus, a lighter filament was tested, and a heavier filament was applied in the absence of a response. At least two positive withdrawal responses out of three trials were required to describe the positive response [31].
Thermal algesia
Both hot-plate (supraspinal pain) and tail withdrawal (spinal pain) tests were used to evaluate thermal hyperalgesia. The hot plat machinery was set at a temperature of 53 ± 0.5°C. Each animal was placed individually on the heated surface, and then the time interval between placement and the licking of any hind paws was recorded. The cut-off time, selected to avoid any probable tissue damage, was 40 s [32].
Rats were gently restrained by placed in a restrainer, and then the distal 5 cm of their tail was dipped into the hot water bath maintained at 48 ± 0.5°C. The time or latency (second), between exposure to the hot water and the tail sudden withdrawal, was recorded. To minimize the possibility of tissue damage from heat exposure, a cut-off time of 40 s was established, at which time the animal was removed from the test [33].
Blood sampling
At the end of the study, all rats were anesthetized under i.p. injection of 80 mg/kg ketamine and 10 mg/kg xylazine. Blood samples were taken from the inferior vena cava and then centrifuged at 3500 g for 10 min. Then sera were separated and stored at −20 °C until the analysis.
Statistical analysis
Statistical analysis was done using SPSS version 21 statistic software package. Data were represented as mean ± SEM. All data are expressed as mean ± SEM. A One-way ANOVA analysis followed by Tukey’s post-hoc tests, were used to determine the significance of differences in behavioral tests measurements. Comparisons between the different times in all groups were performed by Bonferroni post hoc-test in behavioral tests. For all other results, One-way ANOVA analysis followed by Tukey’s post-hoc test was done. A p value less than 0.05 was considered as statistically significant.
Results
Effect of sodium nitrate preconditioning on blood glucose level and body weight of diabetic rats
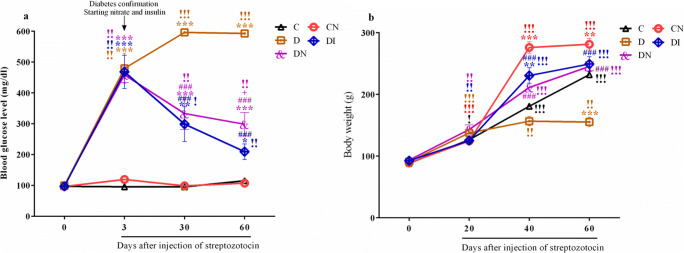
As shown in Fig. 2, there were no significant differences in either blood glucose or body weight among the groups before treatment. Three days after diabetic induction blood glucose significantly increased in diabetic rats (D, DI and DN groups) compared to the control group (Fig. 2a, p < 0.001). Surprisingly, both the sodium nitrate and insulin administration significantly prevented the increase of blood glucose compared to diabetic rats (p < 0.001). In addition, blood glucose levels were elevated in the diabetic group over time (p < 0.001). Nitrate and insulin preconditioning in diabetic groups immediately after diabetes confirmation led to a gradual decrease in blood glucose level which persisted until the end of the study (p < 0.01). Blood glucose in DN group on 60th day after STZ was higher than that of DI group (p < 0.05).
Fig. 2.
Sodium nitrate preconditioning rescued the increase of blood glucose and decrease of body weight in diabetic rats. a Changes of blood glucose among groups at 0th, 3th, 30th, and 60th days. b Changes of body weight among groups at 0th, 20th, 40th, and 60th days. Data represent mean ± SEM of 8 rats per group. One-way ANOVA followed by Tukey post hoc test was used for multiple comparisons. *p < 0.05, **p < 0.01 ***p < 0.001 versus control group; ###p < 0.001 versus D group; +p < 0.05 between the diabetic received insulin group and the diabetic received nitrate group. Comparisons between the different times in each group were performed by Repeated measures one-way ANOVA and Bonferroni post-test. !p < 0.05, !!p < 0.01, and !!!p < 0.001
The effect of nitrate preconditioning on body weight was evaluated in male rats. Sixty days after STZ, body weight was significantly lowered in diabetic group compared to control rats (p < 0.001). In diabetic animals treated with nitrate or insulin, we observed a statistically significant normalization of body weight (Fig. 2b, p < 0.001). Moreover, nitrate preconditioning in CN group caused significant increase in body weight compared to control group (p < 0.001 to p < 0.01). Weight gain in the control and diabetic received nitrate group, as well as diabetic received insulin group was continued over time (p < 0.001).
Effect of sodium nitrate preconditioning on serum insulin and NOx level of diabetic rats
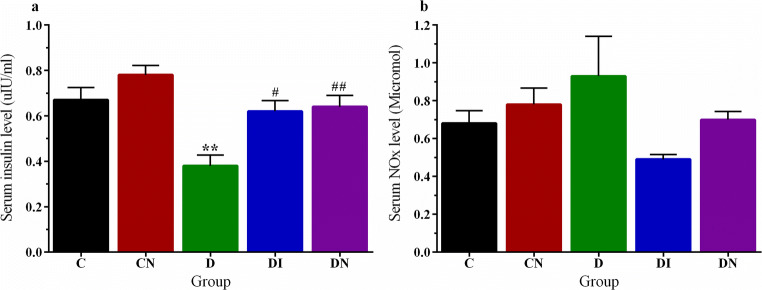
In order to elucidate how nitrate preconditioning was therapeutically effective at blood glucose, we measured the serum level of insulin at 60 days after STZ. As shown in Fig. 3 panel a, the serum level of insulin of diabetic rats was reduced compared to controls (p < 0.01). Both insulin and nitrate preconditioning were able to prevent insulin level reduction compared to D group (p < 0.05 and p < 0.01, respectively). There was no significant difference in the levels of serum NOx between the groups (Fig. 3b).
Fig. 3.
Sodium nitrate preconditioning increased the serum insulin level in diabetic rats. a Changes of serum insulin level among groups. b Changes of serum NOx level among groups. Data represent mean ± SEM of 8 rats per group. One-way ANOVA followed by Tukey test was used for multiple comparisons. **p < 0.01versus control group; #p < 0.05, ##p < 0.01 versus D group
Effect of sodium nitrate preconditioning on mechanical sensitivity of diabetic rats
Since the sensory alterations in diabetic neuropathy are often different, we decided to test the action of nitrate preconditioning on mechanical hyperalgesia immediately after diabetes confirmation. The mechanical allodynia was evidence in STZ-diabetic animals compared to the control group at 60th day compared to controls (p < 0.001). The mechanical threshold in diabetic animals was decreased and maintained significantly lower up to the end of the study (p < 0.01). Nitrate preconditioning was able to provide a fast and persisted antiallodynic effect compared to D group (Fig. 4, p < 0.001). Insulin therapy also counteracted mechanical allodynia in DI group compared to diabetic group (p < 0.001). There was no significant difference in the levels of the mechanical allodynia between diabetic received insulin and diabetic received nitrate groups.
Fig. 4.
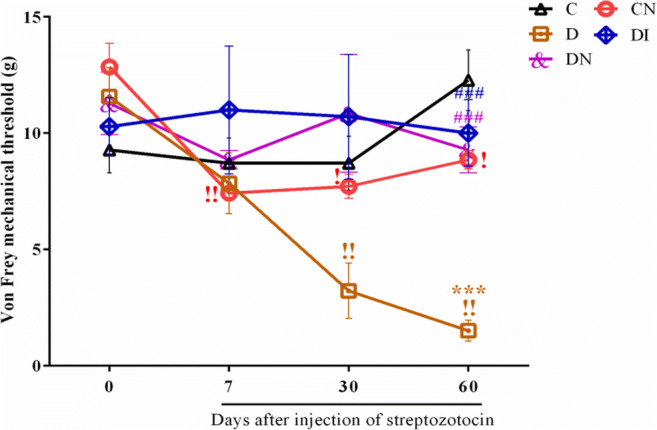
Sodium nitrate preconditioning reduces allodynia in diabetic rats. Changes of Von Frey mechanical threshold at 0th, 7th, 30th, and 60th days. Data represent mean ± SEM of 8 rats per group. One-way ANOVA followed by Tukey test was used for multiple comparisons. ***p < 0.001 versus control group; ###p < 0.001 versus D group. Comparisons between the different times in each group were performed by Repeated measures one-way ANOVA and Bonferroni post-test. !p < 0.05 and !!p < 0.01
Effect of sodium nitrate preconditioning on thermal sensitivity of diabetic rats
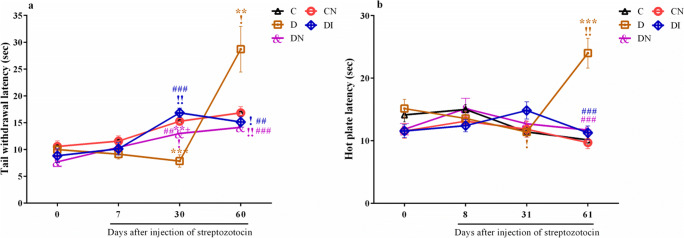
Both tail immersion and hot plate tests were performed for spinal and supraspinal pain, respectively. As reported in Fig. 5, panel a, the tail withdrawal thresholds (TWT) of D group at 30 days after STZ were reduced compared to controls (p < 0.001). After that, we observed a significant and fast increase in tail withdrawal latency in the 60th day compared to 30th day (p < 0.05). Furthermore, insulin treatment was able to significantly correct tail withdrawal thresholds compared to diabetic animals (p < 0.001 at 30th day and p < 0.01 at 60th day).
Fig. 5.
Sodium nitrate preconditioning reduces thermal algesia alterations in diabetic rats. a Changes of tail withdrawal latency among groups at 0th, 7th, 30th, and 60th days. b Changes of hot plate latency among groups at 0th, 8th, 31th, and 61th days. Data represent mean ± SEM of 8 rats per group. One-way ANOVA followed by Tukey test was used for multiple comparisons. **p < 0.01, ***p < 0.001 versus control group; ##p < 0.01, ###p < 0.001 versus D group; +p < 0.05 versus diabetic received insulin group. Comparisons between the different times in each group were performed by Repeated measures one-way ANOVA and Bonferroni post-test. !p < 0.05 and !!p < 0.01
As seen in Fig. 5, panel b, a significant reduction of thermal sensitivity was evident at 31th day compared to 0th in STZ-diabetic group (p < 0.05). At day 61 after STZ, a significant increase in hot plate latency was detectable in D group compared to controls (p < 0.001). Both treatments at that time were able to significantly reduce thermal sensitivity compared to diabetic rats (p < 0.001).
Discussion
This is the first research to investigate the effects of dietary sodium nitrate preconditioning on the diabetic-induced peripheral neuropathy of Wistar rats with type 1 diabetes. The principal findings of this study were that dietary nitrate preconditioning reduced the blood glucose level, increased the body weight and serum insulin level and recovered the thermal and mechanical algesia. We also found that nitrate preconditioning had no effect on the serum NOx level. The pathophysiological factors leading to peripheral neuropathy in diabetes are included chronic hyperglycemia, insulin deficiency, and increased oxidative stress [16, 34, 35]. Studies have shown a close relationship between neurovascular flow and diminished NO bioavailability [36, 37]. The possible explanations for the beneficial effects in the present study related to elevated plasma insulin concentration and subsequent reduced serum blood glucose.
Our study shows that in the STZ model of type 1 diabetes, serum insulin levels were significantly lower than in controls; sodium nitrate preconditioning increases serum insulin level in diabetic rats. Findings from in vitro and in vivo investigations have supported the hypothesis that the nitrate and nitrite signaling pathway plays a significant role in insulin secretion, insulin signaling, and glucose homeostasis [38, 39]. Intraperitoneal administration of sodium nitrite, increased pancreatic islet blood flow and also enhanced plasma insulin concentration in rats [39]. However, oral supplementation with nitrate or infusion of sodium nitroprusside in healthy men had no effect on plasma insulin or glucose levels [40]. Nitrate and nitrite elevated insulin secretion via various mechanisms including increased pancreatic islet blood flow and activation of guanylyl cyclase and the cGMP pathway [39, 41]. However, in contrast with our results, Gheibi and colleagues have shown that administration of sodium nitrite to rats with type 2 diabetes, induced by the combination of a low-dose STZ and high-fat diet, reduced insulin secretion, a discrepancy may be associated with the type of diabetes [42].
In this study, sodium nitrate preconditioning for 60 days decreased blood glucose levels in diabetic rats, findings that also reported by previous studies. In this context, it has also been shown that nitrite decreased serum glucose concentration in diabetic rats by 27.6% in type 2 diabetic rats [43]. Moreover, infusion of sodium nitroprusside in type 2 diabetic patients resulted in higher glucose uptake independent of plasma insulin level [44]. However, there is some evidence that reported these anions to have no effects on glucose level [45]. A discrepancy may be associated with the duration of nitrate/nitrite administration, study design, and differences in the animal and clinical methods. It has been suggested that nitrate and nitrite decreased glucose level, possibly owing to increased glucose uptake in skeletal muscle, glucose transporter type 4 (GLUT4) translocation to the membrane, insulin secretion, and improving insulin signaling [39, 46]. Insulin-independent stimulatory effect of nitrate and nitrite on GLUT4 translocation suggested that nitrite could improve insulin signaling via restoration of NO-dependent nitrosation of GLUT4 signaling [39].
In our study, STZ-induced diabetic rats had decreased body weight. Nitrate preconditioning increased body weight in both control and diabetic rats. Although a number of animal investigations reported increased body weight following nitrate administration in type 1 diabetic models [28, 29], other researchers observed reduced [38] or no significant changes [47] in body weight at various doses of nitrate and nitrite. There are several probable explanations for these discrepancies, including the type of diabetes, duration, and a dose of nitrate/nitrite administration; a dose-dependent decrease in body weight has been shown following 5 months of nitrate treatment with various doses of 50, 150, and 500 mg/L in rats [48]. Mechanisms underlying weight-gaining effects of nitrite are not exactly understood and may be at least in part owing to increased food intake [39]. Moreover, in line with our findings, Seethalakshmi and coworkers reported that insulin supplementation recovered body weight [49].
Decreased [24], increased [50], and no change [51] in serum NOx concentrations of diabetes have been documented by previous reports. In our study, neither diabetes nor any of the treatments led to significant alterations in serum NOx concentrations. Although these findings cannot describe from the results of this study, it may be associated with the duration of diabetes. Dissimilar to our findings, increased NOx levels have been reported following both nitrate and nitrite administration in aorta, heart, lung, brain, liver, kidney, and skeletal muscles [52, 53]. A discrepancy may be related to the duration of their administration.
Although the detection of “pain” is challenging because of its mental nature in experimental models [53], there are a number of behavioral tests such as tail withdrawal, von Frey, and hot-plate tests that allow for the reproducible, rapid, and sensitive determination of the mechanical and thermal nociceptive thresholds in rats [54]. Present study displayed that STZ-induced diabetic rats developed considerable thermal and mechanical hyperalgesia and then thermal hypoalgesia. It has been documented that mechanical and thermal hyperalgesia is mediated by A-fibers and C-fibers, respectively [55, 56]. Moreover, downregulation of GABAB receptors on nociceptive fibers in the spinal cord may contribute to the development of allodynia in diabetic rats [57].
The reduced threshold of harmful tactile stimuli was detected in the von Frey test for diabetic rats in hind paws after 60 days of STZ injection. Hind paw withdrawal threshold was also progressively decreased during this study in diabetic rats, indicating this group of rats experienced allodynia throughout the experiment period. In line with our findings, tactile allodynia and spontaneous pain have been reported in a number of patients with diabetes mellitus [58]. Moreover, Hong Gong et al. have documented that STZ-induced diabetic rats developed considerable mechanical hyperalgesia 4 days after STZ injection [59]. A dose of 100 mg/l/day inorganic nitrate or 2–4 U/day NPH insulin preconditioning for 60 days considerably modified the diabetes-induced mechanical allodynia. A number of beneficial effects of nitrate/nitrite have been demonstrated, including improved endothelial dysfunction and pancreatic islet function, reversal of features of metabolic syndrome, increased islet blood flow, and insulin secretion, as well as lowering blood glucose [41]. It is clear that chronic hyperglycemia leads to peripheral nerve damage through activation of several key metabolic pathways including polyol, hexosamine, and AGE/RAGE pathways [60]. Therefore, we suggest that these beneficial effects of sodium nitrate preconditioning in diabetic rats appear to be more related to the increase in serum insulin level and a decrease in blood glucose concentrations. In line with our findings Calcutt and coworkers have shown that diabetes-induced allodynia can be prevented and recovered by insulin replacement in STZ-diabetic rats [61]. Furthermore, a recent review has demonstrated that excess insulin or insulin deficiency has a key role in neuropathic changes in sensory neurons [35].
It is possible that various types of peripheral nerve fibers are affected in rat models of the diabetic neuropathy since paw withdrawal reactions to thermal signals are associated with supraspinal sensory processing [62]. In our study, diabetic rats revealed a significant decrease in hot plate latency at 31st day compared to the basal value as well as a marked increase at 61st day compared to the control group. Results from the tail withdrawal test showed that diabetes causes a significant reduction in tail withdrawal latency (hyperalgesia) at 30th day compared with control group, however, a thermal hypoalgesia was observed on the 60th day in the tail withdrawal latency test. In line with our findings, the alteration pattern of thermal withdrawal threshold in diabetic rats remained inconsistent in previous studies, and both decrease and increase in thermal withdrawal threshold have been observed [59, 63]. Prnova et al. have reported that type 2 diabetes male rats (Zucker Diabetic Fatty) expanded symptoms of thermal hypoalgesia as showed by increased tail-flick latencies [63]. Moreover, Hong Gong et al. demonstrated thermal hyperalgesia occurred following STZ-induced diabetes [59]. Hyperglycemia-induced overproduction of oxidative stress, as well as pro-inflammatory agents, may contribute to the alterations in behavioral responses in peripheral diabetic neuropathy [64, 65]. Our results show that both insulin and nitrate preconditioning prevented thermal hyperalgesia and hypoalgesia in diabetic rats, probably mediated by the modulation of serum insulin and blood glucose level. Insulin may directly supply strong support of neurons via its effects on brains insulin receptors or may indirectly by its beneficial effects on glycemia [66]. The results are in agreement with our previous findings that showed nitrate supplementation after 1 month of STZ-induced diabetes confirmation improved peripheral neuropathy [30].
Conclusion
In conclusion, this study revealed that long-term nitrate preconditioning in streptozotocin-induced diabetic rats had beneficial and preventive effects against diabetes-induced peripheral nerve damage. Improved blood glucose and serum insulin level may contribute to the favorable effects of sodium nitrate on diabetes-induced peripheral neuropathy.
Acknowledgements
This article is a part of database from the investigation entitled “The effect of inorganic nitrate on the prevention and treatment of peripheral diabetic neuropathy in typ-1 diabetic male rats” written by Hajar Oghbaei, Faculty of Medicine, Tabriz University of Medical Sciences and was funded by the student research committee of Tabriz University of Medical Sciences (No. IR.TBZMED. REC.1395.960), Tabriz, Iran.
Compliance with ethical standards
Ethical issues
All of protocols were approved by the Ethics Committee of Animal Research of Tabriz University of Medical Sciences (No. IR.TBZMED.REC.1395.960).
Conflict of interests
Authors declare no conflict of interests.
Footnotes
Highlights
• Diabetes decreased mechanical threshold (mechanical allodynia)
• Diabetes caused the first reduction and then a secondary increase in tail withdrawal and hot plate latency
• Sodium nitrate preconditioning decreased the blood glucose and increased the serum insulin level in diabetic rats.
• Sodium nitrate preconditioning reduced allodynia and thermal algesia in diabetic rats
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hajar Oghbaei, Email: hoghbaei1988@gmail.com.
Gisou Mohaddes, Email: gmohades@yahoo.com.
GholamReza Hamidian, Email: ghamidian@yahoo.com.
Rana Keyhanmanesh, Email: r_keyhanmanesh@yahoo.com.
References
- 1.Organization WH. Global report on diabetes. World Health Organization 2016. 2017.
- 2.Urbancic-Rovan V. Causes of diabetic foot lesions. Lancet. 2005;366(9498):1675–1676. doi: 10.1016/S0140-6736(05)67673-8. [DOI] [PubMed] [Google Scholar]
- 3.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1(3):168–188. [Google Scholar]
- 4.Cermenati G, Abbiati F, Cermenati S, Brioschi E, Volonterio A, Cavaletti G, Saez E, de Fabiani E, Crestani M, Garcia-Segura LM, Melcangi RC, Caruso D, Mitro N. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res. 2012;53(2):300–310. doi: 10.1194/jlr.M021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinik AI. Diabetic neuropathies. Controversies in treating diabetes. Springer 2008. p. 135–156.
- 6.Rojas DR, Kuner R, Agarwal N. Metabolomic signature of type 1 diabetes-induced sensory loss and nerve damage in diabetic neuropathy. arXiv preprint arXiv:180306740 2018. [DOI] [PubMed]
- 7.Han JW, Sin MY, Y-s Y. Cell therapy for diabetic neuropathy using adult stem or progenitor cells. Diabetes Metab J. 2013;37(2):91–105. doi: 10.4093/dmj.2013.37.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowicki M, Kosacka J, Serke H, Blüher M, Spanel-Borowski K. Altered sciatic nerve fiber morphology and endoneural microvessels in mouse models relevant for obesity, peripheral diabetic polyneuropathy, and the metabolic syndrome. J Neurosci Res. 2012;90(1):122–131. doi: 10.1002/jnr.22728. [DOI] [PubMed] [Google Scholar]
- 9.Fioretto P, Dodson PM, Ziegler D, Rosenson RS. Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol. 2010;6(1):19–25. doi: 10.1038/nrendo.2009.213. [DOI] [PubMed] [Google Scholar]
- 10.Oates PJ. Polyol pathway and diabetic peripheral neuropathy. Int Rev Neurobiol. 2002;50:325–392. doi: 10.1016/s0074-7742(02)50082-9. [DOI] [PubMed] [Google Scholar]
- 11.Du X-L, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97(22):12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichberg J. Protein kinase C changes in diabetes: is the concept relevant to neuropathy? Int Rev Neurobiol. 2002;50:61–82. doi: 10.1016/s0074-7742(02)50073-8. [DOI] [PubMed] [Google Scholar]
- 13.Mišur I, Žarković K, Barada A, Batelja L, Miličević Z, Turk Z. Advanced glycation endproducts in peripheral nerve in type 2 diabetes with neuropathy. Acta Diabetol. 2004;41(4):158–166. doi: 10.1007/s00592-004-0160-0. [DOI] [PubMed] [Google Scholar]
- 14.Miyauchi Y, Shikama H, Takasu T, Okamiya H, Umeda M, Hirasaki E, Ohhata I, Nakayama H, Nakagawa S. Slowing of peripheral motor nerve conduction was ameliorated by aminoguanidine in streptozocin-induced diabetic rats. Eur J Endocrinol. 1996;134(4):467–473. doi: 10.1530/eje.0.1340467. [DOI] [PubMed] [Google Scholar]
- 15.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the epidemiology of Diabetes Interventions, and Complications (EDIC) study. Diabetes Care. 2010. [DOI] [PMC free article] [PubMed]
- 16.Jin HY, Lee KA, Song SK, Liu WJ, Choi JH, Song CH, Baek HS, Park TS. Sulodexide prevents peripheral nerve damage in streptozotocin induced diabetic rats. Eur J Pharmacol. 2012;674(2–3):217–226. doi: 10.1016/j.ejphar.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chopp M, Szalad A, Lu X, Jia L, Lu M, Zhang RL, Zhang ZG. Tadalafil promotes the recovery of peripheral neuropathy in type II diabetic mice. PLoS One. 2016;11(7):e0159665. doi: 10.1371/journal.pone.0159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Chopp M, Szalad A, Jia L, Lu X, Lu M, Zhang L, Zhang Y, Zhang R, Zhang ZG. Sildenafil ameliorates long term peripheral neuropathy in type II diabetic mice. PLoS One. 2015;10(2):e0118134. doi: 10.1371/journal.pone.0118134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gally JA, Montague PR, Reeke GN, Edelman GM. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci. 1990;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncada S, Higgs E. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(S1):S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garry P, Ezra M, Rowland M, Westbrook J, Pattinson K. The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396(1):39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- 23.Muenzel T, Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vasc Pharmacol. 2018;102:1–10. doi: 10.1016/j.vph.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Keyhanmanesh R, Hamidian G, Alipour MR, Oghbaei H. Benefiial treatment effects of dietary nitrate supplementation on testicular injury in streptozotocin-induced diabetic male rats. Reprod BioMed Online. 2019. [DOI] [PubMed]
- 25.Lee-Kubli CA, Mixcoatl-Zecuatl T, Jolivalt CG, Calcutt NA. Animal models of diabetes-induced neuropathic pain. Behavioral Neurobiology of Chronic Pain. Springer 2014. p. 147-. [DOI] [PubMed]
- 26.Oghbaei H, Asl NA, Sheikhzadeh F, Alipour MR. The effect of regular moderate exercise on miRNA-192 expression changes in kidney of streptozotocin-induced diabetic male rats. Adv Pharm Bull. 2015;5(1):127–132. doi: 10.5681/apb.2015.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oghbaei H, Asl NA, Sheikhzadeh F. Can regular moderate exercise lead to changes in miRNA-146a and its adapter proteins in the kidney of streptozotocin-induced diabetic male rats? Endocr Regul. 2017;51(3):145–152. doi: 10.1515/enr-2017-0015. [DOI] [PubMed] [Google Scholar]
- 28.Keyhanmanesh R, Hamidian G, Alipour MR, Ranjbar M, Oghbaei H. Protective effects of sodium nitrate against testicular apoptosis and spermatogenesis impairments in streptozotocin-induced diabetic male rats. Life Sci. 2018;211:63–73. doi: 10.1016/j.lfs.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Oghbaei H, Alipour MR, Hamidian G, Ahmadi M, Ghorbanzadeh V, Keyhanmanesh R. Two months sodium nitrate supplementation alleviates testicular injury in streptozotocin-induced diabetic male rats. Exp Physiol. 2018;103(12):1603–1617. doi: 10.1113/EP087198. [DOI] [PubMed] [Google Scholar]
- 30.Oghbaei H, Alipour MR, Mohaddes G, Hamidian GR, Keyhanmanesh R. Evaluation of ameliorative effect of sodium nitrate in experimental model of streptozotocin-induced diabetic neuropathy in male rats. Endocr Regul. 2019;53(1):14–25. doi: 10.2478/enr-2019-0003. [DOI] [PubMed] [Google Scholar]
- 31.Dobson C, Tohyama Y, Diksic M, Hamel E. Effects of acute or chronic administration of anti-migraine drugs sumatriptan and zolmitriptan on serotonin synthesis in the rat brain. Cephalalgia. 2004;24(1):2–11. doi: 10.1111/j.1468-2982.2004.00647.x. [DOI] [PubMed] [Google Scholar]
- 32.Kubo K, Nishikawa K, Ishizeki J, Hardy-Yamada M, Yanagawa Y, Saito S. Thermal hyperalgesia via supraspinal mechanisms in mice lacking glutamate decarboxylase 65. J Pharmacol Exp Ther. 2009;331(1):162–169. doi: 10.1124/jpet.109.156034. [DOI] [PubMed] [Google Scholar]
- 33.Zeng P, Li S, Zheng Y-h, Liu F-Y, Wang J-l, Zhang D-l, et al. Ghrelin receptor agonist, GHRP-2, produces antinociceptive effects at the supraspinal level via the opioid receptor in mice. Peptides. 2014;55:103–109. doi: 10.1016/j.peptides.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015. [DOI] [PMC free article] [PubMed]
- 35.Grote CW, Wright DE. A role for insulin in diabetic neuropathy. Front Neurosci. 2016;10:581. doi: 10.3389/fnins.2016.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cellek S. Point of NO return for nitrergic nerves in diabetes: a new insight into diabetic complications. Curr Pharm Des. 2004;10(29):3683–3695. doi: 10.2174/1381612043382792. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki T, Yasuda H, Maeda K, Kikkawa R. Hyperalgesia and decreased neuronal nitric oxide synthase in diabetic rats. Neuroreport. 1998;9(2):177. doi: 10.1097/00001756-199801260-00013. [DOI] [PubMed] [Google Scholar]
- 38.Gheibi S, Jeddi S, Carlström M, Gholami H, Ghasemi A. Effects of long-term nitrate supplementation on carbohydrate metabolism, lipid profiles, oxidative stress, and inflammation in male obese type 2 diabetic rats. Nitric Oxide. 2018;75:27–41. doi: 10.1016/j.niox.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Nyström T, Ortsäter H, Huang Z, Zhang F, Larsen FJ, Weitzberg E, Lundberg JO, Sjöholm Å. Inorganic nitrite stimulates pancreatic islet blood flow and insulin secretion. Free Radic Biol Med. 2012;53(5):1017–1023. doi: 10.1016/j.freeradbiomed.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Henstridge DC, Duffy SJ, Formosa MF, Ahimastos AA, Thompson BR, Kingwell BA. Oral nitrate therapy does not affect glucose metabolism in healthy men. Clin Exp Pharmacol Physiol. 2009;36(11):1086–1092. doi: 10.1111/j.1440-1681.2009.05195.x. [DOI] [PubMed] [Google Scholar]
- 41.Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F. Beneficial effects of inorganic nitrate/nitrite in type 2 diabetes and its complications. Nutr Metab. 2015;12(1):16. doi: 10.1186/s12986-015-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gheibi SBF, Jeddi S, Farrokhfall K, Zardooz H, Ghasemi A. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide. 2017;1(64):39–51. doi: 10.1016/j.niox.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Varzandi T, Abdollahifar MA, Rohani SAH, Piryaei A, Zadeh-Vakili A, Jeddi S, et al. Effect of long-term nitrite administration on browning of white adipose tissue in type 2 diabetic rats: a stereological study. Life Sci. 2018;207:219–226. doi: 10.1016/j.lfs.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Henstridge D, Kingwell BA, Formosa MF, Drew B, McConell GK, Duffy S. Effects of the nitric oxide donor, sodium nitroprusside, on resting leg glucose uptake in patients with type 2 diabetes. Diabetologia. 2005;48(12):2602–2608. doi: 10.1007/s00125-005-0018-1. [DOI] [PubMed] [Google Scholar]
- 45.Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med. 2013;60:89–97. doi: 10.1016/j.freeradbiomed.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi J. Nitric oxide and insulin resistance. Immunoendocrinology. 2015;2.
- 47.Ogur R, Coskun O, Korkmaz A, Oter S, Yaren H, Hasde M. High nitrate intake impairs liver functions and morphology in rats; protective effects of α-tocopherol. Environ Toxicol Pharmacol. 2005;20(1):161–166. doi: 10.1016/j.etap.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 48.Chaoui A, Zaki A, Talibi A, Chait A, Derouiche A, Aboussaouira T, Benabdjlil F, Himmi T. Effects of inorganic nitrates on thyroid gland activity and morphology in female rats. Therapie. 2004;59(4):471–475. doi: 10.2515/therapie:2004079. [DOI] [PubMed] [Google Scholar]
- 49.Seethalakshmi L, Menon M, Diamond D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J Urol. 1987;138(1):190–194. doi: 10.1016/s0022-5347(17)43042-4. [DOI] [PubMed] [Google Scholar]
- 50.Assmann TS, Brondani LA, Boucas AP, Rheinheimer J, de Souza BM, Canani LH, et al. Nitric oxide levels in patients with diabetes mellitus: a systematic review and meta-analysis. Nitric Oxide. 2016;61:1–9. doi: 10.1016/j.niox.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Schmetterer L, Findl O, Fasching P, Ferber W, Strenn K, Breiteneder H, Adam H, Eichler HG, Wolzt M. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes. 1997;46(4):653–658. doi: 10.2337/diab.46.4.653. [DOI] [PubMed] [Google Scholar]
- 52.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1(5):290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 53.Ashmore T, Roberts LD, Morash AJ, Kotwica AO, Finnerty J, West JA, et al. Nitrate enhances skeletal muscle fatty acid oxidation via a nitric oxide-cGMP-PPAR-mediated mechanism. BMC Biol. 2015;13(1):110. doi: 10.1186/s12915-015-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 55.Khan G, Chen S-R, Pan H-L. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114(2):291–299. doi: 10.1016/s0306-4522(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 56.Shir Y, Seltzer ZE. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci Lett. 1990;115(1):62–67. doi: 10.1016/0304-3940(90)90518-e. [DOI] [PubMed] [Google Scholar]
- 57.Wang X-L, Zhang Q, Zhang Y-Z, Liu Y-T, Dong R, Wang Q-J, Guo YX. Downregulation of GABAB receptors in the spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett. 2011;490(2):112–115. doi: 10.1016/j.neulet.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 58.Calcutt NA. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol. 2002;50:205–228. doi: 10.1016/s0074-7742(02)50078-7. [DOI] [PubMed] [Google Scholar]
- 59.Gong Y-H, Yu X-R, Liu H-L, Yang N, Zuo P-P, Huang Y-G. Antinociceptive effects of combination of tramadol and acetaminophen on painful diabetic neuropathy in streptozotocin-induced diabetic rats. Acta Anaesthesiol Taiwanica. 2011;49(1):16–20. doi: 10.1016/j.aat.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Yagihashi S, Mizukami H. Diabetic Neuropathy. Diabetes and aging-related complications: Springer; 2018. p. 31–43.
- 61.Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68(2–3):293–299. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- 62.Gilliatt R, Willison R. Peripheral nerve conduction in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1962;25(1):11. doi: 10.1136/jnnp.25.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prnova MS, Svik K, Bezek S, Kovacikova L, Karasu C, Stefek M. 3-Mercapto-5H-1, 2, 4-Triazino [5, 6-b] Indole-5-acetic acid (Cemtirestat) alleviates symptoms of peripheral diabetic neuropathy in Zucker diabetic fatty (ZDF) rats: a role of aldose reductase. Neurochem Res. 2019:1–9. [DOI] [PubMed]
- 64.Chen Y-W, Hsieh P-L, Chen Y-C, Hung C-H, Cheng J-T. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116(2):482–490. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 65.Ismail CAN, Aziz CBA, Suppian R, Long I. Imbalanced oxidative stress and pro-inflammatory markers differentiate the development of diabetic neuropathy variants in streptozotocin-induced diabetic rats. J Diabetes Metab Disord. 2018;1. [DOI] [PMC free article] [PubMed]
- 66.Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53(7):1824–1830. doi: 10.2337/diabetes.53.7.1824. [DOI] [PubMed] [Google Scholar]