Abstract
Introduction
Watermelon is one of the commonly eaten fruit in most homes in Nigeria and has been used in the management of diabetes mellitus traditionally. This study was carried out to explore the antidiabetic potential of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats.
Methods
Watermelon juice was used for the determination of in vitro parameters such as 1,1-diphenyl-2-picryl-hydrazil (DPPH), nitric oxide and ferric reducing antioxidant potential (FRAP) as well as phytochemicals such as total phenol, total flavonoids. In vitro, α-glucosidase and α-amylase inhibitory activities were also accessed using standard procedures. Diabetes was induced in the rats by a single intraperitoneal (I.P) injection of freshly prepared alloxan (150 mg/kg body weight). The animals were randomly grouped into five groups of normal control, untreated diabetic control, diabetic rats administered 200 mg/kg body weight of metformin, diabetic rats administered 500 mg/kg body weight of watermelon (Citrullus lanatus) juice and diabetic rats administered 1000 mg/kg body weight of watermelon juice. The rats were sacrificed on the 14th day of the experiment and various in vivo biochemical parameters were also evaluated in the serum and tissue homogenates of diabetic rats.
Results
The watermelon juice exhibits anti-oxidant properties and inhibitory activities against α-glucosidase and α-amylase in a dose-dependent manner. Added to this, the administration of different doses of the watermelon juice significantly (p < 0.05) reduced the fasting blood glucose level, serum lipid profile, glucose-6-phosphatase, lipid peroxidation and anti-inflammatory activities in alloxan-induced diabetic rats. There was a significant (p < 0.05) increase in antioxidant enzyme activities, hexokinase activity as well as glucose transporters (GLUT 2 and GLUT 4) levels in diabetic rats administered different doses of Citrullus lanatus.
Conclusion
Taken together, this study demonstrates that watermelon (Citrullus lanatus) juice exhibits its antidiabetic potential in experimental diabetic animal model via multiple pathways involving modulation of glucose transporters, anti-inflammatory activities as well as antioxidant defense system and inhibition of α-glucosidase and α-amylase. This suggests that the watermelon (Citrullus lanatus) juice may have a useful clinical application in the management of diabetes mellitus and its metabolic complications if developed as adjuvant therapy.
Keywords: Glucose transporters, Citrullus lanatus, α-glucosidase, α-amylase, Metformin
Introduction
Diabetes mellitus (DM) is recognized as one of the five leading causes of death globally. It is a clinically and genetically heterogeneous metabolic disorder characterized by hyperglycaemia and disturbances of carbohydrate, protein and lipids metabolism due to deficiency in insulin secretion, insulin function or both [1]. It has been estimated that about 1.3% of the world population suffers from this disease [2]. Diabetes has been classified into two main categories, which include type 1 diabetes mellitus (T1DM), characterized by a near-absolute deficiency of insulin secretion and type II diabetes mellitus (T2DM) where the cause is a combination of insulin resistance and an insulin secretory defect. Uncontrolled hyperglycaemia leads to the progressive development of long-term microvascular and macrovascular complications in diabetes which causes morbidity and mortality among those affected [3].
The multiple mechanisms implicated in the pathogenesis and management of T1DM as well T2DM have been documented in the literature [4]. Basically, hyperglycaemia causes tissue damage through five major mechanisms: Increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end-products (AGEs), increased expression of the receptor for advanced glycation end products and its activating ligands, activation of protein kinase C (PKC) isoforms and overactivity of the hexosamine pathway. Several lines of evidence indicate that all five mechanisms are activated by a single upstream event; mitochondrial overproduction of reactive oxygen species [5].
The generation of free radicals notably, reactive oxygen species (ROS) and reactive nitrogen species (RNS) which may lead to oxidative stress are considered critical factors for the pathogenesis of diabetes mellitus. Oxidative stress is the outcome of an imbalance between the production and neutralization of reactive oxygen and nitrogen species such that the antioxidant capacity of the cell is overwhelmed [6, 7].
Diabetes mellitus is a manageable disorder, in which the level of blood glucose is monitored daily. Conventional management of diabetes mellitus includes modification of lifestyles, such as diet and exercise and the use of insulin or oral hypoglycaemic drugs [8]. In recent years there has been an increased inclination towards the herbal formulations and nutraceuticals from medicinal plants, due to the complexity, side effects and costly management associated with the allopathic medicines. This has caused both the health care practitioners and the majority of world populations to turn towards finding alternative therapies since they are believed to be free from side effects and are affordable [2].
Watermelon (Citrullus lanatus) is one of such medicinal plant that has attracted scientific interest due to its bioactivities. C. lanatus sp. is a natural source of antioxidants such as beta carotene, vitamin C, citrulline etc. Watermelon with red flesh is also an excellent source of lycopene. The tissue-protective effects of watermelon juice have been reported. Furthermore, the protective effects of watermelon juice against hepatotoxins such as carbon tetrachloride (CCl4) and paracetamol have been demonstrated [9]. But there is scanty information on the anti-diabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats, which is the aim of this study.
Materials and methods
Chemicals and reagents
All chemicals used were obtained from Sigma chemicals, U.S.A., and were known to be of analytical grade. Chemicals and reagents were procured from Sigma- Aldrich, Inc., (St Louis, MO), dinitrophenyl hydrazine (DNPH) from ACROS Organics (New Jersey, USA), hydrogen peroxide, methanol, acetic acid and FeCl3 was sourced from BDH Chemicals Ltd., (Poole, England), H2SO4, sodium carbonate, AlCl3, potassium acetate, Tris-HCl buffer, FeSO4, potassium ferricyanide and ferric chloride. Total protein and enzyme assay kits were produced by Randox Laboratories Ltd., Antrim, UK. All other chemicals were of analytical grade and prepared in all-glass apparatus using sterilized distilled water (BDH, UK).
Sample collection and preparation
Fresh fruits of the watermelon were bought from Sasha-market along Ado-Ikere road, Ikere-Ekiti, Nigeria. Identification and authentication were carried out by a senior taxonomist at Forestry Research Institute of Nigeria, FRIN, Ibadan, Nigeria, with Herbarium number FHI: 112517. Each watermelon was washed and cut into small pieces. The thick epicarp layer and the seeds were removed, the remaining red-coloured endocarp was put inside a fruit extractor where the water was obtained and subject to freeze-drying.
Experimental animals
Forty (40) matured female Wistar albino rats weighing between 120 g to 130 g were used for this experiment, the animals were bought from Suria Amos farm, Ikere-Ekiti. The rats were randomly divided into 5 groups of 8 animals each. The animals were acclimatized for a period of 2 weeks to the laboratory conditions prior to the experiment at the Animal House of the Department of Biochemistry, Afe Babalola University, Ado-Ekiti. The animals were housed in a cage at room temperature with free access to drinking water and rat feed.
Induction of diabetes
Diabetes was induced in the rats by a single intraperitoneal injection of freshly prepared alloxan of 150 mg/kg body weight in normal saline. Forty-eight hours (48 h.) after alloxan administration, blood samples were obtained from the tips of the rat’s tail and the fasting blood glucose levels were determined using OneTouch Ultra glucometer (Life Scan, Milpitas, CA, USA) to confirm diabetes. Animals with blood glucose levels ranging between 250 and 300 mg/dL were considered diabetic and used for the experiments [10]. This study was approved by Afe Babalola University Ethical Committee with approval number ABUAD/SCI/PG/1012.
Experimental design
The animals were grouped as shown in Table 1. Watermelon juice was administered orally to diabetic rats and the experiment lasted for a period of 14 days.
Table 1.
Experimental grouping of the animals
| Groups | Animals |
|---|---|
| Group 1 | Normal control rats |
| Group 2 | Untreated diabetic rats |
| Group 3 | Diabetic rat + metformin (200 mg/kg) |
| Group 4 | Diabetic rat + watermelon juice (500 mg/kg) |
| Group 5 | Diabetic rat + watermelon juice (1000 mg/kg) |
Preparation of tissue homogenates
Blood samples were collected from overnight fasted rats (only water allowed) after 2 weeks of treatment by cardiac puncture into non-heparinized tubes, for biochemical analyses. For serum samples, blood was allowed to coagulate, followed by centrifugation at 3000 rpm for 15 min at 4 °C to separate serum. Sera were divided into aliquots and stored at −20 °C for biochemical assay. The liver of each rat was rapidly excised, placed in saline solution in a sterile container and placed on ice. The organs were weighed after being dried with a paper towel. The liver tissues were subsequently homogenized in cold Tris- HCl buffer (1:5 w/v). The homogenate was centrifuged for 10 min at 3000 rpm to yield a clear supernatant which was collected using a Pasteur pipette.
In vitro parameter studied
DPPH (1, 1, Diphenyl 2- Picryl Hydrazyl) determination
This assay was carried out according to the method of Re et al. [11] and the absorbance was read at 517 nm.
Determination of ferric (Fe3+) reducing antioxidant power assay (FRAP)
The FRAP level of the extract was measured by the direct reduction of [Fe(CN)6]3− to [Fe(CN)6]2− and was determined by absorbance measurement of the formation of the Perls Prussian Blue complex following the addition of excess Fe3+ as described previously by Benzie and Strain [12].
Determination of total flavonoid
The total flavonoid of the watermelon juice extract was determined using the method described by Hatamnia et al. [13].
Determination of Total phenol
The total phenol content of the extract was determined by the method of Olajire and Azeez [14].
Estimation of α-glucosidase inhibition
The effect of the plant extract on α- glucosidase activity was determined according to the method described by Shai et al. [15] using α - glucosidase from Saccharomyces cerevisiae.
Estimation of α-amylase inhibition
This assay was carried out using a modified procedure of Apostolidis et al. [16].
In vivo parameters studied
Estimation of fasting blood glucose level
Fasting blood glucose was determined using OneTouch Ultra glucometer [17]. In this method, blood was collected from the tips of the rat’s tail and a drop was placed on the glucometer to produce the fasting blood glucose of the rat.
Serum insulin determination
This was estimated using an ultrasensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Upsala, Sweden) according to the manufacturer’s manual.
Determination of liver glycogen
This was determined according to the method of Lo et al. [18].
Determination of some carbohydrate metabolizing enzymes
Determination of hexokinase activity
The activity of hexokinase was measured using the modified method of Akinyosoye et al. [19].
Determination of glucose-6-phosphatase activity
The determination of glucose-6-phosphatase activity was done using the glucose dehydrogenase coupled reaction. The assay measures the rate of glucose released in the glucose-6- phosphatase reaction. The glucose is then oxidized to beta-D-gluconolactone by glucose dehydrogenase in a coupled reaction that uses NADP+ [20].
Determination of anti-inflammatory concentration
Interleukin 2 and 6 (IL-2 and IL-6) and tumour necrosis factor- α (TNF-α) determined using an ultrasensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Upsala, Sweden) according to the manufacturer’s manual.
Determination of oxidative stress biomarkers
The activities of catalase (CAT), superoxide dismutase (SOD), GPx, GSH and, GST, as well as level of lipid peroxidation, were estimated using their respective assay kits.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0 (Version 5, Software Program, GraphPad Prism Inc., San Diego, CA) and presented as mean ± S.D. One-way analysis of variance (ANOVA) was used for the analysis of the biochemical indices followed by Tukey’s posthoc test. Results were considered significant at p < 0.05.
Results
The in vitro inhibitory activity of watermelon juice (WMJ) is presented in Fig. 1. The extract (watermelon juice) shows satisfactory α-amylase inhibitory activity in a concentration-dependent manner.
Fig. 1.
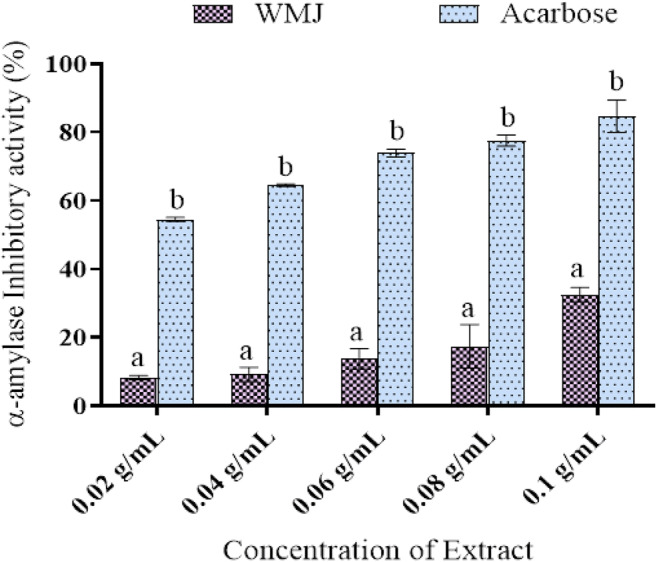
In vitro α-amylase inhibitory activity of watermelon juice. Data are presented as mean ± SEM of triplicate determinations (n = 3). a mean is significantly different compared to acarbose at p < 0.05. bmean is significantly different compared to watermelon juice (WMJ) at p < 0.05
Figure 2 shows the in vitro α-glucosidase inhibitory activity of watermelon juice (WMJ). The extract (watermelon juice) possesses inhibition potential which is significantly lower (p < 0.05) than that of the standard (Acarbose).
Fig. 2.
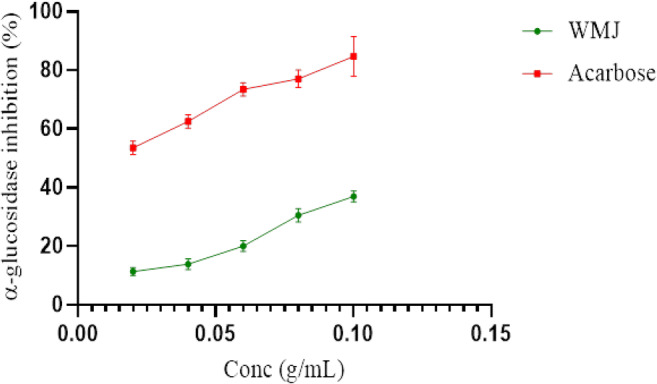
In vitro α-glucosidase inhibitory activities of WMJ (watermelon Juice). Data are presented as mean ± SEM of triplicate determinations (n = 3)
Watermelon juice (WMJ) was evaluated for nitric oxide inhibition activity. The result shown in Fig. 3 reveals the inhibitory ability of the watermelon juice which increases with the concentration of the extract (p < 0.05).
Fig. 3.
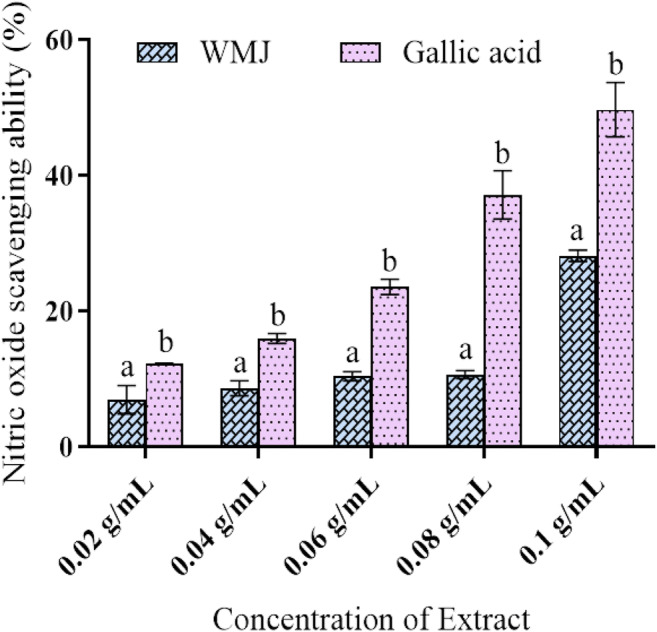
Nitric oxide scavenging ability of Watermelon Juice. Data are presented as mean ± SEM of triplicate determinations (n = 3). aMean is significantly different compared to gallic acid at p < 0.05. bMean is significantly different compared to watermelon juice (WMJ) at p < 0.05
The DPPH free radical scavenging activity of watermelon juice is depicted in Fig. 4. Vitamin C was used as the standard. Watermelon juice has appreciable DPPH free radical scavenging activity compared to the standard used in this study. The radical scavenging ability of the watermelon juice was reported as the percent of DPPH scavenged.
Fig. 4.
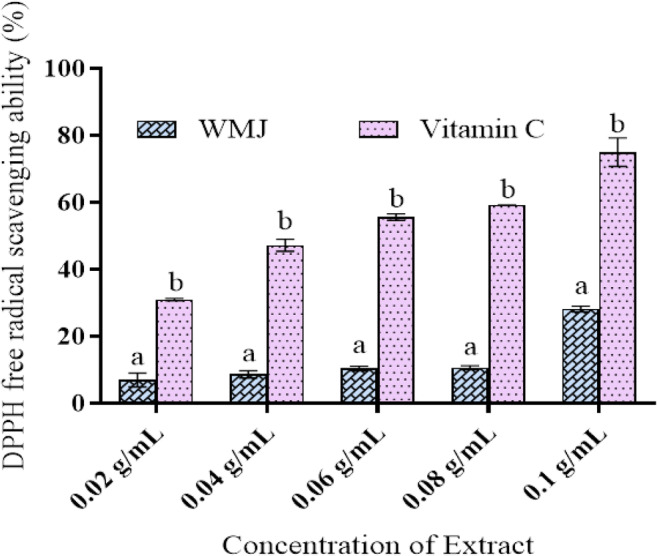
DPPH scavenging ability of Watermelon Juice. Data are presented as mean ± SEM of triplicate determinations (n = 3). aMean is significantly different compared to Vitamin C at p < 0.05. b Mean is significantly different compared to watermelon juice (WMJ) at p < 0.05. Legends: WMJ: Watermelon Juice; Vitamin C: Standard drug
Ferric reducing-antioxidant power (FRAP), phenolic and flavonoids contents of watermelon juice are presented in Table 2. The FRAP, phenolic and flavonoids content of watermelon juice is comparable well with what is documented in the literature. Results presented show that watermelon juice has significant antioxidant properties.
Table 2.
Ferric reducing-antioxidant power (FRAP), phenolic and flavonoids contents of watermelon juice
| Parameters | Content (ug/g) |
|---|---|
| FRAP | 29.57 ± 4.16 |
| Phenolic | 52.44 ± 4.11 |
| Flavonoids | 3.19 ± 2.26 |
Values represent means ± SEM (n = 3)
The effect of watermelon juice (WMJ) on fasting blood glucose levels is shown in Fig. 5. Repeated administration of WMJ, for 14 days, significantly (p˂0.05) decrease the fasting blood glucose level of diabetic rats in a dose-dependent manner as compared with the diabetic control group.
Fig. 5.
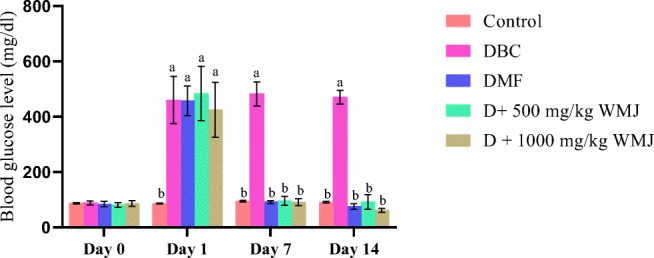
Effect of WMJ on fasting blood glucose level in alloxan-induced diabetic rats. Data are presented as the mean ± standard error of the mean (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (BBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered metformin
Figure 6 and Table 3 revealed that diabetic animals had a significantly (p < 0.05) reduced level of insulin and glycogen respectively when compared with the control group. Administration of WMJ for 14 days significantly (p < 0.05) increased the insulin levels in diabetic treated groups.
Fig. 6.
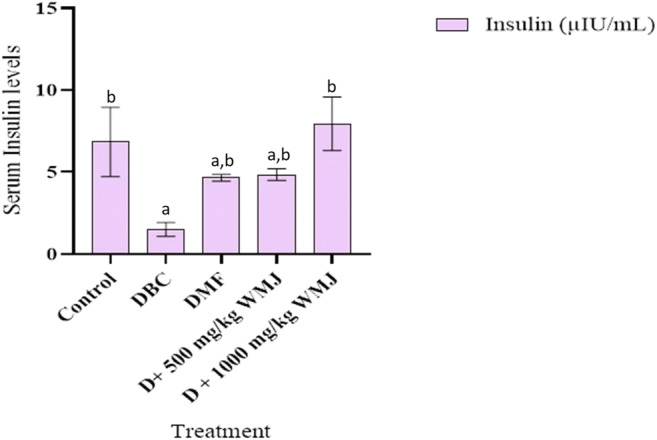
Effect of WMJ on serum insulin levels in alloxan-induced diabetic rats. Data are presented as mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered metformin
Table 3.
Effect of WMJ on liver glycogen in alloxan-induced diabetic rats
| Groups | Liver glycogen (mg/g tissue) |
|---|---|
| Control | 10.85 ± 3.21b |
| Diabetic control | 2.47 ± 1.53a |
| DMF | 7.10 ± 2.13b |
| D + 500 mg/kg WMJ | 6. 76 ± 3.04b |
| D + 1000 mg/kg WMJ | 9.96 ± 4.31b |
Data are presented as mean ± SEM (n = 8)
aMean is significantly different compared to Control at p < 0.05
bMean is significantly different compared to Diabetic control (DBC) at p < 0.05
Legends: WMJ: Watermelon Juice; DMF: Diabetic rats administered metformin
The activity of hexokinase was significantly (p < 0.05) lower in diabetic animals compared to control. On the other hand, there was a significant (p < 0.05) increase in glucose-6-phosphatase in diabetic control compared to the normal control. The administration of WMJ reversed the observed effect (Fig. 7).
Fig. 7.
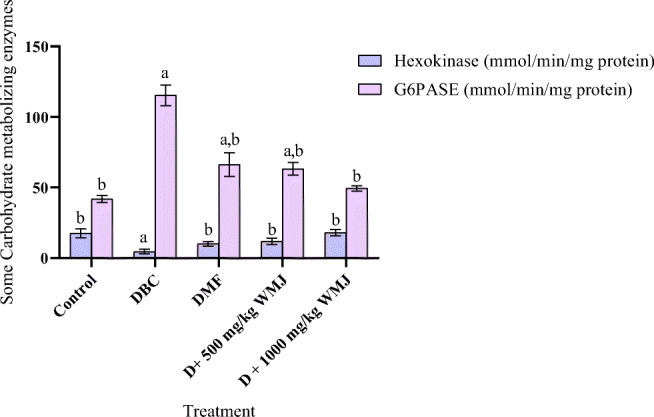
Effect of WMJ on some Liver carbohydrate metabolizing enzymes in alloxan-induced diabetic rats. Data are presented as mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered metformin; G6PASE: glucose-6-phosphatase
Figure 8 demonstrated that diabetic animals had a significantly (p < 0.05) decreased in both GLUT 2 and 4 when compared with other groups. However, the administration of WMJ to diabetic animals for 14 days significantly (p < 0.05) increased the levels of the two GLUTs.
Fig. 8.
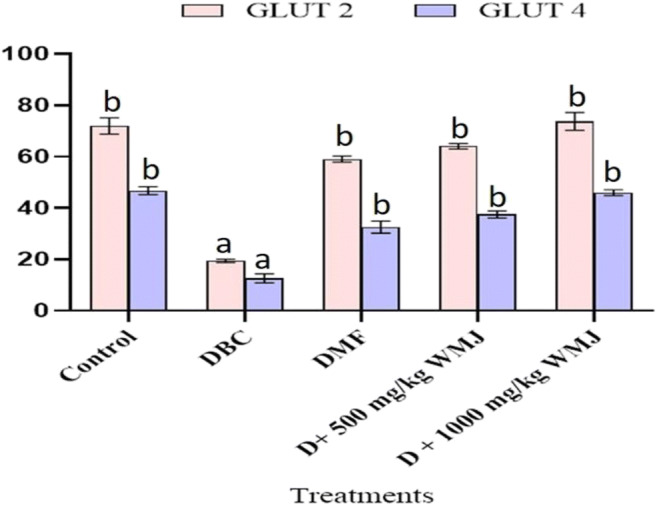
Effect of WMJ on Glut 2 and Glut 4 in alloxan-induced diabetic rats. Data are presented as the mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered with metformin
The effects of WMJ on some serum lipid profile in experimental animals are presented in Fig. 9. Diabetic rats showed a significant (p < 0.05), increase in the levels of triglyceride and total cholesterol and a corresponding decrease in high-density lipoprotein (HDL-c) in comparison with the normal control. Treatment with WMJ significantly reversed the observed effect of alloxan on serum lipid profile in experimental animals in the present study.
Fig. 9.
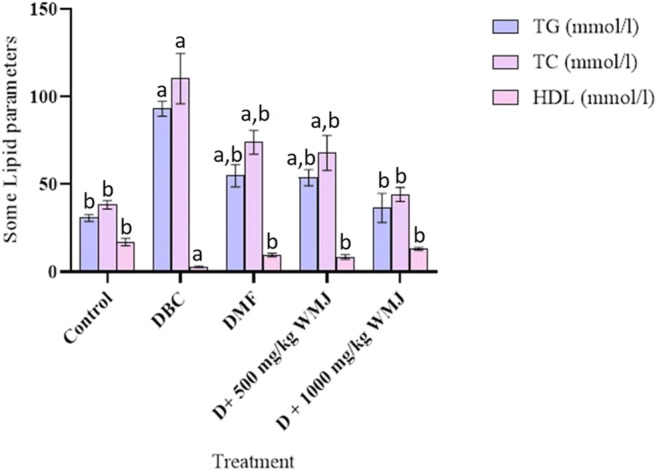
Effect of WMJ on some serum lipid profile parameters in alloxan-induced diabetic rats. Data are presented as mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered with metformin; TG: triglycerides; TC: Total cholesterol; HDL: High-density lipoprotein
Induction of diabetes led to an elevation in the level of pro-inflammatory parameters examined in this study. Treatment with WMJ at the tested doses reversed the elevations observed as presented in Fig. 10. Also, presented in Fig. 11 is the effect of watermelon juice (WMJ) on liver enzymatic and non-enzymatic antioxidants in alloxan-induced diabetic rats. WMJ was able to ameliorate the effect of alloxan on diabetic rats and restore them near normal in a dose-dependent manner as well as illustrated in Table 4.
Fig. 10.
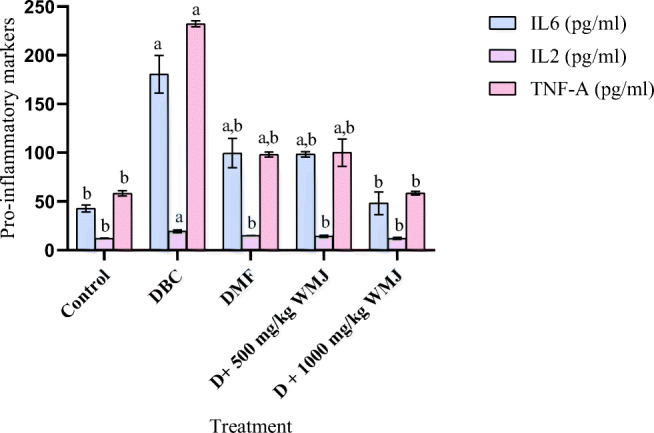
Effect of WMJ on some pro-inflammatory markers in alloxan-induced diabetic rats. Data are presented as mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered with metformin; IL6: Interleukin-6; IL2: Interleukin-2; TNF-α: Tumour necrosis factor-alpha
Fig. 11.
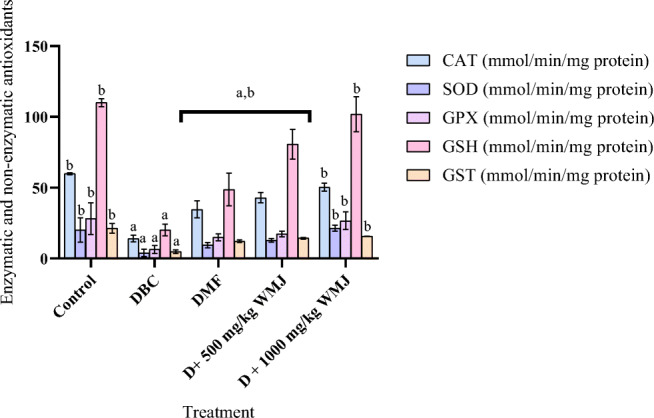
Effect of WMJ on liver enzymatic and non-enzymatic antioxidants in alloxan-induced diabetic rats/ Data are presented as mean ± SEM (n = 8). aMean is significantly different compared to Control at p < 0.05. bMean is significantly different compared to Diabetic control (DBC) at p < 0.05. Legends: WMJ: Watermelon Juice; DBC: Diabetic control; DMF: Diabetic rats administered with metformin; CAT: catalase; SOD: superoxide dismutase; GPX: glutathione peroxidase; GSH: glutathione reduced; GST: glutathione transferase
Table 4.
Effect of WMJ on lipid peroxidation in alloxan-induced diabetic rats
| Groups | Lipid peroxidation (mg/g tissue) |
|---|---|
| Control | 1.21 ± 0.64b |
| Diabetic control | 9.34 ± 3.33a |
| DMF | 4.65 ± 1.34b |
| D + 500 mg/kg WMJ | 3. 74 ± 1.09b |
| D + 1000 mg/kg WMJ | 1.65 ± 0.10b |
Data are presented as mean ± SEM (n = 8)
aMean is significantly different compared to Control at p < 0.05
bMean is significantly different compared to Diabetic control (DBC) at p < 0.05
Legends: WMJ: Watermelon Juice; DMF: Diabetic rats administered with metformin
Discussion
All over the world, diabetes mellitus (DM), one of the most common metabolic disorders, has been considered as one of the most serious diseases threatening human health. DM is characterized by the destruction or dysfunction of islet cells which caused not enough insulin or no response to the insulin. Hyperglycaemia is considered as one of the main features of DM and causes several diabetes complications in the body [21]. Over the years medicinal plants and fruits have been employed in the treatment of diabetes mellitus. Watermelon (Citrullus lanatus) is one of such medicinal plant that has attracted scientific interest due to its bioactivities [9].
In the present study, the ability of watermelon juice (WMJ) to protect against alloxan-induced diabetes mellitus was investigated. Prior to the in vivo experiments, some in vitro parameters were examined. One management option for diabetes mellitus is by decreasing postprandial hyperglycaemia and this could be achieved by delaying the digestion of carbohydrates in the digestive tract through inhibiting carbohydrate hydrolyzing enzymes (α-amylase and whereas α-glucosidase). Pancreatic α-amylase plays a crucial role in the breakdown of starch into disaccharides and oligosaccharides while intestinal α-glucosidase catalyses the breakdown of disaccharides to liberate glucose which is later absorbed into the blood circulation [1]. By inhibiting these key enzymes, minimal amounts of glucose would be absorbed into the bloodstream, hence the plasma glucose will not spike after a meal [22, 23]. This study shows that watermelon juice extracts moderately inhibited α-amylase and significantly inhibited α-glucosidase activity in a concentration-dependent manner and could be useful in the management of postprandial hyperglycaemia.
Under normal physiological conditions, nitric oxide (NO) acts as a necessary component in the regulation of various physiological functions such as blood pressure, immune response, and neural communication. However, overproduction of NO can induce tissue damage and is associated with inflammatory diseases including atherosclerosis and hypertension [24]. Therefore, researchers have paid more attention to discovering natural antioxidants that may act as potent inhibitors of NO production in relation to the treatment of chronic inflammatory diseases [24]. NO is known to be a ubiquitous free-radical moiety, which is distributed in tissues or organ systems and is supposed to have a vital role in neuromodulation or as a neurotransmitter in the CNS [25]. In this study, watermelon juice showed a significant NO radical scavenging ability comparable with what has been reported in the literature Fig. 3.
The effect of antioxidants on DPPH is believed to be due to their hydrogen-donating ability. The DPPH assay measures the antioxidant activity of water-soluble phenolics [26]. Figure 4 shows the dose-response ability of DPPH radical scavenging activity of WMJ compared with vitamin C. Although the DPPH radical scavenging abilities of the WMJ were significantly lower than that of vitamin C, it was evident that the extracts showed proton-donating ability and this could serve as free radical inhibitors or scavengers, acting possibly as primary antioxidants.
The ferric reducing antioxidant power (FRAP), an assay that is employed in the assessment of the antioxidant component in plants [27]. Iron (Fe) is essential in this regard and it acts as a trace element required for the survival of almost all organisms. Its involvement in haem and sulphur mediated reactions with proteins gives room for the participation of iron in diverse essential functions such as DNA synthesis, metabolic energy, oxygen transport and cellular respiration [28]. On the other hand, the ability of iron to interchange electrons such as in singlet oxygen with some substrates can lead to the formation of reactive oxygen species [29] which the WMJ was able to inhibit with its ability to chelate interconversion of iron to toxic form.
Phenols are very important plant constituents. There is a linear relationship between total phenol and antioxidant activity of plant species, because of the scavenging ability of their phenolic hydroxyl groups. Phenolic compounds are also reported to be effective hydrogen donors, making them very good antioxidants. Thus, it was reasonable to determine the total amount in WMJ [30]. The antioxidant activity of medicinal plants could be attributed to its flavonoid content. Flavonoids act as scavengers of various oxidizing species i.e. superoxide anion, hydroxyl radical or peroxy radicals, they also act as quenchers of singlet oxygen [30]. The relationship between phenol and flavonoid contents and free radical scavenging activity have so far been the subject of many studies describing the roles of both these groups of phytoconstituents in the manifestation of free radical inhibition properties by herbal extracts [31]. The results obtained in this study suggest that WMJ is rich in phenol and flavonoid and is similar to what has been reported in the literature.
Glucose is an important fuel for all cells and organs, but at high concentrations, it can cause many problems. Consequentially, blood glucose may be controlled in order to thwart the diverse damages produced by elevated glucose levels. The oral administration of WMJ decreases the blood glucose level in alloxan-induced diabetic rats. This hypoglycaemic effect was more pronounced for the WMJ at a dose of 1000 mg/kg/BW and correlated with the study reported by Mann et al. [32]. This finding suggests that WMJ exhibited a potent antihyperglycemic activity in diabetic rats and may be due to the richness of the extract in alkaloids, flavonoids and saponins which could act synergistically and/or independently as a stimulant for the release of insulin following the repair of pancreatic β-cells by the extract or by inhibition of the intestinal absorption glucose [33]. In DM, insulin is not or inadequately incorporated, creating hyperglycaemia, which leads to a change in glucose synthesis. A previous report proposes that to facilitate normal plasma insulin plays a vital role in keeping up the glucose equilibrium through upgrading glycolysis. Similarly decreased insulin and increased glucose level were observed in STZ induced diabetic rats; oral administration of WMJ results in recovery of blood glucose and plasma insulin significantly.
Glycogen is the storage form of glucose in the human body system and its level in the liver is a direct signal of insulin activity because insulin enhances intracellular glycogen deposition by stimulating glycogen synthase and inhibiting glycogen phosphorylase [10]. In this study, the reduction in liver glycogen levels was observed in diabetic control rats due to insufficient insulin secretion, which was ameliorated in diabetic control rats administered WMJ probably through insulin secretion to recover the glycogen synthase system.
According to Naik [1] insulin plays an important role in stimulating liver to store glucose in the form of glycogen, by activating the activity of hexokinase and inhibit the activity of glucose-6-phosphatase. Glucose-6-phosphatase is one of the important key enzymes in gluconeogenesis and glycogenolysis, while hexokinase is an important enzyme in the glycolysis pathway. Hence, the increase in glucose-6-phosphatase activity in diabetic rats, maybe due to an increase in gluconeogenesis during diabetes mellitus state [10]. The decrease in the activity of hexokinase in diabetic rats may be due to a decrease in insulin secretion. However, diabetic rats administered WMJ were able to ameliorate the abnormalities in these two enzymes probably because of increase in insulin secretion.
The observed increases in the levels of GLUT 2 and GLUT 4 in diabetic rats administered WMJ may be due to an increase in insulin secretion. While the decrease in these glucose transporters may be linked to a decrease in insulin secretion in diabetic mellitus state [34]. These results are in accordance with the previous research. The observed effect of the WMJ in decreasing the serum lipid and lipoprotein such as cholesterol, and triglyceride, as well as increasing HDL-c, could be beneficial in the prevention of diabetes and its associated complications. On the other hand, the results obtained from this study demonstrate a reduced level of pro-inflammatory cytokines such as IL-2, IL-6 and TNF-α in diabetic animals treated with WMJ. The decrease in the levels of these pro-inflammatory cytokines in diabetic rats upon treatment with WMJ as revealed in this study, may reduce insulin resistance and ameliorate pancreatic β-cell destruction in DM [35].
Hyperglycaemia in diabetes mellitus induces the generation of reactive oxygen species (ROS), which causes cellular damage by oxidizing nucleic acid, protein and membrane lipids [36]. Uncontrolled ROS impairs the activities of endogenous antioxidant defense systems such as SOD, catalase, GPx, glutathione reductase and glutathione transferase (GST), are known to counterbalance the toxic effect of free radicals [37]. The general decline in the antioxidant status (both enzymatic and non-enzymatic) in this study maybe as a result of the overwhelming effect of the ROS generated due to STZ exposure. The obtained results were also supported by a reduction in the levels of lipid peroxidation. The result obtained agrees with previous reports that natural products possess antioxidative capacity that can mitigate alloxan-induced toxicity in rats [38]. It is believed that one of the anti-diabetic mechanism of action of WMJ could be explained by its ability to enhance endogenous enzymatic and non-enzymatic antioxidant production and possibly by reducing and effectively scavenging mitochondrial ROS generation. Although further studies are required to firmly establish this mechanism of actions.
Conclusion
Plant-derived compounds possessing therapeutic potential have been leading the way in the search for novel antidiabetic drugs. The findings from this study show that watermelon juice exhibits antioxidant, anti-inflammatory, and anti-hyperglycaemic activities, as all biochemical parameters examined were all ameliorated when compared with the control. The results obtained further support the ethnomedical use of this plant to promote good health especially in the management of diabetes and its associated complications.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naik P. Biochemistry textbook. 3. New Delhi, India: Jaypee Brothers Medical Publishers Ltd.; 2010. [Google Scholar]
- 2.Mukundi MJ, Mwaniki NEN, Piero NM, Murugi NJ, Daniel AS, Peter GK, Alice MN. In Vivo anti-diabetic effects of aqueous leaf extracts of Rhoicissus tridentata in Alloxan induced diabetic mice. Journal of Developing Drugs. 2015;4:131. [Google Scholar]
- 3.Ayepola OM, Brooks NL, Oguntibeju OO. Oxidative stress and diabetic complications: the role of antioxidant vitamins and flavonoids. In: Oguntibeju OO, editor. Antioxidant-antidiabetic agents and human health. IntechOpen: Rijeka; 2014. [Google Scholar]
- 4.Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrology Dialysis Transplantation. 2016;31(2):206–213. doi: 10.1093/ndt/gfu405. [DOI] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chikezie PC, Ojiako OA, Ogbuji AC. Oxidative stress in diabetes mellitus. Int J Biol Chem. 2015;9:92–109. [Google Scholar]
- 7.Maiese K. New insights for oxidative stress and diabetes mellitus. Oxidative Med Cell Longev. 2015;875961. [DOI] [PMC free article] [PubMed]
- 8.Tankoy Y, Mahdi M, Yaro AH, Musa KY, Mohamed A. Hypoglycemic activity of methanolic stem bark of Adansonia digitata extract on blood glucose levels of streptozocin induced diabetic Wistar rats. International Journal of Applied Research in Natural Products. 2008;2:32–36. [Google Scholar]
- 9.Oyenihi OR, Afolabi BA, Oyenihi AB, Ogunmokuna OJ, Oguntibeju OO. Hepato- and neuro-protective effects of watermelon juice on acute ethanol-induced oxidative stress in rats. Toxicol Rep. 2016;2016(3):288–294. doi: 10.1016/j.toxrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajiboye OB, Ojo A, Akuboh O, Abiola O, Idowu O, Amuzat A. Antihyperglycemic and antiinflammatory activities of polyphenolic-rich extract of Syzygim cumini leaves in alloxan-induced diabetic rats. J Evid Based Integr Med. 2018;23:1–8. doi: 10.1177/2515690X18770630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, RiceEvans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 12.Benzie IEF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 13.Hatamnia AA, Abbaspour N, Darvishzadeh R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014;145:306–311. doi: 10.1016/j.foodchem.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Olajire AA, Azeez L. Total antioxidant activity, phenolic, flavonoid and ascorbic acid contents of Nigerian vegetables. African Journal of Food Science and Technology. 2011;2(2):022–029. [Google Scholar]
- 15.Shai LJ, Masoko P, Mokgotho MP, Magano SR, Mogale A, Boaduo N, Eloff J. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S Afr J Bot. 2010;76(3):465–470. [Google Scholar]
- 16.Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. [Google Scholar]
- 17.Ahmad MS, Pischetsrieder M, Ahmed N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007;561:32–38. doi: 10.1016/j.ejphar.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Lo S, Russell JC, Taylor A. Determination of glycogen in small tissue samples. J Appl Physiol. 1970;28(2):234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 19.Akinyosoye F, Fawole M, Akinyanju J. Studies on some enzymes of carbohydrate metabolism in Geotrichum candidum. Nig J Microbiol. 1987;7:154–161. [Google Scholar]
- 20.Alegre M, Ciudad CJ, Fillat C, Guinovart JJ. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem. 1988;173(1):185–189. doi: 10.1016/0003-2697(88)90176-5. [DOI] [PubMed] [Google Scholar]
- 21.Altas S, Kizil G. Protective effect of Diyarbakır watermelon juice on carbon tetrachloride-induced toxicity in rats. Food and Chem Toxicol. 2011;49:2433–2438. doi: 10.1016/j.fct.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 22.Karakaya S, Gözcü S, Güvenalp Z, Özbek H, Yuca H, Dursunoğlu B, Kazaz C, Kılıç CS. (2018). The α-amylase and α-glucosidase inhibitory activities of the dichloromethane extracts and constituents of Ferulago bracteata roots. Pharm. Biol. 2018;56:18–24. doi: 10.1080/13880209.2017.1414857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type II diabetes from normal or impaired glucose tolerance. J Diabetes. 2004;53:160–165. doi: 10.2337/diabetes.53.1.160. [DOI] [PubMed] [Google Scholar]
- 24.Chen HL, Lan XZ, Wu YY, Ou YW, Chen TC, Wu WT. The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. BioMedicine. 2017;7(4):22. doi: 10.1051/bmdcn/2017070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulati K, Ray A, Masood A, Vijayan VK. Involvement of nitric oxide (NO) in the regulation of stress susceptibility and adaptation in rats. Indian J Exp Biol. 2006;44:809–815. [PubMed] [Google Scholar]
- 26.Chun SS, Vattem DA, Lin YT, Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against helicobacter pyroli. Process Biochem. 2005;40:809–816. [Google Scholar]
- 27.Xang JK, Barrera G, Guldbakke B, Munday R, Lenzen S. Importance of the GLUT2 glucose transporter for pancreatic beta cell toxicity of alloxan. Diabetologia. 2015;45:1542–1549. doi: 10.1007/s00125-002-0955-x. [DOI] [PubMed] [Google Scholar]
- 28.Lindhul DD, Roseiro GU, Hanseleit BY. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: FRAP assay. Anal Biochem. 2014;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012;2012:26. [Google Scholar]
- 30.Subedi L, Timalsena S, Duwadi P, Thapa R, Paude A, Parajuli K. Antioxidant activity and phenol and flavonoid contents of eight medicinal plants from Western Nepal. J Tradit Chinese Med. 2014;34:584–590. doi: 10.1016/s0254-6272(15)30067-4. [DOI] [PubMed] [Google Scholar]
- 31.Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8:96. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann S, Singh PK, Gubta AAA. Antidiabetic effects of Ricinus communis on the blood biochemical parameters in streptozotocin induced rat. International Journal of Pharma and Biosciences. 2013;4(2):382–388. [Google Scholar]
- 33.Gad-Elkareem MAM, Abdelgadir EH, Badawy OM, Kadr A. Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ. 2019;7:e6441. doi: 10.7717/peerj.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czech MP. Insulin action and resistance in obesity and type II diabetes. Nat Med. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masola B, Oguntibeju OO, Oyenihi AB. Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed Pharmacother. 2018;101:447–457. doi: 10.1016/j.biopha.2018.02.115. [DOI] [PubMed] [Google Scholar]
- 36.Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci. 2015;16(10):25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belayneh YM, Birhanu Z, Birru EM, Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L. (Solanaceae) J Exp Pharmacol. 2019;11:29–38. doi: 10.2147/JEP.S192264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]


