Abstract
Purpose
The aim of the study was to determine the efficacy of whole body vibration (WBV) therapy on pain, neuropathy disability score, balance, proprioception and quality of life (QOL) in patients with painful diabetic peripheral neuropathy (PDPN).
Methods
Twenty-six (16 males and 10 females) patients with PDPN were selected on the basis of inclusion and exclusion criteria. Subjects were randomly allocated to an experimental group (n = 13, age = 60.69 ± 5.08) and a control group (n = 13, age = 59.54 ± 4.25). The experimental group was given WBV therapy for six weeks (3 days/week) in addition to standard medical care, dietary advice and lifestyle modifications. Control group was provided only standard medical care, dietary advice and lifestyle modifications. Outcome measures included numeric pain rating scale (NPRS), Leeds assessment of neuropathic symptoms and signs (LANSS), vibration perception threshold (VPT), neuropathy disability score (NDS), proprioception, single-leg stance test (SLST), timed up and go test (TUGT) and short form 36 questionnaire (SF-36).
Results
NPRS, LANSS, NDS, SLST and TUGT showed significant time effect (p ≤ 0.022) and time×group interaction (p ≤ 0.007), whereas group effect was found to be significant only in LANSS (p = 0.001). VPT showed significant group effect (p ≤ 0.045) and time×group interaction (p ≤ 0.007) at great toe, metatarsal head and total average score. SF-36 was found to be significant time effect (p ≤ 0.024) in all domains except limitations due to physical health (p = 0.461). SF-36 average score was found be significant for group effect (p = 0.002) and time×group interaction (p < 0.001).
Conclusion
WBV improves sensory sensations like pain and vibration perception, neuropathy disability score, balance measures and health-related QOL in PDPN.
Keywords: Diabetic neuropathy, Neuropathy disability score, Single leg stance, Timed up and go, SF-36
Introduction
Painful diabetic peripheral neuropathy (PDPN) is one of the long-term complications of diabetes mellitus. The prevalence of PDPN varies from 26.4 to 65.3% in all diabetes patients [1, 2]. Davies et al. found that every sixth diabetic patient is affected with neuropathic pain associated to long-standing peripheral neuropathy [1]. Annual health care cost for patients with PDPN has been observed to be almost three times higher than the matched control populations [3], and it increases to four times in populations with severe painful peripheral neuropathy [4].
Neuropathic pain was diagnosed in PDPN patients by using Leeds assessment of neuropathic symptoms and signs (LANSS) score [5]. It has been validated to identify patients suffering from dominant neuropathic pain among chronic pain patients [6]. PDPN patients imposes moderate to high levels of pain, interference with function and work/activity limitations [7].
PDPN has a major impact on physical and mental functioning, thereby compromising the ability to work, attend to household responsibilities and enjoy social relationships [8]. Up to 44% of patients with PDPN show symptoms of depression compared with 26% of patients with painless DPN and 10% diabetics without neuropathy [9]. PDPN has substantial detrimental effect on the quality of life (QOL) as it often associates with other problems such as loss of physical function, anxiety, depression, disturbed sleep and impaired cognition [10]. Benbow et al. found that QOL was more impaired in patients with PDPN when compared with non-painful DPN and healthy controls, and it was reported that patients with PDPN had more impaired in physical mobility, emotional reactions, energy, pain and sleep measures [11]. PDPN individuals report interference with sleep, mobility, employment, recreational activities, social relations and enjoyment of life [12]. Although neuropathic pain in diabetes has not been determined as a significant cause for mortality, but severe chronic pain was found association with increases risk of mortality [13]. However, mortality attributed to pain can be traced to analgesic overdose and suicides caused by comorbid depression [14].
Whole body vibration (WBV) therapy is used for the management of patients with type 2 diabetes mellitus [15, 16]. It is an effective tool for increasing mobility, balance and exercise capacity in older adults with type 2 diabetes mellitus [17, 18]. WBV therapy produces neuromuscular activation of leg muscular structure in response to an acute progressive vibratory stimulus [19]. A pilot study showed reduction in pain levels on both visual analog scale (VAS) and neuropathic pain scale (NPS) after WBV therapy in patients with PDPN [20]. Another pilot study by Stambolieva et al. showed that eight weeks of plantar vibratory stimulation improved pain, tingling and weakness in patients with DPN [21]. Many of these pilot studies did not have either control groups or the credibility attached with randomized controlled trial. However, a quasi-randomized controlled trial conducted by Yoosefinejad et al. found that WBV improved balance and muscle strength in patients with DPN [22]. Hence, there is the need for more research concerning the effect of WBV on pain, disability and balance measures in PDPN population. Also, while WBV has been shown to improve health-related QOL in older population [23], patients with metabolic syndrome [24], and other chronic conditions [25]. To the best of our knowledge, the effect of WBV on health-related QOL in patient with PDPN remains largely unexplored.
Therefore, the aim of the present study was to evaluate the effect of WBV therapy on pain, neuropathy disability score (NDS), proprioception, balance measures and health-related QOL in patients with PDPN.
Methods
Participants
A total of 26 subjects, aged between 50 and 70 years, who were referred to the Diabetic Centre, Ansari Medical Centre and outpatient department, Centre for Physiotherapy and Rehabilitation Sciences, Jamia Millia Islamia (a central university) were selected. Diabetic patients with symptoms of distal symmetrical sensorimotor polyneuropathy with pain for ≥6 months [26], NDS ≥3, LANSS ˃12, and who could stand on both their feet were included in the study. Participants with a history of diabetic complications such as advanced cardiovascular, renal, or hepatic diseases; diabetic retinopathy; nephropathy; open wounds/ulcers on the weight bearing surface of the feet; and who were unable to ambulate independently were excluded.
After the procedure and possible risks were explained, written consent was taken in English/Hindi from each patient who participated in the study. Ethical clearance for the study was obtained from the Institutional Ethics Committee of Jamia Millia Islamia, New Delhi, India, and the study was conducted in accordance with the Helsinki Declaration, 1964.
Design
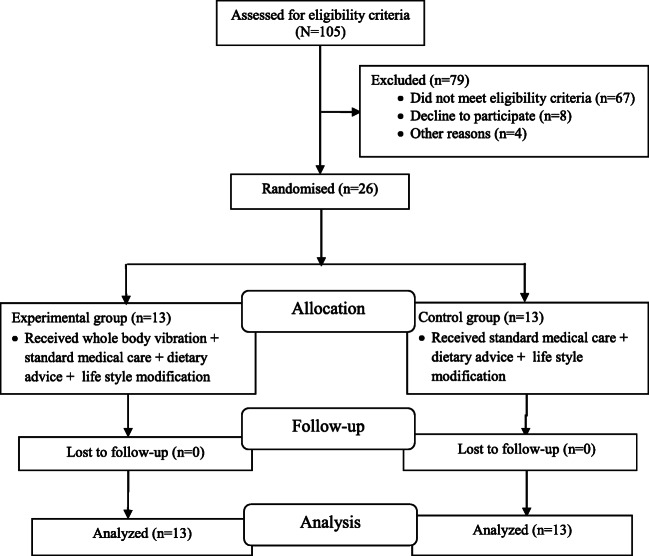
The study was a two arm, parallel-group randomized controlled trial with single blinding (blinding of outcome assessor). Subjects who met inclusion and exclusion criteria were randomly allocated to either an experimental group (n = 13) or the control group (n = 13) using 1:1 ratio through the coin method [27]. Both groups were assessed at baseline and after six weeks. Enrollment and assignment of patients were done by an investigator who was neither part of assessment nor implementation of exercise. Study design is presented in Fig. 1.
Fig. 1.
Flow chart of the study
Procedure
Baseline measurements of pain, NDS, proprioception, static and dynamic balance and health related QOL were recorded for all subjects. Both groups continued their standard medical care, dietary advice and lifestyle modifications during the course of study. Additionally, subjects of the experimental group received WBV therapy thrice a week for six weeks. Prior to the first session of WBV therapy, subjects were allowed to get familiar with the procedure. At the end of the six week program, subjects were assessed again for the same variables to analyze the difference from baseline measurements.
Interventions
Each session comprised of warm-up followed by WBV therapy. Warm-up included self-static stretching for hamstring, gastrocnemius, and quadriceps muscles, body twist and stationary cycling for 5–10 min. Each subject received WBV therapy under supervision of one of the researchers. Participants were trained by standing on the vibration platform (KH 75 Crazy Fit, VIVA Fitness, India) with 12 Hz frequency and 5 mm amplitude [18]. Subjects were asked to stand barefoot on the platform evenly distributing their body weight on both feet with knees bent at 20 degree as flexing of knees is a common postural adaptation used to minimize transmission of vibration to the head [20]. All participants received four bouts of three minute sessions with 60 s rest between bouts, three times a week. This was continued for six weeks. During the six week study period, subjects in the control group did not receive WBV therapy or any other form of physical rehabilitation or exercise program.
Outcome measurements
Numeric pain rating scale (NPRS) is a segmented numeric version of the visual analog scale (VAS) in which the respondent selects a number from 0 to 10 that best reflects the intensity of his/her pain [28]. It is described as an 11-point scale with scores from ‘0’ representing no pain to ‘10’ representing extreme pain (e.g., ‘pain as bad as you can imagine’ or ‘worst pain imaginable’). The test–retest reliability for the NPRS has been demonstrated to be moderate to high; ICC value varying from 0.67 to 0.96 [29, 30].
LANSS pain scale comprises of questionnaire and sensory testing with seven items; the highest achievable score is 24. The pain questionnaire includes sensations such as pricking, tingling, pins and needles, skin discoloration, light touch pain, electric shocks, jumping and bursting, and feeling of altered skin temperature including hot and burning [31]. Sensory testing includes allodynia and altered pinprick threshold. If the pain symptom is consistent with the description, the subjects answer ‘yes’, and if the pain symptom is inconsistent, the subjects answer ‘no’. The cut-off value is 12. If the total score is ≥12, neuropathic mechanisms could be responsible for the pain experienced by the patient. LANSS has good sensitivity and specificity range from 80.17 to 94.29% and 88.57 to 100% respectively [5, 32].
Vibration perception threshold (VPT) was evaluated using a digital biothesiometer-vibrometer (Diabetik Foot Care India Pvt. Limited). Patients were asked to lay prone with foot out of the bed. The biothesiometer probe was applied perpendicularly without any pressure (the weight of the biothesiometer probe provides the required pressure) to the site of the testing. Patients were familiarized about feeling of first vibration sensation on their distal palmar surface of the hand. VPT was then measured at the distal plantar surface of great toe, base of first, third, fifth metatarsals, midpoint of medial arch and heel. The voltage was slowly increased at the rate of 1 mV/s and VPT was recorded when the subject indicated the first felt vibration sense. VPT at great toe, base of first metatarsal and the average of all six sites were recorded for the analysis. VPT has shown excellent reliability (ICC = 0.93) and validity (sensitivity 86% and specificity 83%) in previous studies [33, 34].
NDS consists of vibration perception (check by means of a 128 Hz tuning fork), pin-prick and temperature perceptions in the great toe, and the presence or absence of ankle reflexes. The sensory modalities were scored as either present (0) or reduced or absent [1] for each leg; ankle reflexes were scored as normal (0), present with reinforcement [1], or absent [2] for each leg. The total maximum abnormal score is 10. NDS is an acceptable, reproducible and validated tool for measuring DPN [35].
Proprioception was examined using Pedalo®-Sensamove Balance-test Pro with miniboard. Miniboard comprises of a circular board with hemispherical sensors below the board. Subjects were asked to stand on the miniboard with a cushion placed below; they were then asked to tilt as much as they could in four directions (front, back, left and right), called maximal tilting angle. The reference stimulus lies within maximal tilting angle. Each subject was asked to move his/her center of pressure on the given coloured spot with the help of a marker displayed on the screen and asked to remember the spot. The subject was then asked to reach the given coloured spot without the marker on the screen. The difference in the angle between the reference and the actual position was measured and recorded. Prior to actual testing, patients were familiarized with the procedure. The computer screen was kept at eye level. The reliability of the device has been reported in older subjects with ICC value 0.91 [36].
Single leg stance test (SLST) is a commonly used measure of postural balance capabilities, and is also a significant predictor of falling in peripheral neuropathy [37]. The subjects were tested first with eyes open and then with eyes close on the dominant limb. They were asked to stand on their dominant leg and maintain the position as long as they could. A digital stopwatch was used to measure time as this approach has previously been shown to exhibit near perfect inter-rater reliability [38]. The test was terminated till the raised foot touched the ground. Three trials of SLST were performed and the highest score was recorded for analysis.
Timed up and go test (TUGT) is a balance test used to examine functional mobility in community-dwelling frail older adults. Many studies have already shown high intra-rater and inter-rater reliability [39, 40]. In this test, the patient had to stand up from a stable chair, walk at a regular pace for 3 m, turn around, walk back to the chair and sit down. The subjects were allowed to wear their regular footwear and can use their gait aid that they normally used during ambulation. Before performing the test they were first allowed to get familiar with the procedure. Average of three readings was recorded.
The Medical Outcomes Study Short Form 36 (SF-36) involves eight domains of general health: physical function, social function, role–emotional, role–physical, mental health, vitality, pain and general health. The maximum score in each domain is 100 and a high score is desirable and indicative of better well-being or less pain. Changes in score of 5 units have been shown to be clinically relevant [41]. SF-36 has shown consistently high levels of reliability (test-retest, internal consistency) and validity (content, concurrent, criterion, construct, predictive) [42].
Sample size
The number of subjects was determined using Software G*Power 3.1.9.2, using data of changes in physical function component of SF-36 questionnaire from the study done by Bruyer et al. [43]. A total of 13 subjects (including 10% of drop-outs) per group was shown to be necessary based on the effect size of 1.25, alpha level of 0.05 and power (1-beta) of 0.80.
Statistical analysis
Data was analyzed using SPSS Version 21. Shapiro Wilk test was used to assess the normality of distribution for all the outcome measures. Variables that were found to be non-normal were log transformed for further analysis. Demographic characteristics and outcome variables were compared at baseline using independent t-test. 2 × 2 mixed model ANOVA was used considering within-group factors (baseline and post-intervention values) and between-group factors (experimental and control groups) to find out the main effect (time and group effect) and time×group interaction. p ≤ 0.05 was considered to be statistically significant. Data are presented as mean ± SD, unless otherwise indicated.
Results
Demographic characteristics are reported in Table 1. There was no statistically significant difference for demographic variables between the groups. NPRS, LANSS, VPT, NDS, proprioception, SLST, TUGT and SF-36 showed no significant difference between the groups at baseline (Table 1).
Table 1.
Comparison of demographic characteristics and outcome measures at baseline
| Variables | Experimental group (n = 13) | Control group (n = 13) | Independent t-test |
|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | (p- value) | |
| Age (years) | 60.69 ± 5.08 | 59.54 ± 4.25 | 0.536 |
| Gender (male/female) | 9/4 | 7/6 | |
| Height (m) | 162.23 ± 7.2 | 162.23 ± 7.55 | 1 |
| Weight (kg) | 69.38 ± 3.3 | 69.53 ± 4.31 | 0.92 |
| BMI (kg/m2) | 26.48 ± 2.4 | 26.52 ± 2.31 | 0.966 |
| FBG (mg/dL) | 139.23 ± 16.36 | 134.3 ± 15.86 | 0.444 |
| SBP (mm Hg) | 129.61 ± 14.2 | 128.07 ± 11.99 | 0.768 |
| DBP (mm Hg) | 81.92 ± 8.04 | 84.23 ± 8.37 | 0.481 |
| NPRS | 5.46 ± 2.33 | 5.07 ± 2.13 | 0.665 |
| LANSS | 15.92 ± 3.68 | 18.23 ± 4.28 | 0.154 |
| Vibration perception threshold | |||
| Total | 22.15 ± 5.41 | 28.23 ± 12.99 | 0.133 |
| Great toe | 21.61 ± 6.99 | 29.61 ± 13.94 | 0.077 |
| Metatarsal head | 22.61 ± 6.23 | 27.15 ± 12.81 | 0.262 |
| Neuropathy disability score | 7.07 ± 1.84 | 6.46 ± 1.98 | 0.421 |
| Proprioception (angle difference) | |||
| Front | 7.78 ± 4.8 | 8.38 ± 4.24 | 0.739 |
| Back | 12.03 ± 8.3 | 12.14 ± 9.22 | 0.975 |
| Left | 11.5 ± 6.83 | 11.52 ± 7.64 | 0.994 |
| Right | 9.63 ± 6.92 | 10.63 ± 6.84 | 0.717 |
| Single leg stance EO (sec) | 5.61 ± 3.12 | 7.07 ± 3.14 | 0.246 |
| Single leg stance EC (sec) | 3.3 ± 1.49 | 3 ± 1.77 | 0.637 |
| Time up and go test (sec) | 15.46 ± 4.57 | 13.61 ± 3.12 | 0.241 |
| SF-36 | |||
| Average score | 33.76 ± 15.7 | 32.02 ± 7.48 | 0.723 |
| Physical functioning | 38.84 ± 19.16 | 31.61 ± 12.73 | 0.268 |
| Limitations (physical health) | 34.61 ± 19.19 | 32.69 ± 12 | 0.762 |
| Limitations (emotional problems) | 35.89 ± 25.32 | 30.76 ± 16.45 | 0.546 |
| Energy/Fatigue | 26.92 ± 19.09 | 29.61 ± 16.76 | 0.706 |
| Emotional well being | 45.84 ± 26.81 | 29.5 ± 13.94 | 0.063 |
| Social functioning | 30.05 ± 18.6 | 34.78 ± 14.15 | 0.473 |
| Pain | 32.49 ± 14.54 | 33.43 ± 12.85 | 0.863 |
| General health | 27.29 ± 18.54 | 33.45 ± 12.61 | 0.332 |
BMI body mass index, FBG fasting blood glucose, SBP systolic blood pressure, DBP diastolic blood pressure, NPRS numeric pain rating scale, LANSS leeds assessment of neuropathic symptoms and signs, SF-36 36-Item short form survey, EO eyes open, EC eyes closed
NPRS, LANSS and NDS showed significant time effect (p ≤ 0.016) and time×group interaction (p ≤ 0.007), whereas group effect was found to be significant only in LANSS (p = 0.001). VPT at all sites showed no significant difference in time effect whereas group effect (p ≤ 0.045) and time×group interaction (p ≤ 0.007) were found to be significant (Table 2).
Table 2.
Changes in the Pain, VPT, NDS, Proprioception and Balance after Six Weeks of Intervention
| Variables | Intervention group (n = 13) | Control group (n = 13) | Time (T) effect | Group (G) effect | T × G effect | ||
|---|---|---|---|---|---|---|---|
| Baseline | 6th week | Baseline | 6th week | ηp2 (p value) | ηp2 (p value) | ηp2 (p value) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| NPRS | 5.46 ± 2.33 | 2.92 ± 1.55 | 5.07 ± 2.13 | 5.46 ± 1.94 | 0.3 (0.004)* | 0.08 (0.145) | 0.44 (<0.001)* |
| LANSS | 15.92 ± 3.68 | 10.23 ± 3.7 | 18.23 ± 4.28 | 18.84 ± 3.93 | 0.42 (<0.001)* | 0.38 (0.001)* | 0.53 (<0.001)* |
| Vibration perception threshold | |||||||
| Total | 22.15 ± 5.41 | 17.53 ± 4.96 | 28.23 ± 12.99 | 29.38 ± 13.29 | 0.13 (0.062) | 0.18 (0.028)* | 0.31 (0.003)* |
| Great toe | 21.61 ± 6.99 | 18.07 ± 6.53 | 29.61 ± 13.94 | 30.46 ± 13.66 | 0.12 (0.083) | 0.19 (0.023)* | 0.26 (0.007)* |
| Metatarsal head | 22.61 ± 6.23 | 17.69 ± 3.88 | 27.15 ± 12.81 | 29 ± 12.89 | 0.11 (0.095) | 0.15 (0.045)* | 0.37 (0.001)* |
| Neuropathy disability score | 7.07 ± 1.84 | 5.84 ± 1.57 | 6.46 ± 1.98 | 6.53 ± 1.85 | 0.21 (0.016)* | 0.00 (0.955) | 0.26 (0.007)* |
| Proprioception | |||||||
| Front | 7.78 ± 4.8 | 6.8 ± 5.67 | 8.38 ± 4.24 | 6.94 ± 3.62 | 0.07 (0.172) | 0.002 (0.819) | 0.002 (0.794) |
| Back | 12.03 ± 8.3 | 8.73 ± 7.97 | 12.14 ± 9.22 | 8.98 ± 4.91 | 0.11 (0.095) | 0.001 (0.941) | 0.00 (0.969) |
| Left | 11.5 ± 6.83 | 7.4 ± 5.04 | 11.52 ± 7.64 | 13.95 ± 6.14 | 0.01 (0.625) | 0.11 (0.099) | 0.13 (0.063) |
| Right | 9.63 ± 6.92 | 5.53 ± 2.72 | 10.63 ± 6.84 | 10.83 ± 7.26 | 0.05 (0.263) | 0.11 (0.085) | 0.06 (0.217) |
| Single leg stance EO (sec) | 5.61 ± 3.12 | 8.23 ± 3.16 | 7.07 ± 3.14 | 6.84 ± 2.64 | 0.46 (<0.001)* | 0.001 (0.974) | 0.54 (<0.001)* |
| Single leg stance EC (sec) | 3.3 ± 1.49 | 4.69 ± 2.05 | 3 ± 1.77 | 2.53 ± 1.61 | 0.19 (0.022)* | 0.12 (0.074) | 0.49 (<0.001)* |
| Time up and go test (sec) | 15.46 ± 4.57 | 10.38 ± 3.09 | 13.61 ± 3.12 | 15.46 ± 4.57 | 0.26 (0.007)* | 0.05 (0.27) | 0.62 (<0.001)* |
*Significant difference
NPRS numeric pain rating scale, LANSS leeds assessment of neuropathic symptoms and signs, EO eyes open, EC eyes closed
Proprioception showed no significant time effect, group effect and time×group interaction in all directions. SLST was found to be significant for time effect (p ≤ 0.022) and time×group interaction (p < 0.001) during eyes open as well as eyes closed condition. TUGT was found significant for time effect (p = 0.007) and time×group interaction (p < 0.001) (Table 2).
SF-36 showed significant time effect (p ≤ 0.024) in all domains except limitations due to physical health (p = 0.461). Group effect was found to be significant in SF-36 average score (p = 0.002), physical functioning (p = 0.003), limitations due to physical health (p = 0.046), emotional problems (p = 0.045), emotional well-being (p = 0.001) and pain (p = 0.002). Time×group interaction (p < 0.001) was found to be significant in SF-36 average score, physical functioning, fatigue, social functioning, pain and general health (Table 3).
Table 3.
Changes in the SF-36 after Six Weeks of Intervention
| Variables | Intervention group (n = 13) | Control group (n = 13) | Time (T) effect | Group (G) effect | T × G effect | ||
|---|---|---|---|---|---|---|---|
| Baseline | 6th week | Baseline | 6th week | ηp2 (p value) | ηp2 (p value) | ηp2 (p value) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||||
| SF-36 | |||||||
| Average score | 33.76 ± 15.7 | 58.06 ± 13.11 | 32.02 ± 7.48 | 32.1 ± 6.32 | 0.62 (<0.001)* | 0.33 (0.002)* | 0.61 (<0.001)* |
| Physical functioning | 38.84 ± 19.16 | 63.88 ± 17.41 | 31.61 ± 12.73 | 33.15 ± 12.75 | 0.6 (<0.001)* | 0.31 (0.003)* | 0.54 (<0.001)* |
| Limitations (inadequate physical health) | 34.61 ± 19.19 | 44.23 ± 14.97 | 32.69 ± 12 | 28.84 ± 9.38 | 0.02 (0.461) | 0.15 (0.046)* | 0.11 (0.093) |
| Limitations (emotional problems) | 35.89 ± 25.32 | 57.36 ± 20.14 | 30.76 ± 16.45 | 33.33 ± 23.57 | 0.19 (0.023)* | 0.15 (0.045)* | 0.13 (0.067) |
| Fatigue | 26.92 ± 19.09 | 50.76 ± 15.25 | 29.61 ± 16.76 | 28.84 ± 11.02 | 0.38 (0.001)* | 0.11 (0.089) | 0.41 (<0.001)* |
| Emotional well being | 45.84 ± 26.81 | 63.69 ± 15.95 | 29.5 ± 13.94 | 35.13 ± 18.37 | 0.19 (0.024)* | 0.38 (0.001)* | 0.06 (0.223) |
| Social functioning | 30.05 ± 18.6 | 56.73 ± 14.97 | 34.78 ± 14.15 | 32.49 ± 14.46 | 0.32 (0.002)* | 0.13 (0.062) | 0.4 (<0.001)* |
| Pain | 32.49 ± 14.54 | 71.88 ± 15.23 | 33.43 ± 12.85 | 34.36 ± 14.95 | 0.76 (<0.001)* | 0.34 (0.002)* | 0.7 (<0.001)* |
| General health | 27.29 ± 18.54 | 49.35 ± 13.43 | 33.45 ± 12.61 | 30.69 ± 10.75 | 0.34 (0.002)* | 0.06 (0.207) | 0.46 (<0.001)* |
SF-36: 36 item short form survey; *Significant difference
Discussion
The present investigation revealed that WBV improves in the pain as well as vibration perception, neuropathy disability score, static and functional balance and each domain of SF-36 in patients with PDPN.
The results of the current study show significant decrease in pain as reflected in NPRS as well as in LANSS score in intervention group when compared with control group, indicating that WBV had beneficial effects on general as well as neuropathic pain. This findings was found consistent with findings of previous pilot studies that revealed significant reduction in VAS and NPS variables [20, 44].
A plausible mechanism by which WBV reduces pain is the gate control theory of pain given by Melzack and Wall [45]. According to Longe et al. [46], “The mechanism of action of these procedures is generally explained by the gate-control theory of pain inhibition in which large diameter sensory fibers (Aβ fibers) conducting impulses from the selective activation of low threshold mechanoreceptors, reduce the painful input of the small diameter nociceptive afferents (C fibers) by triggering local inhibitory circuits in the substantia gelatinosa of the dorsal horn.” Thus, chronic pain as reduced by WBV is a presynaptic inhibition of nociceptive stimuli at dorsal horn of the spinal cord. This theory was supported by the results of Armstrong et al. and Kipp et al., which showed depression of Hoffman reflex in healthy subjects after bouts of WBV, because Hoffman reflex directly arises from dorsal motor neurons [47, 48]. They explained that the decrease in Hoffman reflex was due to reduction in spinal excitability, which occurs due to presynaptic inhibition [47, 48]. Kessler and Hong described the above mechanism in detail as an acute and chronic effect of WBV [20]. Another mechanism is the adaptation in spinal reflex system, resulting in prolonged presynaptic inhibition in response to chronic exposure to vibration [49]. The basis for this theory is a phenomenon known as central sensitization, which involves increase in central nervous system reactivity to pain stimuli in cases of chronic pain disorders such as fibromyalgia and DPN [50].
VPT, in the present study was found to have decreased after the WBV intervention, which indicates that vibration receptors such as Merkel’s disk, Meissner’s corpuscle and Pacinian corpuscle are facilitated or activated at the level of sole. The cerebral cortex area contains vibro-tactile and pain sensations which are in proximity to one another [51]. The facilitation of vibration sensation influences the area of cerebral cortex receiving the pain sensation and this could be one more reason for the reduction in pain and facilitation of vibration sense. We suggest that the vibratory pain relief is not simply at the level of the dorsal horn but also in the cerebrum. Regardless of which mechanism is responsible, further research is needed to confirm the physiological effects of vibration.
NDS was used to grade the severity of neuropathy in peripheral neuropathy patients with diabetes, includes the assessment of sensation and reflexes. To best of our knowledge, this study is the first to show improvement in NDS after WBV intervention. Our study found improvement in the pin-prick sensitivity, thermal sensation and vibration sense, indicating WBV improves the sensitivity of the mechanoreceptors mediated by Merkel’s disc, Pacinian corpuscle and Ruffini endings. Improvement in the glycemic control or nerve function like nerve conduction velocity could be a reason for changes in the NDS. WBV improves fasting glucose and HbA1c level in patients with type 2 diabetes [15, 52]. A case study found increased sural sensory nerve conduction velocity in both lower limbs after WBV therapy [21]. HbA1c was linearly related with thermal sensations [53] and nerve conduction studies found good association with the NDS score [54].
The present investigation also revealed improvement in static and functional balance in experimental patients after WBV therapy, when compared with the control group patients. Following WBV therapy, patients improved in their SLST by 46.7% with eyes open and 42.12% with eyes closed as well as in TUGT by 32.85%, whereas deterioration of static as well as dynamic balance was observed in the control group. The results were in line with a previous study which found improvement in SLST with eyes open and eyes closed, and TUGT in type 2 diabetes patients with peripheral neuropathy [22, 52]. Stambolieva et al. and del Pozo-Cruz et al. also found that WBV improves static balance by improving postural stability [21, 55]. However, del Pozo-Cruz et al. found no significant difference in TUGT after WBV in type 2 diabetes [55, 56]. Other studies had shown improvement in TUGT score after WBV intervention in older population [43, 57].
Balance improvements following WBV can be attributed to vibration-induced sensory stimulation that activates tactile receptors in the soles, and mechanoreceptors in the skin and joints that provide the necessary information to improve balance [58]. We found no significant difference in proprioception after WBV, the given intervention supported the principle of specificity in patients with PDPN. We can say that the WBV is not capable of enhancing sensitivity of mechanoreceptors present in the muscle spindle and golgi tendon organs. So, the improvement in balance is due to tactile sense and muscle performance. Improvement in the SLST with eyes closed indicates that WBV decreases visual dependency for balance, and a probable explanation of this could be the vibratory noise enhancement of foot sole sensory information, which improves signal detection and transmission from the tactile sensors. Increase in lower limb muscular strength might be another reason that improved the SLST and TUGT results. Various other studies have also shown significant effects of vibration in improving lower-limb muscle strength [22, 59]. A previous publication has shown that muscle strength is an independent predictor of decreasing or loss of balance in older subjects [60]. Although the current study did not directly assess muscular performance while SLST and TUGT are used, it could be considered a surrogate assessment of muscle function.
WBV therapy improved scores in all domains, namely pain, general and mental health, vitality, physical, emotional and social functioning, of SF-36 QOL health survey. Existing evidence suggests that PDPN has a significant negative impact on QOL associated with depression and anxiety [7], and relief of neuropathic pain helps in improving patient-reported functioning and QOL in PDPN [61]. Studies have also shown that WBV improves health-related QOL in older population [23, 43] and other chronic conditions [25]. Yet, our study is the first to suggest that a controlled WBV intervention improved self-rated health-related QOL in patients with PDPN. The reason for this improvement could be the resultant improvement of pre-mentioned outcome variables. As already discussed, WBV decreases pain, which is the most distressing symptom of this population [7, 11, 61, 62], thus helping improve mental and emotional components of QOL. Further, due to the progress in static and functional balance measures, patients’ physical functioning is also enhanced. These physical and emotional components integrate to advance social engagement in patients with PDPN. Thus, after WBV therapy, participants experience an increase in their overall functional capability, indicating a multidimensional positive impact on health and fitness in patients with PDPN.
WBV proved to be beneficial in terms of neuropathic pain, neuropathy disability score, balance measures and QOL in patients with PDPN. For patients with advanced stages of neuropathy, who find it difficult to participate in any exercises programs, WBV therapy is a safer and feasible treatment to improve functional capacity in all domains of SF-36 questionnaire. The technique is very easy to use and has no adverse effect on neuropathic symptoms. Participants showed high compliance rate (100%) in our study suggesting that patients were satisfied with this form of therapy. We believe that it may serve a suitable method as an adjunct therapeutic intervention, along with gylcemic control methods for PDPN patients.
This study has several limitations. To begin with, it did not include follow-up after the intervention. Another limitation is the small sample size, which limits its power considering proprioception measures. There is also scope, to conduct studies in future to ascertain the effect of WBV on muscle activation pattern and nerve function, depending on different levels of neuropathy. Further, studies with longer treatment protocol, follow-up and large number of patients are needed to establish the long-term effects of WBV on the functional capacity and QOL in PDPN patients.
Conclusion
WBV therapy improves sensory sensation like pain and vibration perception, neuropathy disability score, and static and functional balance measures in PDPN. The intervention helps to improve the functional capacity in all domains of health-related QOL in patients with PDPN.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 2.Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(3):143–149. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complicat. 2015;29(2):212–217. doi: 10.1016/j.jdiacomp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Spanos K, Lachanas VA, Chan P, Bargiota A, Giannoukas AD. Validation of the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire and its correlation with visual analog pain scales in Greek population. J Diabetes Complicat. 2015;29(8):1142–1145. doi: 10.1016/j.jdiacomp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Koc R, Erdemoglu AK. Validity and reliability of the Turkish Self-Administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) questionnaire. Pain Med. 2010;11(7):1107–1114. doi: 10.1111/j.1526-4637.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Gore M, Brandenburg N, Tai K. Burden of illness in painful diabetic peripheral neuropathy (DPN): the patients’ perspectives. J Pain. 2005;6(3):S28. doi: 10.1016/j.jpain.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Nathan HJ, Poulin P, Wozny D, Taljaard M, Smyth C, Gilron I, et al. Randomized trial of the effect of mindfulness-based stress reduction on pain-related disability, pain intensity, health-related quality of life, and A1C in patients with painful diabetic peripheral neuropathy. Clin Diabetes. 2017;35(5):294–304. doi: 10.2337/cd17-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Amato C, Morganti R, Greco C, Di Gennaro F, Cacciotti L, Longo S, et al. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Vasc Dis Res. 2016;13(6):418–428. doi: 10.1177/1479164116653240. [DOI] [PubMed] [Google Scholar]
- 10.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Prim. 2017;3(1):17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benbow SJ, Wallymahmed ME, Macfarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733–737. doi: 10.1093/qjmed/91.11.733. [DOI] [PubMed] [Google Scholar]
- 12.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47(2):123–128. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 13.Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur J Pain. 2010;14(4):380–386. doi: 10.1016/j.ejpain.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl_2):S43–S48. doi: 10.1111/j.1526-4637.2011.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum K, Votteler T, Schiab J. Efficiency of vibration exercise for glycemic control in type 2 diabetes patients. Int J Med Sci. 2007;4(3):159–163. doi: 10.7150/ijms.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manimmanakorn N, Manimmanakorn A, Phuttharak W, Hamlin MJ. Effects of whole body vibration on glycemic indices and peripheral blood flow in type II diabetic patients. Malay J Med Sci. 2017;24(4):55–63. doi: 10.21315/mjms2017.24.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes-Neto M, da Cunha de Sá-Caputo D, Paineiras-Domingos LL, Brandão AA, Neves MF, Marin PJ, et al. Effects of whole-body vibration in older adult patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Can J Diabetes. 2019;43(7):524–529. doi: 10.1016/j.jcjd.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231(4):305–314. doi: 10.1620/tjem.231.305. [DOI] [PubMed] [Google Scholar]
- 19.Perchthaler D, Horstmann T, Grau S. Variations in neuromuscular activity of thigh muscles during whole-body vibration in consideration of different biomechanical variables. J Sports Sci Med. 2013;12(3):439–446. [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler NJ, Hong J. Whole body vibration therapy for painful diabetic peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2013;17(4):518–522. doi: 10.1016/j.jbmt.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Stambolieva K, Petrova D, Irikeva M. Positive effects of plantar vibration training for the treatment of diabetic peripheral neuropathy: a pilot study. Somatosens Mot Res. 2017;34(2):129–133. doi: 10.1080/08990220.2017.1332585. [DOI] [PubMed] [Google Scholar]
- 22.Kordi Yoosefinejad A, Shadmehr A, Olyaei G, Talebian S, Bagheri H, Mohajeri-Tehrani MR. Short-term effects of the whole-body vibration on the balance and muscle strength of type 2 diabetic patients with peripheral neuropathy: a quasi-randomized-controlled trial study. J Diabetes Metab Disord. 2015;14(1):45. doi: 10.1186/s40200-015-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santin-Medeiros F, Santos-Lozano A, Cristi-Montero C, Garatachea VN. Effect of 8 months of whole-body vibration training on quality of life in elderly women. Res Sport Med. 2017;25(1):101–107. doi: 10.1080/15438627.2016.1258638. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho-Lima RP, Sá-Caputo DC, Moreira-Marcon E, Dionello C, Paineiras-Domingos LL, Sousa-Gonçalves CR, et al. Quality of life of patients with metabolic syndrome is improved after whole body vibration exercises. Afr J Tradit Complement Altern Med. 2017;14(4S):59–65. doi: 10.21010/ajtcam.v14i4S.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Zhang G, Wang Y, Wang X, Zhou H, Li H, et al. The effect of whole body vibration on health-related quality of life in patients with chronic conditions: a systematic review. Qual Life Res. 2019;28(11):2859–2870. doi: 10.1007/s11136-019-02274-x. [DOI] [PubMed] [Google Scholar]
- 26.Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc. 2006;81(4 SUPPL):S3–11. doi: 10.1016/s0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 27.Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Rodriguez CS. Pain measurement in the elderly: a review. Pain Manag Nurs. 2001;2(2):38–46. doi: 10.1053/jpmn.2001.23746. [DOI] [PubMed] [Google Scholar]
- 29.Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10(2):123–128. [Google Scholar]
- 30.Good M, Stiller C, Zauszniewski JA, Anderson GC, Stanton-Hicks M, Grass JA. Sensation and distress of pain scales: reliability, validity, and sensitivity. J Nurs Meas. 2001;9(3):219–236. [PubMed] [Google Scholar]
- 31.Chen J, Li L. Validation of neuropathic pain assessment tools among Chinese patients with painful diabetic peripheral neuropathy. Int J Nurs Sci. 2016;3(2):139–145. [Google Scholar]
- 32.Hamdan A, Luna JD, Del Pozo E, Gálvez R. Diagnostic accuracy of two questionnaires for the detection of neuropathic pain in the Spanish population. Eur J Pain. 2014;18(1):101–109. doi: 10.1002/j.1532-2149.2013.00350.x. [DOI] [PubMed] [Google Scholar]
- 33.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract. 2001;54(2):115–128. doi: 10.1016/s0168-8227(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 34.van Deursen RWM, Sanchez MM, Derr JA, Becker MB, Ulbrecht JS, Cavanagh PR. Vibration perception threshold testing in patients with diabetic neuropathy: ceiling effects and reliability. Diabet Med. 2001;18(6):469–475. doi: 10.1046/j.1464-5491.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 35.Chawla A, Bhasin G, Chawla R. Validation of Neuropathy Symptoms Score (NSS) and Neuropathy Disability Score (NDS) in the clinical diagnosis of peripheral neuropathy in middle aged people with diabetes. Internet J Fam Pract. 2013;12:1. [Google Scholar]
- 36.Noohu MM, Moiz JA, Dey AB, Hussain ME. A balance device reliability for reaction time and proprioception measurement in older adults. Indian J Gerontol. 2016;30(3):396–403. [Google Scholar]
- 37.Hurvitz EA, Richardson JK, Werner RA. Unipedal stance testing in the assessment of peripheral neuropathy. Rehabil Med Serv. 2001;82(2):198–204. doi: 10.1053/apmr.2001.17830. [DOI] [PubMed] [Google Scholar]
- 38.Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30(1):8–15. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 40.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 41.White CA, Pilkey RM, Lam M, Holland DC. Pre-dialysis clinic attendance improves quality of life among hemodialysis patients. BMC Nephrol. 2002;3(1):3. doi: 10.1186/1471-2369-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHorney The MOS 36-Item Short-Form Health Survey (SF-36): III. Tests of data quality, Scaling Assumptions, and Reliability across Diverse Patient Groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Bruyere O, Wuidart M-A, Di Palma E, Gourlay M, Ethgen O, Richy F, et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–307. doi: 10.1016/j.apmr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Hong J, Barnes MJ, Kessler NJ. Case study: use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. Int J Diabetes Mellit. 2015;3(1):72–75. doi: 10.1016/j.jbmt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 46.Longe SE, Wise R, Bantick S, Lloyd D, Johansen-Berg H, McGlone F, et al. Counter-stimulatory effects on pain perception and processing are significantly altered by attention: an fMRI study. Neuroreport. 2001;12(9):2021–2025. doi: 10.1097/00001756-200107030-00047. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong WJ, Nestle HN, Grinnell DC, Cole LD, Van Gilder EL, Warren GS, et al. The acute effect of whole-body vibration on the Hoffmann reflex. J Strength Cond Res. 2008;22(2):471–476. doi: 10.1519/JSC.0b013e3181660605. [DOI] [PubMed] [Google Scholar]
- 48.Kipp K, Johnson ST, Doeringer JR, Hoffman MA. Spinal reflex excitability and homosynaptic depression after a bout of whole-body vibration. Muscle Nerve. 2011;43(2):259–262. doi: 10.1002/mus.21844. [DOI] [PubMed] [Google Scholar]
- 49.Ginanneschi F, Dominici F, Milani P, Biasella A, Rossi A, Mazzocchio R. Changes in the recruitment curve of the soleus H-reflex associated with chronic low back pain. Clin Neurophysiol. 2007;118(1):111–118. doi: 10.1016/j.clinph.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Nijs J, Van Houdenhove B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: application of pain neurophysiology in manual therapy practice. Man Ther. 2009;14(1):3–12. doi: 10.1016/j.math.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp. 2012:305–14. [DOI] [PubMed]
- 53.Chao CC, Hsieh SC, Yang WS, Lin YH, Lin WM, Tai TY, Hsieh ST. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev. 2007;23(8):612–620. doi: 10.1002/dmrr.734. [DOI] [PubMed] [Google Scholar]
- 54.Feki I, Lefaucheur JP. Correlation between nerve conduction studies and clinical scores in diabetic neuropathy. Muscle Nerve. 2001;24(4):555–558. doi: 10.1002/mus.1040. [DOI] [PubMed] [Google Scholar]
- 55.del Pozo-Cruz J, Alfonso-Rosa RM, Ugia JL, McVeigh JG, del Pozo-Cruz B, Sañudo B. A primary care–based randomized controlled trial of 12-week whole-body vibration for balance improvement in type 2 diabetes mellitus. Arch Phys Med Rehabil. 2013;94(11):2112–2118. doi: 10.1016/j.apmr.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 56.del Pozo-Cruz B, Alfonso-Rosa RM, del Pozo-Cruz J, Sañudo B, Rogers ME. Effects of a 12-wk whole-body vibration based intervention to improve type 2 diabetes. Maturitas. 2014;77(1):52–58. doi: 10.1016/j.maturitas.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Álvarez-Barbosa F, del Pozo-Cruz J, del Pozo-Cruz B, Alfonso-Rosa RM, Rogers ME, Zhang Y. Effects of supervised whole body vibration exercise on fall risk factors, functional dependence and health-related quality of life in nursing home residents aged 80+ Maturitas. 2014;79(4):456–463. doi: 10.1016/j.maturitas.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Nied RJ, Franklin B. Promoting and prescribing exercise for the elderly. Am Fam Physician. 2002;65(3):419–426. [PubMed] [Google Scholar]
- 59.Trans T, Aaboe J, Henriksen M, Christensen R, Bliddal H, Lund H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee. 2009;16(4):256–261. doi: 10.1016/j.knee.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Judge JO, King MB, Whipple R, Clive J, Wolfson LI. Dynamic balance in older persons: effects of reduced visual and proprioceptive input. J Gerontol Ser A Biol Sci Med Sci. 1995;50A(5):M263–M270. doi: 10.1093/gerona/50a.5.m263. [DOI] [PubMed] [Google Scholar]
- 61.Vinik AI, Nevoret M-L, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin N Am. 2013;42(4):747–787. doi: 10.1016/j.ecl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Quattrini C, Tesfaye S. Understanding the impact of painful diabetic neuropathy. Diabetes Metab Res Rev. 2003;19(S1):S2–S8. doi: 10.1002/dmrr.360. [DOI] [PubMed] [Google Scholar]