Abstract
Prebiotics are functional foods with health-promoting properties that are used in many developed countries. Thailand is one of the countries that produces many plants that should have prebiotic properties. In this study, we investigated the potential prebiotic effects of powders obtained from Saba, Pisang Awak Banana and Silver bluggoe in vitro in accordance with their physical, chemical and microbiological properties. These selected plants were found to demonstrate good water-/oil-binding properties. They contained chlorophyll, beta carotene and lycopene and showed good resistance to stomach and small-intestine enzymes. The selected plants were further used to evaluate prebiotic properties by supplementing as a carbon source in culturing broth for growing probiotic bacteria and pathogenetic bacteria. The increase in the number of probiotic bacteria during fermentation of these selected plants correlated with decreased pH. The growth of four strains of probiotic bacteria seemed to be promoted in MRS broth containing these selected plants, but no significant differences in the number of probiotic bacterial groups were detected in response to difference concentrations of all these selected plants. In addition, we noted that a decrease in the number of all four strains of pathogenic bacteria during fermentation of these selected plants correlated with a decreased pH. Moreover, the antimicrobial activity of selected plant prebiotics supported probiotic substance production to inhibit growth of pathogenic bacteria. In conclusion, we have shown that the addition of selected prebiotic plants, indicating that they should be used as a prebiotic food ingredient, represents a potential alternative to available commercial prebiotics.
Keywords: Probiotic, Saba, Pisang Awak, Anti-microbial, Chemical composition, Physical property
Introduction
Today, dairy technology science has made many important advances that have led to foods with high nutritional properties that confer health. Prebiotics are one in a group of nutraceutical and functional food and their health-benefiting effects have been widely studied. (Younis et al. 2015). A prebiotic is ‘a non-digestible and selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microbiota that confers benefits upon host well-being and health’ (Synytsya et al. 2009). These compounds must be able to withstand acid hydrolysis in the stomach; unable digested by pancreatic and small-bowel enzymes in the human gut and therefore, reaches the large bowel. (Thammarutwasik et al. 2009; Younis et al. 2015). If preferred, prebiotics can be considered as a ‘food’ for probiotic purposes. Probiotics have been described as ‘harmless live normal flora/microorganisms food dietary supplements which provide a health benefit on the host, when administered in adequate amounts and it also leads to have nutritional advantages’ (Abatenh et al. 2018).
The banana, belonging to the family Musaceae, is a highly common plant in tropical and subtropical countries. It contains approximately 75% water and 25% carbohydrate along with trace amounts of protein and fat. It also contains high levels of potassium, magnesium, phosphorus, iron and calcium and is known as a source of vitamins (provitamins A, B and C), which are important to health (Mishra and Satur 2010; Jenie et al. 2013). In their green stage, bananas stand out for having a high starch/resistant starch content, attracting industrial interest for developing new products. Moreover, the green banana appear to be a good source of fibers, vitamins (Vit C, B6, provitamin A), minerals (potassium,phosphorus, magnesium, zinc) bioactive compounds such as phenolic compounds, and resistant starch present in both pulp and peel (Wall 2006; Riquette et al. 2019; Falcomer et al. 2019). Banana is a potential carbohydrate source for food consumption, while its starches are relatively low in amylase content and have high resistance to heat and amylase attack, low swelling properties, and low retrogradation, affording application in the food industry as a gelling agent, thickening agent and stabilizer. These characteristics are particularly important to the dairy industry due to providing an odourless and soluble capacity. Thus, the banana could be used to formulate several kinds of desserts and functional foods (Padam et al. 2014).
Among their prebiotic properties, bananas contain about of 60–80% of their carbohydrates as indigestible carbohydrates (resistant starch, cellulose, hemicelluloses, and lignin), which contain prebiotic properties (Sarawong et al. 2014, do Prado Cordoba et al. 2018). Some of indigestible carbohydrates from banana are source of food for probiotic bacteria (e.g. Lactobacilli spp.). Probiotic bacteria can digest such type of carbohydrates produce short-chain fatty acids during fermentation and therefore promote the growth of probiotic itself (Buranawit and Laenoi 2015; Budhisatria et al. 2017).
In developing a prebiotic product, certain types of banana (e.g., Saba, Pisang Awak and Silver bluggoe) were selected for investigation on account of their optimal physical, chemical and preliminary-prebiotic properties.
Materials and methods
Banana samples
Raw banana; Saba (Musa acuminata × balbisiana (ABB Group)), Pisang Awak (Musa sapientum Linn), and Silver bluggoe (Musa (ABB group)) punch was obtained from a local market. Its skin was sliced, peeled and cut into small pieces. Bananas were subjected to hot (dry) air for drying process at 50 °C for 24 min, then ground into powder in a grinder. Then, the powder was strained through a 150-μm sieve and collected in a plastic bag for further testing.
Water-binding capacity
Water-binding capacity was modified from the method described by Gorecka et al. (2000) and Sowbhaya et al. (2007), by adding 0.5 g of selected plant to 15 mL of different buffers to maintain pH at 6.6, 1.8 and 8.7, allowing the mixture to swell in the water bath, then shaking at 37 °C for 7, 135 and 60 min, respectively, to determine the experimental pH conditions under which it may be mimicked in specific parts of the human gastrointestinal tract. The mixture was subjected to centrifugation at 3000g for 15 min and the supernatant carefully removed. The tubes were slanted for 30 min to drain off the excess water. The wet mass in each tube was removed, weighed and dried to constant weight (± 0.05 mg) at 100 °C. The data obtained were expressed as a gram of water bound per gram of dry initial sample, per the following formula:
Oil-binding capacity
Binding capacity in oil was modified from the process described by Sangnark and Noomhorm (2003). Briefly, 0.5 g of the selected plant was weighed and 15 ml of soybean oil added. The samples were incubated in a shaker water bath for 1 h at 37 °C. Each tube was subjected to centrifugation at 3000 rpm for 15 min and supernatant were removed carefully to drain off the excess oil. The data obtained were expressed as a gram of oil bound per a gram of dry initial sample, per the following formula:
Determination of chlorophyll content
The quantity of chlorophyll in a sample was analyzed according to a method modified from that of Whitham et al. (1971) by adding 1 g of selected plant in 10 mL of 80% v/v acetone solution and placed at room temperature for 15 min. The mixture was filtered through Whatman No. 1 filter paper and the volume adjusted with 80% v/v acetone solution to 25 mL. The sample was measured for optical density (OD) at 663 and 645 nm. The blank was 80% v/v acetone solution. After the OD was calculated, the chlorophyll content was calculated in mg/100 mg per the following formula:
By V, the last volume of solution; W, weight of dry subject.
Determination of β-carotene and lycopene
The amount of ß-carotene and lycopene was determined by adding 100 mg of selected plant into a 10 mL aqueous mixture of acetone-hexane (92:3) solution. The mixture was shaken for 1 min and filtered through Whatman No. 4 paper. The sample was subjected to OD measuring at 453, 505 and 663 nm. The amount of β-carotene and lycopene can be calculated per the following formula:
Resistance to human α-amylase and trypsin hydrolysis
The enzyme activity was determined using α-amylase and trypsin. The enzyme was prepared in a solution containing 1 unit/mL of α-amylase or 90 units/mL of trypsin using sodium phosphate buffer (20 mM) pH 5, 6 and 7 in α-amylase or pH 6, 7 and 8 in trypsin, respectively. Sample was prepared as a 1% (w/v) solution in 15 mL of 20 mM sodium phosphate buffer. Fifteen mL of enzyme solution were added to a sample solution. The reaction mixture was incubated in a controlled temperature water bath (37 ± 1 °C) for 6 h. Each 1 mL of sample was taken at 0, 0.5, 1, 2, 4 and 6 h, respectively, to determine reducing sugar using a dinitrosalicyclic acid assay (Miller 1959) and total sugar by the phenol–sulfuric acid method (Dubois et al. 1956). Percentage of hydrolysis of the sample was calculated based on reducing sugar released and total sugar content of the sample, as per the following formula:
Microorganisms
All pathogenic bacterial strains were obtained from the stock culture collection of the Microbiology Laboratory (Faculty of Medical Technology, Rangsit University, Thailand). The prebiotic bacterial strains were obtained from the stock culture collection of the Microbiology Laboratory of Thailand Institute of Scientific and Technological Research. Cultures were maintained at − 20 °C in 20% (v/v) glycerol. Probiotic bacteria were activated by growing them in MRS agar or nutrient agar 24 h at 37 °C prior to use.
Determination of relationship between concentration of selected plant and growth of probiotic strains
Inocula of selected probiotic bacterial strains were prepared by suspending bacteria in sterile 0.85% NaCl (0.5 McFarland standard equivalent concentration of 108 CFU/mL) and serially diluted (1:100; corresponding to ~ 1 × 106 CFU/ml during the colony count assay). The MRS broth (5 mL) was then inoculated with 50 µL of bacterial suspension, which was supplemented with selected plants at a concentration of 0–6% (w/v) under aseptic conditions and incubated at 37 °C. The optical density at 600 nm of each culture was determined at 0, 24, 48 and 72 h, respectively. Subsequently, specific growth rates of the probiotics in each carbohydrate medium were calculated compared with each probiotic standard growth curve plots according to growth curve(cfu/ml) and OD (600 nm). Exponential phase growth was derived from growth plots of the probiotics.
Determination of relationship of selected banana as a carbohydrate source and growth, pH change of each probiotic strain and pathogenic bacterial strains
Inocula of each tested probiotic bacterial strain and pathogenic bacterial strains were prepared using a pure colony suspended in sterile 0.85% NaCl set as a 0.5 McFarland standard (equivalent concentration of 108 CFU/mL) and serially diluted (1:100; corresponding to ~ 1x 106 CFU/mL during colony count assay). Five mL of MRS broth (for probiotic bacteria) or tryptic soy broth (for pathogenic bacteria), supplemented with 3% (w/v) of powder of selected plant, was then inoculated with 50 µL of each probiotic strain or pathogenic bacterial strains under aseptic conditions and incubated at 37 °C. OD was checked at 0, 24, 48 and 72 h. At the same time, the pH of fermented broth over a time interval was determined using a calibrated pH meter (Metrohm, 713 pH meter/Switzerland). All measurements were carried out in triplicate. Subsequently, specific growth rates of the bacteria in each selected plant were calculated and reported as log CFU/mL as described previously.
Determining inhibitory activity of cell-free supernatants from fermented broth of probiotic strains cultured in selected plant by agar diffusion method
Preparation of cell-free supernatants
This technique was modified from that of Fooks and Gibson (2002). Briefly, cell-free supernatants (CFS) were obtained by inoculating 50 µL of 106 CFU/mL probiotic strain into 5 mL of MRS broth cultures supplemented with 3% (w/v) of selected plants. After 48 h of incubation at 30 °C, the culture was centrifuged at 10,000g for 10 min at 4 °C. To rule out inhibition due to pH reduction caused by organic acids, the pH of the CFS was adjusted to 6.2 using 1 N NaOH. Sterile catalase (1000 U/mL; Sigma-Aldrich, Co., Inc., St. Louis, MO, USA) was added to the CFS and filter-sterilized through 0.22-μm pore-size filters. CFS from each probiotic strain was obtained and their inhibitory activity against the pathogenic bacterial strains was assayed.
To determine the inhibitory activity of the CFS, 100 µL of pathogenic bacterial strains (~ 108 CFU/mL) in the late exponential phase was spread over the surface of Muller-Hinton agar with a three-way swab method using a sterile cotton swab. Six-mm diameter wells in agar were made using a sterile cork borer; 30 μL of each CSF was added to each well. Similarly, 30 µL of MRS broth was used as a negative control. The plates were incubated at 37 °C for 24 h. The extent of inhibition was assessed after incubation by measuring the diameter of the clear zone surrounding each well and measured with a scale. The experimental set-up was repeated in triplicate.
Statistical analysis
All experimental results were carried out in triplicate and expressed as means of three analyses ± SD. Comparison of each data set between groups was performed using one-way ANOVA. Duncan’s Multiple Range Test was applied to determine the significant difference between the incubation times at p ≤ 0.05. Student’s t test with Bonferroni correction was used for pair-wise comparisons. Significance (p ≤ 0.05) was considered at the 95% confidence level using SPSS version 22.
Results and discussion
Prebiotics are short-chain carbohydrates that alter the composition or metabolism of the gut microbiota in a beneficial manner and help to improve health. Because prebiotics also imply carbohydrate residues that escape digestion, three types of selected banana plants were assessed for in vitro prebiotic potential. Growth of probiotics using selected banana plants as the only source of carbohydrate was considered as the first biomarker for determination of prebiotic potential.
Water- and oil-binding capacities
It was shown that Saba powder was best able to hold water as 10.13 ± 4.13 g water/g dry matter in stomach conditions (pH 1.8; incubation time, 135 min) with significant differences from other banana plants (p < 0.05); second was Pisang Awak Banana powder, as 3.90 ± 0.75 g water/g dry matter in stomach condition. In addition, powders from Silver bluggoe and Saba had the best oil-binding capacity (15.86 ± 0.12 and 15.86 ± 0.08 g of oil/g dry matter, respectively) (Table 1). However, there was no evidence of a significant difference (p > 0.05) when compared among banana types. Good water-binding capacity means that these plants can help to increase the amount of faeces and also reduce nutrient absorption rates in the intestine. Good oil-binding capacity indicates that a particular plant can be a good emulsifier of high-fat food, thus reducing fat absorption. Similar findings have been observed that, first, fibre from Lady Finger banana pulp has the best water- and oil-binding capacity, as 13.54 ± 0.24 g water/g dry matter, 7.75 ± 0.29 g of oil/g dry matter, respectively; second, Lady Finger banana’s peel powder demonstrated a water-binding capacity of 11.75 ± 0.24 g water/g dry matter (Suksathit and Suksathit 2015).
Table 1.
Water- and oil-binding capacities, chlorophyll, ß-carotene and lycopene of selected plants (n = 3)
| Type of plant | Water binding capacity (g water/g dry matter) | Oil-binding capacity (g oil/g dry matter) | Total chlorophyll (mg/100 mg) | Lycopene (mg/100 mg) | ß-carotene (mg/100 mg) | ||
|---|---|---|---|---|---|---|---|
| Oral cavity pH 6.6 incubation time 7 min | Stomach pH 1.8 incubation time 135 min | Duodenum pH 8.7 incubation time 60 min | |||||
| Saba powder | 1.13 ± 0.25 | 10.13 ± 4.13 | 3.39 ± 1.11 | 15.86 ± 0.08 | 0.0291 ± 0.0124 | 0.403 ± 0.059 | 0.494 ± 0.029 |
| Pisang Awak Banana powder | 1.48 ± 0.66 | 3.90 ± 0.75 | 1.91 ± 0.37 | 14.83 ± 0.15 | 0.0378 ± 0.0124 | 0.284 ± 0.040 | 0.392 ± 0.013 |
| Silver bluggoe powder | 3.07 ± 0.21 | 2.68 ± 0.11 | 2.20 ± 0.02 | 15.86 ± 0.12 | 0.0169 ± 0.0052 | 0.273 ± 0.043 | 0.106 ± 0.026 |
Values expressed are mean ± standard deviation
Chlorophyll, ß-carotene and lycopene content
It was found that Pisang Awak Banana powder has the highest total chlorophyll content as 0.0378 ± 0.0124 mg/100 mg, followed by powders from Saba and Silver bluggoe, which have the values of 0.0291 ± 0.0124 and 0.0169 ± 0.0052 mg/100 mg, respectively. Moreover, Saba powder had the highest concentration of β-carotene and lycopene (0.494 ± 0.029 and 0.403 ± 0.059 mg/100 mg), followed by Pisang Awak Banana powder (0.392 ± 0.013 and 0.284 ± 0.040 mg/100 mg) (Table 1). Chlorophyll, β-carotene and lycopene are a group of phenolic compounds that have antioxidant properties. It was proposed that the antioxidant property is another benefit of prebiotic effect because antioxidant-containing prebiotic can reduce the risk of cardiovascular disease, stroke and cancer (Bazzano et al. 2002; Southon 2000). Taking into account of these results, the banana powder used in this study could have an alternative use as a functional food. This result was correlated with the report that revealed aguamiel (A. atrovirens) used as a prebiotic in México exhibited antioxidant activity against DPPH and ABTS (Romero-López et al. 2015).
Effect of α-amylase and trypsin hydrolysis
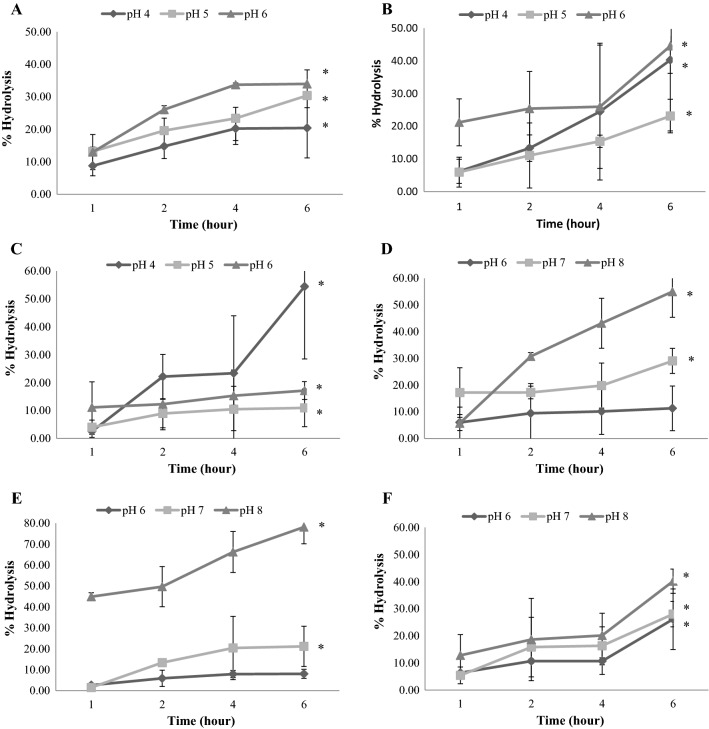
The maximum hydrolysis of powders from Saba, Pisang Awak Banana and Silver bluggoe by α-amylase enzyme at pH 4 were: 8.74 ± 0.13, 6.22 ± 3.69 and 2.53 ± 2.15%; pH 5 were 13.16 ± 1.3, 5.95 ± 4.56 and 4.02 ± 2.58%; and pH 6 were 12.98 ± 5.40, 21.20 ± 7.18 and 11.12 ± 9.16%, respectively, at 1 h incubation. The maximum hydrolysis (33.97 ± 5.40, 44.55 ± 7.18 and 17.15 ± 9.16%, respectively) occurred after 6 h of incubation at pH 6 (Fig. 1). The hydrolysis of various bananas with α-amylase at pH 4–6 showed that higher pH gave a significantly higher degree of hydrolysis (p ≤ 0.05); however, there was no significant difference at pH 4 and 5. However, in the body system, the source of the amylase enzyme was found at pH 6.6. Moreover, the food consumed in the beginning of the gastrointestinal tract was less than 2 h. It was estimated that approximately 50% of the banana consumed would reach the colon because some of them were hydrolysed by α-amylase (8–13%), by acid in the stomach, and by brush-border enzymes in the small intestine. Typically, the carbohydrates were digested in the small intestine (30%), where some brush-border enzymes (i.e., isomaltase, glucoamylase, maltase, sucrase and lactase, and hydrolyse α-1,4- and α-1,6-linked glucosaccharides), present in the small intestine, yield monosaccharides as end-products (Johnson and Schmit 2005). Similar findings have been observed that, using an in vitro technique, at least 34.88% of oligosaccharides of pitaya was hydrolyzed by human α-amylase (Wichienchot et al. 2010). Therefore, the banana used in this experiment appeared to have partial α-amylase enzymatic resistance.
Fig. 1.
Percentage of hydrolysis after incubation with α-amylase and trypsin enzyme at various times (n = 3). Percentage of hydrolysis of each banana powder after incubation with α-amylase: a Saba powder, b Pisang Awak Banana powder, c Silver bluggoe powder; and after incubation with trypsin, d Saba powder, e Pisang Awak Banana powder, f Silver bluggoe powder. Values expressed are mean ± standard deviation. * indicated significantly different at p ≤ 0.05
The maximum hydrolysis of powders from Saba, Pisang Awak Banana and Silver bluggoe by trypsin enzyme at pH 6 were: 5.98 ± 2.78, 2.65 ± 0.89 and 6.39 ± 0.52%; pH 7 were 17.19 ± 9.44, 1.52 ± 0.39 and 5.43 ± 3.10%; and pH 8 were 5.71 ± 3.38, 44.92 ± 1.87 and 12.82 ± 7.67%, respectively, at 1 h incubation. The maximum hydrolysis (55.01 ± 45.70, 78.15 ± 7.96 and 40.17 ± 4.47%, respectively) occurred after 6 h of incubation at pH 8 (Fig. 1). The hydrolysis of various bananas with trypsin at pH 6–8 showed that higher pH gave a significantly higher degree of hydrolysis (p ≤ 0.05). However, the source of the trypsin enzyme in the body system was found at pH 8.7 and the food digested in the last section of the gastrointestinal tract was less than one hour. It was estimated that about 50% of the banana consumed would reach the colon because some of them were hydrolysed by trypsin (5–12%). Therefore, the banana used in this experiment appeared to have partial trypsin enzymatic resistance.
Relationship of concentration of selected plant and growth curves probiotic strains
Changes in log CFU during 0–72 h fermentation was observed in every concentration from 1–6% w/v of selected plant, but no significant effect was observed (Table 2). Each probiotic strain had a significant increase in number (p ≤ 0.05) from 0 to log 7.16 ± 0.05–8.25 ± 0.00 CFU/mL within 24 h fermentation to enter the log phase. However, the log number shows little increase (7.73 ± 0.00–8.33 ± 0.01 CFU/mL) from 48 to 72 h, meaning that the growth curve entered the stationary phase. There were no significant differences in the number of probiotic bacteria detected in response to difference concentration of these entire banana powder and the probiotic strains too.
Table 2.
Relationship of various concentrations of selected banana as a carbohydrate source and growth curves of each probiotic strain (n = 3)
| Probiotic | Incubation time (h) | Banana powder concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1% | 2% | 3% | ||||||||
| Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | ||
|
Lactobacillus acidophilus CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.79 ± 0.01* | 7.58 ± 0.03* | 7.82 ± 0.01* | 7.79 ± 0.01* | 7.71 ± 0.06* | 7.79 ± 0.01* | 7.49 ± 0.01* | 7.72 ± 0.01* | 7.76 ± 0.03* | |
| 48 | 8.28 ± 0.02* | 7.71 ± 0.00* | 7.92 ± 0.01* | 7.83 ± 0.01* | 7.50 ± 0.00* | 7.81 ± 0.01* | 7.80 ± 0.01* | 7.73 ± 0.03* | 7.88 ± 0.00* | |
| 72 | 8.33 ± 0.01* | 7.77 ± 0.00* | 8.04 ± 0.00* | 8.12 ± 0.01* | 7.88 ± 0.01* | 7.98 ± 0.01* | 8.04 ± 0.01* | 7.56 ± 0.00* | 8.01 ± 0.00* | |
|
Lactobacillus casei CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.83 ± 0.00* | 7.71 ± 0.01* | 7.82 ± 0.00* | 7.74 ± 0.00* | 7.70 ± 0.00* | 7.72 ± 0.01* | 7.70 ± 0.01* | 7.70 ± 0.01* | 7.64 ± 0.02* | |
| 48 | 7.91 ± 0.00* | 7.74 ± 0.00* | 7.91 ± 0.00* | 7.84 ± 0.00* | 7.81 ± 0.02* | 7.74 ± 0.00* | 7.85 ± 0.00* | 7.96 ± 0.00* | 7.76 ± 0.01* | |
| 72 | 7.95 ± 0.01* | 8.19 ± 0.00* | 8.22 ± 0.00* | 7.91 ± 0.01* | 8.11 ± 0.00* | 8.08 ± 0.00* | 8.21 ± 0.00* | 8.11 ± 0.00* | 8.19 ± 0.00* | |
|
Lactobacillus fermentum CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.52 ± 0.01* | 7.60 ± 0.02* | 7.69 ± 0.01* | 7.59 ± 0.01* | 7.70 ± 0.01* | 7.80 ± 0.01* | 7.52 ± 0.01* | 7.74 ± 0.02* | 7.70 ± 0.01* | |
| 48 | 7.84 ± 0.01* | 7.81 ± 0.01* | 7.73 ± 0.01* | 7.66 ± 0.00* | 8.19 ± 0.01* | 7.92 ± 0.01* | 7.90 ± 0.00* | 8.13 ± 0.01* | 7.79 ± 0.01* | |
| 72 | 8.08 ± 0.00* | 8.12 ± 0.00* | 8.19 ± 0.00* | 7.89 ± 0.00* | 8.19 ± 0.00* | 7.95 ± 0.00* | 8.25 ± 0.00* | 8.23 ± 0.00* | 7.80 ± 0.01* | |
|
Streptococcus thermophiles CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.79 ± 0.01* | 7.47 ± 0.03* | 7.46 ± 0.01 | 7.37 ± 0.02* | 7.16 ± 0.05* | 7.47 ± 0.02* | 7.71 ± 0.01* | 7.31 ± 0.02* | 7.69 ± 0.01* | |
| 48 | 7.77 ± 0.01* | 7.58 ± 0.01* | 7.50 ± 0.01 | 7.47 ± 0.01* | 7.33 ± 0.02* | 7.64 ± 0.01* | 7.72 ± 0.02* | 7.67 ± 0.01* | 7.90 ± 0.01* | |
| 72 | 8.01 ± 0.00* | 8.28 ± 0.01* | 7.57 ± 0.01 | 7.77 ± 0.01* | 7.98 ± 0.01* | 7.74 ± 0.01* | 7.76 ± 0.01* | 7.89 ± 0.00* | 8.02 ± 0.00* | |
| Probiotic | Incubation time (h) | Banana powder concentration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4% | 5% | 6% | ||||||||
| Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | Saba powder | Pisang Awak Banana powder | Silver bluggoe powder | ||
|
Lactobacillus acidophilus CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.16 ± 0.02* | 7.61 ± 0.00* | 7.57 ± 0.01* | 7.39 ± 0.01* | 7.45 ± 0.04* | 7.84 ± 0.01* | 6.38 ± 0.02* | 7.44 ± 0.00* | 7.54 ± 0.00* | |
| 48 | 8.00 ± 0.01* | 7.67 ± 0.01* | 7.82 ± 0.01* | 7.82 ± 0.01* | 7.58 ± 0.07* | 7.95 ± 0.00* | 7.85 ± 0.00* | 7.78 ± 0.19* | 8.06 ± 0.01* | |
| 72 | 8.21 ± 0.01* | 7.99 ± 0.01* | 8.09 ± 0.00* | 8.24 ± 0.01* | 7.76 ± 0.01* | 8.16 ± 0.00* | 7.90 ± 0.01* | 7.93 ± 0.01* | 8.26 ± 0.00* | |
|
Lactobacillus casei CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.76 ± 0.01* | 7.82 ± 0.00* | 7.65 ± 0.01* | 7.70 ± 0.01* | 7.80 ± 0.00* | 7.59 ± 0.01* | 7.84 ± 0.00* | 7.68 ± 0.00* | 7.65 ± 0.00* | |
| 48 | 7.82 ± 0.00* | 7.89 ± 0.00* | 7.92 ± 0.00* | 7.82 ± 0.01* | 8.01 ± 0.00* | 7.91 ± 0.00* | 7.90 ± 0.00* | 7.75 ± 0.01* | 7.89 ± 0.01* | |
| 72 | 8.13 ± 0.00* | 8.08 ± 0.00* | 7.99 ± 0.00* | 8.07 ± 0.00* | 8.17 ± 0.00* | 8.21 ± 0.00* | 8.07 ± 0.00* | 7.79 ± 0.00* | 8.15 ± 0.00* | |
|
Lactobacillus fermentum CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.70 ± 0.01* | 7.83 ± 0.00* | 7.62 ± 0.01* | 7.70 ± 0.01* | 7.77 ± 0.00* | 7.74 ± 0.01* | 7.53 ± 0.01* | 7.73 ± 0.01* | 7.57 ± 0.01* | |
| 48 | 7.96 ± 0.00* | 8.18 ± 0.01* | 8.04 ± 0.00* | 7.82 ± 0.01* | 7.83 ± 0.01* | 7.76 ± 0.01* | 7.69 ± 0.01* | 7.76 ± 0.01* | 7.82 ± 0.01* | |
| 72 | 8.18 ± 0.00* | 8.19 ± 0.00* | 8.18 ± 0.00* | 8.07 ± 0.00* | 7.86 ± 0.01* | 8.07 ± 0.01* | 7.73 ± 0.00* | 8.06 ± 0.01* | 7.87 ± 0.01* | |
|
Streptococcus thermophiles CFU/mL |
0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24 | 7.41 ± 0.02* | 7.44 ± 0.01* | 7.42 ± 0.02* | 7.42 ± 0.02* | 7.16 ± 0.00* | 7.42 ± 0.02* | 7.29 ± 0.02* | 7.37 ± 0.01* | 7.73 ± 0.01* | |
| 48 | 7.64 ± 0.01* | 7.58 ± 0.01* | 7.63 ± 0.02* | 7.67 ± 0.01* | 7.77 ± 0.01* | 7.71 ± 0.01* | 7.74 ± 0.01* | 7.71 ± 0.01* | 7.74 ± 0.01* | |
| 72 | 7.67 ± 0.01* | 8.17 ± 0.00* | 7.94 ± 0.00* | 7.75 ± 0.01* | 8.12 ± 0.01* | 8.14 ± 0.00* | 7.97 ± 0.01* | 8.32 ± 0.01* | 7.77 ± 0.01* | |
*p ≤ 0.05 denotes an increase of described parameter. A significant increase of probiotic numbers from baseline (0 h fermentation)
Relationship of selected plant as a carbohydrate source and growth curves, pH change of each probiotic strain and pathogenic bacterial strains
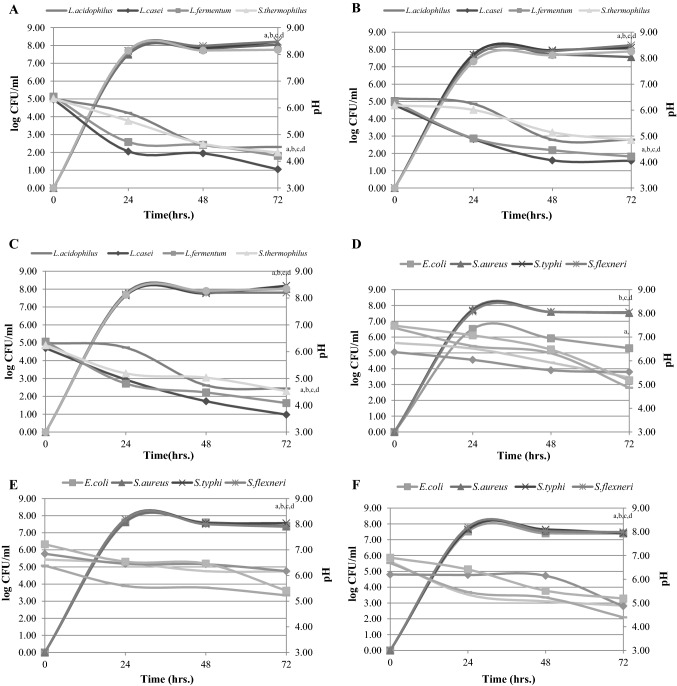
Increases in the log number of growth curves of each probiotic strain in each kind of banana were revealed from 0 to 72 h. At 24 h fermentation, the log number showed an increase in number from 0 to log 7.31 ± 0.01–7.76 ± 0.01 CFU/mL and gradually increased until 72 h (log 7.56 ± 0.01–8.25 ± 0.00 CFU/mL). There was no significant difference in the number of probiotic bacteria detected in response to different types of banana samples (p > 0.05). Moreover, changes in pH during the 0–72 h fermentation were observed and a significant decrease (p ≤ 0.05) for L. casei (pH 3.65 ± 0.01–4.05 ± 0.00 at 72 h) and L. fermentum (pH 4.08 ± 0.02–4.21 ± 0.04 at 72 h) was noticed in all banana samples (Fig. 2a–c), even though no significant difference in pH of probiotic bacteria was detected in response to different types of banana samples (p > 0.05). Therefore, a correlation could be made between the observed decrease in pH and the probiotic strains. Culture pH decrease was generally observed after fermentation for 24 h and continued decreasing until 72 h. This observation may be because some Lactobacillus spp. can produce cellulase and hemicellulase enzymes (Sharp et al. 1992); thus, carbohydrate from all banana samples can promote growth of probiotic strains. According to a previous study, raw bananas contain cellulose and hemicellulose. It has been reported that dietary fibre from banana contained high levels of hemicellulose and cellulose up to 42.27 ± 0.70 and 17.99 ± 0.48 g/100 g dry matter, respectively (Suksathit and Suksathit 2015).
Fig. 2.
Relationship of selected banana as a carbohydrate source and growth curves; pH change of each probiotic strain and pathogenic strain (n = 3). Relationship of each selected banana with each probiotic strain: a Saba powder, b Pisang Awak Banana powder, c Silver bluggoe powder, and each selected banana with each pathogenic strain, d Saba powder, e Pisang Awak Banana powder, f Silver bluggoe powder. Mean with difference superscript indicated significantly different from baseline (0 h fermentation) at p ≤ 0.05
Increased log number of each pathogenic bacterial strain was observed early, from 0 to 24 h (log 6.52 ± 0.02 –7.80 ± 0.02 CFU/mL) (Fig. 2d–f). However, at a later time, from 24 to 72 h fermentation, lowering of pathogen numbers was observed because the log number gradually decreased (log 5.30 ± 0.01–7.56 ± 0.01 CFU/mL), even though there was no evidence of a significant difference in log number in response to different types of banana sample. Furthermore, a significantly decreased culture pH (p ≤ 0.05) was observed in fermentation with S. aureus (pH 4.39 ± 0.01–5.23 ± 0.01) and S.flexneri (pH 5.17 ± 0.69–5.41 ± 0.97 at 72 h) in the presence of all types of banana samples (Fig. 2d–f). Nevertheless, there was no significant difference in pH value in response to different types of banana samples (p > 0.05). Similar findings have been observed by Fooks and Gibson (2002), that the culture pH effect on the growth of the pathogens when medium pH decreases to 3 or below can completely inhibit growth of E. coli and of C. jejuni and S. enteritidis at pH values of 4 and below (Fooks and Gibson 2002).
Antimicrobial activity cell free supernatants (CFS) from the fermented broth of probiotic strains cultured in selected plant powder
Banana samples showed other functional properties besides their prebiotic effect, such as anti-microbial activity to pathogenic bacterial strains, verified by an in vitro technique (Table 3). The results demonstrated that CFS from culture of L. casei supplemented with Saba powder and Pisang Awak Banana powder gave the significantly widest inhibition zones of 20.7 ± 2.3 and 20.3 ± 1.2 mm, respectively, to S. flexneri (p < 0.05). Secondly, CFS from culture of S. thermophiles supplemented with Silver bluggoe powder gave inhibition zones of 16.3 ± 0.6 mm to S. flexneri. Considering the culture system, it was found that L. casei and L. fermentum supplemented all types of banana samples, showing the most inhibition effect to all indicated pathogenic bacterial strains. Confirmatory evidence of pathogen inhibition was observed when the cell supernatant of L. plantarum and L. pentosus in combination of FOS, inulin and XOS (and mixtures thereof) have a significantly higher inhibitory effect on E. coli, C. jejuni and S. enteritidis by disc assay technique (Fooks and Gibson 2002). It was proposed that lactobacilli spp. were best known to utilize prebiotics in the gastrointestinal tract, based on the fact that they contain relatively high amounts of β-fructosidase, β-fructofuranosidases and glycotransferases, respectively, which enable them to break down polymers of a prebiotic into smaller units and make it available as a substrate (Lee et al. 2002; Vergara et al. 2010). In fact, a number of mechanisms that probiotic bacteria use to suppress the growth of pathogens have been proposed: decreasing the luminal pH via the production of volatile short-chain fatty acids; rendering specific nutrients unavailable to pathogens; and/or producing specific inhibitory compounds mainly due to organic acids, such as lactic acid, formic acid and the acetic acids reuterin, proteinaceous compounds, cyclic dipeptides, ethanol, acetone, hydrogen peroxide, diacetyl and bacteriocins (Delgado et al. 2001; Oliveira et al. 2008). In the normal intestinal flora, these mechanisms are essential to the component bacterial populations as a means of gaining advantage over competing bacteria. Therefore, we can conclude that banana can be used as a prebiotic, which can be a good food source for all types of probiotic bacteria, regardless of the type of banana.
Table 3.
Inhibitory activity of cell-free supernatants (CFS) from fermented broth of probiotic strains cultured in selected plant (n = 3)
| Probiotic | Prebiotic plant | Escherichia coli | Staphylococcus aureus | Salmonella typhi | Salmonella flexneri | Total inhibition |
|---|---|---|---|---|---|---|
| Lactobacillus acidophilus | Saba powder | 9.7 ± 0.6 | – | – | – | 1 |
| Pisang Awak Banana powder | 9.3 ± 0.6 | – | 11.0 ± 1.0 | – | 2 | |
| Silver bluggoe powder | 8.7 ± 0.6 | – | 10.0 ± 0.0 | – | 2 | |
| Lactobacillus casei | Saba powder | 12.0 ± 1.0 | 9.7 ± 2.5 | 13.0 ± 1.0 | 20.7 ± 2.3 | 4 |
| Pisang Awak Banana powder | 11.3 ± 0.6 | 11.3 ± 0.6 | 14.0 ± 0.0 | 20.3 ± 1.2 | 4 | |
| Silver bluggoe powder | 11.0 ± 1.0 | – | 9.3 ± 0.6 | 12.3 ± 0.6 | 3 | |
| Lactobacillus fermentum | Saba powder | – | 11.0 ± 1.0 | 11.0 ± 2.0 | 10.0 ± 1.0 | 3 |
| Pisang Awak Banana powder | – | 10.7 ± 0.6 | 11.0 ± 1.0 | 10.0 ± 1.0 | 3 | |
| Silver bluggoe powder | – | 11.0 ± 1.0 | 12.3 ± 0.6 | 11.7 ± 1.5 | 3 | |
| Streptococcus thermophilus | Saba powder | – | 8.7 ± 0.6 | 10.0 ± 2.0 | 12.0 ± 1.0 | 3 |
| Pisang Awak Banana powder | – | – | 11.7 ± 0.6 | 12.0 ± 1.0 | 2 | |
| Silver bluggoe powder | – | 9.3 ± 0.6 | 11.3 ± 0.6 | 16.3 ± 0.6 | 3 |
Conclusion
Carbohydrate content in the banana, which, as used in this study, showed prebiotic properties, which included water- and oil-holding ability, antioxidant contenting, resistance to α-amylase and trypsin, the capability of stimulating the growth of lactobacilli and inhibiting the growth of pathogenic bacteria. Thus, these bananas are a potential source of prebiotics, which may be used as an ingredient in functional food and nutraceutical products.
Acknowledgments
The author (P. Powthong) would like to express her sincere gratitude and profound appreciation to Research Institute, Rangsit University, under grant number 56/2559, for providing funds to P. Powthong. The authors would also like to extend their thanks to Dr. Kamlai Laohaphatanalert for kindly providing banana samples.
Compliance with ethical standards
Conflict of interest
The work described in the manuscript is original. It has never been presented, submitted or published in any publication before and does not encroach on any patents.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abatenh E, Gizaw B, Tsegay Z, Tefera G, Aynalem E. Health benefits of probiotics. J Bacteriol Infec Dis. 2018;2(1):8–27. [Google Scholar]
- Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey epidemiologic follow up study. Am J Clin Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- Budhisatria R, Jap R, Jan TT. In vitro and in vivo prebiotic activities of purified oligosaccharides derived from various local bananas (Musa sp.): Tanduk, Uli, Raja Sereh, and Cavendish. Microbiol Indonesia. 2017;11(2):55–61. doi: 10.5454/mi.11.2.3. [DOI] [Google Scholar]
- Buranawit K, Laenoi W. Effects of supplementing banana (Musa spp.) as prebiotic, probiotic (Toyocerin®) and their combination on growth performance, Carcass and meat quality in broilers. KKU Res J. 2015;20(4):419–427. [Google Scholar]
- Delgado A, Brito D, Fevereiro P, Marques JF. Antimicrobial activity of L. plantarum, isolated from atraditional lactic acid fermentation of table olives. Lait. 2001;81:203–215. doi: 10.1051/lait:2001124. [DOI] [Google Scholar]
- do Prado Cordoba L, da Silva RG, de Souza Gomes D, Schnitzler E, Waszczynskyj N. Brazilian green banana. J Therm Anal Calorim. 2018;134:2065–2073. doi: 10.1007/s10973-018-7374-9. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hhamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Falcomer AL, Riquette RFR, de Lima BR, Ginani VC, Zandonadi RP. Health benefits of green banana consumption:a systematic review. Nutrients. 2019;11(6):1–22. doi: 10.3390/nu11061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooks JL, Gibson RG. In vitro investigations of the effect of probiotics and prebiotics on selected human intestinal pathogens. FEMS Microbiol Ecol. 2002;39:67–75. doi: 10.1111/j.1574-6941.2002.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Gorecka D, Lampart-Szczapa E, Janitz W, Sokolowska B. Composition of fractional properties of dietary fibre of lupines (L. luteus and L. albus) Nahrung. 2000;44(4):229–232. doi: 10.1002/1521-3803(20000701)44:4<229::AID-FOOD229>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jenie SBSL, Saputra YM, Widaningrum D. Sensory evaluation and survival of probiotics in modified banana flour yoghurt during storage. J Teknol dan Industri Pangan. 2013;24(1):40–48. doi: 10.6066/jtip.2013.24.1.40. [DOI] [Google Scholar]
- Johnson CD, Schmit GD. Mayo clinic gastrointestinal imaging review. Rochester: Mayo Clinic Scientific Press; 2005. [Google Scholar]
- Lee HW, Park YS, Jong JS, Shim WS. Chitosan oligosaccharides, dp 2-8, have prebiotic effect on the Bifidobacterium bifidum and Lactobacillus sp. Anaerobe. 2002;8:319–324. doi: 10.1016/S1075-9964(03)00030-1. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mishra S, Satur N. Banana and its byproduct utilization: an overview. J Sci Ind Res. 2010;69:323–329. [Google Scholar]
- Oliveira RBP, de Oliveira LA, Gloria MBA. Screening of lactic acid bacteria from vacuum packaged beef for antimicrobial activity. Braz J Microbiol. 2008;39:368–374. doi: 10.1590/S1517-83822008000200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padam BS, Tin HS, Chye FY, Abdullah MI. Banana by-products: an under utilized renewable food biomass with great potential. J Food Sci Technol. 2014;51:3527–3545. doi: 10.1007/s13197-012-0861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquette RFR, Ginani VC, dos Santos Leandro E, de Alencar ER, Maldonade IR, de Aguiar LA, de Souza Acácio GM, Mariano DRH, Zandonadi RP. Do production and storage affect the quality of green banana biomass? LWT. 2019;111:190–203. doi: 10.1016/j.lwt.2019.04.094. [DOI] [Google Scholar]
- Romero-López MR, Osorio-Díaz P, Flores-Morales A, Robledo N, Mora-Escobedo R. Chemical composition, antioxidant capacity and prebiotic effect of aguamiel (agave atrovirens) during in vitro fermentation. Rev Mex de Ingenierıa Quımica. 2015;14(2):281–292. [Google Scholar]
- Sangnark A, Noomhorm A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003;80:221–229. doi: 10.1016/S0308-8146(02)00257-1. [DOI] [Google Scholar]
- Sarawong C, Schoenlechner R, Sekiguchi K, Berghofer E, Ng PKW. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014;143:33–39. doi: 10.1016/j.foodchem.2013.07.081. [DOI] [PubMed] [Google Scholar]
- Sharp R, O’Donnell AG, Gilbert HG, Hazlewood GP. Growth and survival of genetically manipulated Lactobacillus plantarum in silage. Appl Environ Microbiol. 1992;58:2517–2522. doi: 10.1128/AEM.58.8.2517-2522.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southon S. Increased fruit and vegetable consumption within the EU: potential health benefits. Food Res Int. 2000;33:211–217. doi: 10.1016/S0963-9969(00)00036-3. [DOI] [Google Scholar]
- Sowbhaya HB, Sampathu SR, Krishnamurthy N. Evaluation of size reduction on the yield and quality of celery seed oil. J Food Eng. 2007;80:1255–1260. doi: 10.1016/j.jfoodeng.2006.09.019. [DOI] [Google Scholar]
- Suksathit P, Suksathit S. Chemical composition, functional Properties and preliminary prebiotic properties of dietary fiber and resistant starch from green Banana ‘Kluay Khai’ peel and pulp. King Mongkut’s Univ Technol Thonburi J. 2015;33:49–60. [Google Scholar]
- Synytsya A, Mickova K, Synytsya A, Jablonsky I, Spevacek J, Erban V, Kovarikova E, Copikova J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohydr Polym. 2009;76:548–556. doi: 10.1016/j.carbpol.2008.11.021. [DOI] [Google Scholar]
- Thammarutwasik P, Hongpattarakere T, Chantachum S, Kijroongrojana K, et al. Prebiotics—a review. Songklanakarin J Sci Technol. 2009;31(4):401–408. [Google Scholar]
- Vergara CMAC, Honorato TL, Maia GA, Rodrigues S. Prebiotic effect of fermented cashew apple (Anacardium occidentale L) juice. LWT-Food Sci Technol. 2010;43:141–145. doi: 10.1016/j.lwt.2009.06.009. [DOI] [Google Scholar]
- Wall MM. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J Food Compos Anal. 2006;19:434–445. doi: 10.1016/j.jfca.2006.01.002. [DOI] [Google Scholar]
- Whitham FH, Blaydes DH, Devin RM, Van D. Experiments in plant physiology. New York: Nostrand company; 1971. p. 245. [Google Scholar]
- Wichienchot S, Jatupornpipat M, Rastall RA. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010;120:850–857. doi: 10.1016/j.foodchem.2009.11.026. [DOI] [Google Scholar]
- Younis K, Ahmad S*, Jahan K. Health benefits and application of prebiotics in foods. J Food Process Technol. 2015;6(4):1–7. [Google Scholar]