Abstract
Modification of the surface properties of materials is a promising approach to reduce the uptake of oils during frying. In this work, the quality characteristics of plantain chips subjected to a pretreatment consisting of osmotic drying in sugar solutions (29 and 45° Brix; 40 and 80 °C) were evaluated. True density, apparent density, porosity and moisture content were measured in pretreated samples (PS) before frying. Image ESEM was used to evaluate microstructural changes on the surface and in cross-sections at different depths. Global oil absorption (GOA) and fatty acid profile were monitored in surface and deep cross-sections (DCS). The color parameters of chips (L*, a*, b*, ΔE), browning index, crispness, crunchiness and hardness were evaluated during frying. Oil absorption in the crust was lower in samples subjected to pretreatments with a higher temperature. PS showed high gelatinization in both the surface and DCS, thus changing crust physical properties, total oil uptake and fatty acid profile. An exponential correlation between porosity (ε) and GOA was found, while a second order correlation was found between ε and the fatty acid profile. The characteristics of texture and color, as desired by the consumer, were reached more quickly in the PS at 29° Brix and 40 °C.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04298-5) contains supplementary material, which is available to authorized users.
Keywords: Plantain, Oil uptake, Fried, Osmotic drying, Fatty acid profile, Microstructure
Introduction
There is a growing concern amongst consumers about high intakes of oil and saturated fats, and this has led to the investigation into process technologies that minimize the absorption of oils and fats in foods subjected to frying processes. Many methods have been studied to control the final oil content of fried products, including modifications of the surface properties of the materials (Mellema 2003; Pinthus et al. 1995).
Food subjected to the frying process undergoes physical and chemical changes. The development of pores is a major structural change, followed by shrinkage. Furthermore, chemical changes form toxic components such as acrylamide, produced by the Maillard reaction, which together with other degradation products are absorbed by the food (Bordin et al. 2013).
Among the foods that are preferably eaten fried are potatoes and bananas. Banana is a general term embracing a number of species or hybrids in the genus Musa of the family Musaceae. The genomic groupings AAA of the subspecies M. acuminate and M. balbisiana (Happi et al. 2007) are chiefly eaten raw as table fruit, while some bananas belonging to genomic groupings AAB and ABB (plantain) have to be cooked (Wanna et al. 2002).
Plantains are mostly cooked to be consumed at different stages of maturity. When the fruit has reached physiological maturity, but the skin is green, the plantain is starchy and hard and is treated like a vegetable. Unlike ripe fruits with yellow or even black skin, green fruits are preferably consumed cooked by frying, in the form of slices (chips) or thin strips (snacks).
Consumers prefer plantains that are cooked by deep fat frying, because this improves the quality characteristics and the sensory properties of color, flavor, texture and palatability (Karizaki et al. 2013). Some efforts have been made to improve the frying process by controlling and lowering the final fat content through techniques to reduce the oil content of snacks and plantain chips (Ziaiifar et al. 2008).
Pretreatments, such as convective, osmotic and assisted ultrasound dehydration, have been applied with the purpose of reducing the absorption of oil in fried products (Ikoko and Kuri 2007; Krokida et al. 2001; Yamsaengsung et al. 2011). Osmotic solutions of glucose and salt have been used as a pretreatment for plantain, reducing the oil absorption content by up to 38% (Ikoko and Kuri 2007), while in French fries, Krokida et al. (2001) reported an absorption of less than 10% using osmotic solutions of sugar.
Changes in the microstructure during frying are a determining factor in the absorption of oil and it has been shown that most of the absorbed oil is confined to the surface of fried products. This phenomenon has been explained by the formation of cavities and capillary pathways, weakening the cellular structure, and an increase in porosity caused by the rapid evaporation of water during the frying process (Vitrac 2000).
To reduce the initial porosity, pretreatments have been proposed to modify the physical properties of the food, however, contradictory results have been reported regarding the uptake and final content of oil (Moreno and Bouchon 2008; Karizaki et al. 2013).
In foods with a high starch content, surface modifications using thermal treatments favor gelatinization and porosity. On the other hand, the absorption of oil has also been studied as a global concentration, where the depth of oil absorption is influenced by the properties of the crust. Primo-Martín et al. (2007, 2010) reported differences between the properties of crust and crumb, with the crust having a lower degree of starch gelatinization.
Fatty acid profiles of in-depth cross-sections have been used to analyze the degradation of fatty acids at different depths during frying. The application of pretreatments modifies the surface and layers located at different depths that make up the crust and will influence both the amount of oil absorbed and the degradation of fatty acids.
The objective of this work was to evaluate the effect of pretreating with osmotic drying on plantain slice microstructure and on the moisture kinetics, surface and depth fatty acid profile in cross-sections, total oil uptake and physical and textural properties of fried plantain.
Methods
Materials
Plantain (Musa paradisiaca), of the musa ABB genomic group at physiological maturity and with the skin green in color, were harvested in a plantation located in Oaxaca, México (18° 04′ 52″ N, − 96° 07′ 07 W). The fruit was harvested in the morning and immediately transported to the laboratory and analyses were carried out immediately.
Moisture content
The moisture content was determined according to the AOAC Official Method 934.06.
Color and browning index
An MiniScan EZ (Mod. 4500L, Hunter Lab) was used to measure color. The color was reported on the CIE Lab scale (L*, a*, b*), ΔE was calculated with Eq. 1 and the browning index (BI) was calculated with Eq. 2 (Gökçe et al. 2007).
| 1 |
| 2 |
In this work, a BI value of 100 is considered a preferred BI by the consumer. In fresh samples, measurements were taken in quadruplicate at 25 °C. In fried samples, measurements were taken at the surface, immediately after each frying time, in triplicate. The mean of the data was reported.
Osmotic pretreatment
Osmotic solutions of 29 and 45° Brix were prepared with sucrose and maintained with constant agitation at a temperature of 40 and 80 °C. Slices of plantain of 1.6 ± 0.6 mm thickness were cut with a manual slicer and submerged in osmotic solutions in a ratio sample:solution 1:15. To ensure that the sample reached equilibrium, slices were removed from the solution after 285 min of treatment (Gallegos-Marin et al. 2016) and gently shaken on absorbent paper.
Frying
The pretreated samples (PS) and a reference without treatment (R) were placed in a fryer containing 3 L of oil. The ratio sample:oil was 1:50. Soybean oil was used as a means of frying.
The frying temperature was 170 °C. Samples were taken at 30, 60, 90, 120, 150 and 180 s. Triplicates of the plantain chips were removed from the fryer and allowed to cool to room temperature on a paper towel. R samples were removed from the fryer when they reached the color and crispness desirable for the consumer.
Global oil absorption and fatty acid profile
The oil content was determined in all samples using the Soxhlet according AOAC Official Method 945.16.
For the determination of fatty acid profiles, each chip was frozen using liquid nitrogen and cut at three depths using a microtome. The oil was extracted from each of the layers (surface, 3 and 5 mm) and from a global sample. The oil obtained was subjected to transesterification as follows.
100 mg of oil was weighed into a 1.5 mL glass vial and 800 μL of chloroform–methanol solution (2:1 v/v) was added. Then, 200 μL of this solution was transferred to a test tube and 1 mL of 8% (w/v) solution of HCl in methanol was added. Immediately afterwards the tube was placed in a dry bath at 80 °C for 20 min. The solution was cooled and 200 μL of distilled water was added. The organic phase was extracted with hexane in two portions (1 mL each), filtered and dried with anhydrous sodium sulfate, then centrifuged at 3000 rpm for 5 min. The samples were decanted in a glass vial and the hexane was evaporated with a stream of nitrogen. Finally, the precipitates were resuspended in 1.4 mL of hexane.
The methyl esters of the fatty acids were analyzed on a Perkin Elmer Clarus 580 (Perkin Elmer, Shelton, CT, USA) equipped with a flame ionization detector (FID), using a fused silica capillary column (SP™-2380, 30 m i.d. × 0.25 mm f.d. with a 0.20 μm film thickness) from Supelco (Bellefonte, PA, USA). The column oven temperature was programmed to 60 °C, 2.0 min; 60–186 °C, 4.0 °C/min; 185 °C, 16.0 min. The injector temperature was 220 °C. The flow rate of carrier gas helium was 1.2 mL/min, and the injector split ratio was 100:1. The detector temperature 220 °C. Methyl esters of the sample were identified by their retention times and in comparison, with FAME references (Supelco Inc., Bellefonte, PA, USA).
For the quantification of fatty acid profiles, methyl heptadecanoate was used as an internal standard and Eqs. 3 and 4 were used for quantification.
| 3 |
where
| 4 |
True density
True density (ρp) is defined as the mass contained in a given volume, excluding pores in the material, and was calculated with Eq. 5, as described by Méndez-Lagunas et al. (2017).
| 5 |
Particle volume was calculated using a stereopicnometer (SPY-5DC, Quantachrome, Boynton Beach, FL, USA) and mass of the sample. The pressure inside the stereopicnometer was set at 1.195 ± 0.003 kgf/m2.
Apparent density
Apparent density (ρb) was measured in 1 ± 0.25 g of non-coated sample, using the volume displacement method with n-heptane and a density kit (SDK 01, Boeco, Germany). Measurements were carried out within ≈ 10 s to prevent the immersion liquid from being absorbed and apparent density was calculated with Eq. 6.
| 6 |
Porosity
Porosity was calculated using the data for true and apparent densities (Zogzas et al. 1994) as expressed by Eq. 7.
| 7 |
Texture
A TA1 Ametek Lloyd texture analyzer (Florida, USA), equipped with a 12.5 mm stainless steel cilinder and a 30 mm diameter hollow cylindrical base. The samples were fractured within 30 s after frying. The probe velocity was 1 mm s−1, and preload/stress was 0.10 N.
The maximum force is the indication for the overall hardness (N). Crispness was calculated as the length of the curve (a linear distance) and is a unitless value for comparation purposes. Crunchiness (N mm) was calculated as the total work used during the test (Valenzuela-Lagarda et al. 2018). All calculations are automatically made by the equipment. All fracture tests were performed in triplicate.
Environmental scanning electron microscopy (ESEM)
A Quanta 3D FEG (FEI, Japan) environmental scanning electron microscope (ESEM) was used to observe changes in surface tissue and in slices cut at 100, 200 and 800 µm depth of fresh and OD-treated samples.
Experiment design and data analysis
A completely random factorial design was performed. All tests were performed in triplicate and the average values and standard deviations are reported. The results were compared by one-way analysis of variance (ANOVA) followed by the Tukey method to determine the differences by pairs (P < 0.05) using the Minitab statistical software (Minitab, Inc., State College, PA, USA).
Results
Physical properties of PS
Table 1 shows the physical properties of plantain before the frying process. All the properties have a significant difference between the PS and R sample. A high concentration of sugar in the osmotic solution decreases the final water content in PS.
Table 1.
Physical properties of pretreated plantain
| Without treatment | 29° Brix 40 °C | 29° Brix 80 °C | 45° Brix 40 °C | 45° Brix 80 °C | |
|---|---|---|---|---|---|
| Moisture content (%db) | 58.16 | 46.76 | 50.01 | 41.61 | 44.86 |
| Solid content (%db) | 41.84 | 53.24 | 50.00 | 58.24 | 55.20 |
| ρp (kg/m3) | 1390 | 1382.43 | 1411.12 | 1443.03 | 1403.36 |
| ρb (kg/m3) | 1073.66 | 1109.95 | 1210.37 | 1196.19 | 1313.32 |
| ε | 0.22 | 0.19 | 0.14 | 0.17 | 0.06 |
The porosity and true and apparent density of untreated samples (R) indicates that the material is less compact and more porous than pretreated samples (PS). In PS, the porosity and true and apparent density shows a similar behavior, and the same range of values, as those reported by Krokida et al. (2001) for French fries pretreated with sugar solutions.
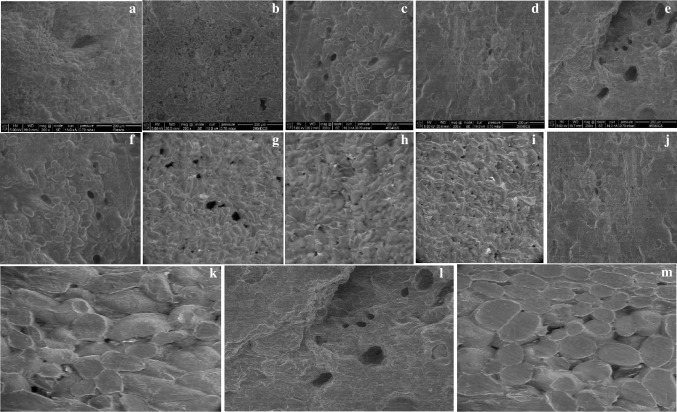
Figure 1a–e shows the microstructural differences between fresh plantain tissues and those pretreated with OD. Fresh plantain shows a uniform cellular structure with intact oval-like shape starch granules, with an average size of 24.46 ± 5 µm, in accordance with the results reported by Hernández-Jaimes et al. (2013). Micrographs of samples pretreated at a high temperature showed greater gelatinization as compared to those subjected to low temperatures and fresh samples. OD pre-treatment with 45° Brix 40° C sugar solutions caused the cells to swell until 55% larger than those from fresh samples.
Fig. 1.
ESEM images (200 ×) of surface plantain after OD pretreatments. a Fresh plantain; pretreated plantain slices at b 29° Brix 40 °C, c 45° Brix 40 °C, d 29° Brix 80 °C, e 45° Brix 80 °C. Surface and cross-sectional slices of plantain after OD pretreatment to 45° Brix 40 °C: f surface; g depth 0.2 mm; h depth 0.1 mm; i depth 0.8 mm. Surface and cross-sectional slices of plantain after OD pretreatment to 29° Brix 80 °C: j surface; k depth 0.8 mm; and after OD pretreatment to 45° Brix 80 °C: l surface; m depth 0.8 mm
Moisture kinetics
Moisture kinetics during frying (Fig. 2) showed that all pretreatments reached a moisture content below 10% (wb) at 30 s while R reached the same moisture content at 90 s of frying. Samples pretreated at 29 and 45° Brix and 40 °C reached a lower moisture content (< 0.06 gw/gds) more quickly (60 s).
Fig. 2.
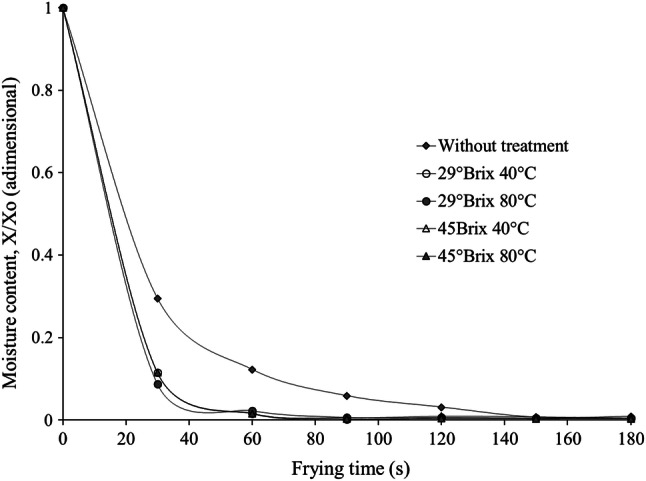
Moisture kinetics
Color kinetics and browning index of chips
A strong negative correlation was found between L parameter and moisture content. The color kinetics of R showed a greater luminosity (L) than PS (Fig. 3). This is consistent with the initial moisture content of the samples (Table 1), which has a strong relationship with lightness due to a reflection of light in the water. All pretreated samples showed the lowest lightness values due to non-enzymatic browning (Krokida et al. 2001).
Fig. 3.
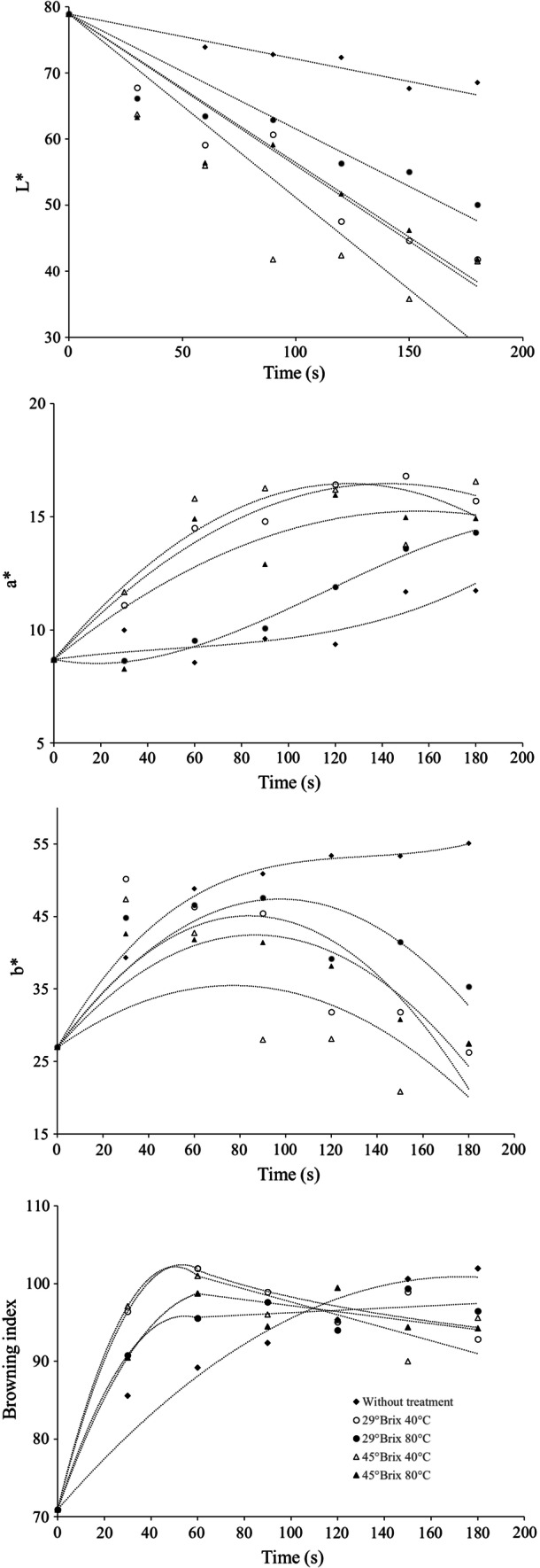
Kinetics of the change of color parameter and browning index as a function of frying time
The a* parameter is used to determine the optimum frying point (Heredia et al. 2014). High values could indicate an orange hue as a result of non-enzymatic browning. The behavior of the kinetics of the a* parameter (Fig. 3) showed, at the end of the process, a difference between R samples and those that received an OD pretreatment. All the samples underwent changes with an increase in reddish tones, however, samples pretreated at 29° Brix 80 °C showed a similar behavior to R samples during the first 100 s.
The kinetics of the b* parameter (Fig. 3) increases during the first 100 s, followed by a decrease, reaching a lower value than the initial value for most of the PS, except those subjected to osmotic conditions of 29° Brix 80 °C, which reached the same value as the initial one. On the other hand, R samples maintained an increase until the end of the process, indicating that the yellowish hue increased. Yellow-gold is the desirable color for fried products (Heredia et al. 2014) high values of the b* parameter indicate a more yellow color and most of PS reached the highest value of b* at 100 s of frying. The trend of the color parameters is similar to that reported by Ikoko and Kuri (2007) in green plantain chips subjected to pretreatments with glucose and salt.
The browning index (BI) of the pretreated samples showed, at the end of the frying time, a value lower than that reached by R. PS subjected to 29° Brix 40 °C and 45° Brix 40 °C reached a BI value similar to R samples at 60 s, after which all PS maintained constant BI until the end of the fry (Fig. 3). This shows a similar behavior to that reported by Mba et al. (2015) for fried unripe green plantain crisps. The Maillard reaction is the most important process involved in browning during frying. This non-enzymatic reaction affects sugars, forming intermediate products that rapidly polymerize to create dark-colored molecules (Bordin et al. 2013).
R samples reached the color quality characteristics desired by the consumer in 180 s, while PS only required 60 s to have the same characteristics (supplementary material). After that time all pre-treated samples were burned. This is consistent with ΔE values calculated (Eq. 1) using the color parameters of the R after 180 s of frying and the PS after 30 s of frying. The greatest difference was found in the treatment 45° Brix 80 °C (ΔE = 14.4) and the lowest in the treatment 29° Brix 40 °C (ΔE = 5.1). This last treatment at 60 s is the closest to the BI value of 100, which is an indicator of consumer preference. An increase in the browning index (Fig. 3) was also observed in PS; however, it was lower than that reached by R after 180 s. This behavior is consistent with the presence of a high solids content, such as sucrose and other sugars, which favor Maillard reactions and caramelization during frying (Roe and Faulks 1991). In general, gelatinization does not favor the BI value and samples with a lower initial moisture content reached the highest BI values more quickly.
Total oil uptake and fatty acid profile in surface and depth cross-sections
Table 2 shows the total oil uptake and fatty acid profile of soy oil and the surface of plantain chips. Soy oil, used in plantain fry, contains mostly linoleic acid (63.07%) and oleic acid (23.2%). The total oil uptake was lower in all the PS. Notably, samples pretreated to high temperature absorbed less oil by between 20 and 23%. Similar results were reported by Krokida et al. (2001) and Gupta et al. (2000) for pre-dried French fries. The low oil absorption found in the pretreated samples can be explained by the lower porosity, high surface gelatinization and short frying times compared to R samples. There is abundant proof that oil hardly penetrates in the cooked core and that the microstructure of the crust is the main determining factor in oil uptake (Pinthus et al. 1995).
Table 2.
Total oil content and fatty acid profile in soy oil, surface, 3 and 5 depth cross-section of plantain chips
| Treatment | Total oil content (%) | Palmitic | Stearic | Oleic | Linoleic | Linolenic |
|---|---|---|---|---|---|---|
| Fatty acid content in soy oil and surface of plantain chips (mg/100 g) | ||||||
| Soy oil | 12.37 ± 3.06a | 2.94 ± 0.40a | 23.23 ± 5.23a | 63.07 ± 11.3a | 7.19 ± 2.01a | |
| WP | 31.2 ± 0.6d | 6.03 ± 0.49b | 2.56 ± 0.59a | 19.86 ± 1.42a | 43.18 ± 1.19b | 5.23 ± 0.48a |
| 29° Brix 40 °C | 18.7 ± 0.5c | 2.37 ± 0.20bc | 0.67 ± 0.08b | 3.68 ± 0.26b | 7.77 ± 0.45c | 1.02 ± 0.00b |
| 29° Brix 80 °C | 07.2 ± 0.5a | 1.67 ± 0.12bc | 0.12 ± 0.03b | 1.25 ± 0.13b | 2.97 ± 0.44c | 0.31 ± 0.04b |
| 45° Brix 40 °C | 13.1 ± 0.6b | 0.76 ± 0.04c | 0.71 ± 0.04b | 5.01 ± 0.32b | 10.66 ± 1.39c | 1.31 ± 0.10b |
| 45° Brix 80 °C | 06.3 ± 0.2a | 0.63 ± 0.05c | 0.04 ± 0.05b | 0.95 ± 0.01b | 2.30 ± 0.07c | 0.27 ± 0.03b |
| Fatty acid in 3 depth cross-section (mg/100 g) | ||||||
| WP | 6.071 ± 0.50b | 0.89 ± 0.25b | 8.75 ± 0.75b | 18.74 ± 1.26b | 2.85 ± 1.01b | |
| 29° Brix 40 °C | 1.56 ± 0.12bc | 0.36 ± 0.05b | 2.90 ± 0.32c | 6.65 ± 0.77c | 0.95 ± 0.10b | |
| 29° Brix 80 °C | 0.78 ± 0.10c | 0.11 ± 0.00b | 1.28 ± 0.13c | 2.93 ± 0.39c | 0.33 ± 0.01b | |
| 45° Brix 40 °C | 1.18 ± 0.03bc | 0.57 ± 0.20b | 2.07 ± 0.08c | 4.54 ± 0.15c | 0.59 ± 0.08b | |
| 45° Brix 80 °C | 0.61 ± 0.01c | 0.08 ± 0.00b | 0.97 ± 0.02c | 2.16 ± 0.06c | 0.24 ± 0.00b | |
| Fatty acid in 5 depth cross-section (mg/100 g) | ||||||
| WP | 1.53 ± 0.08b | 0.29 ± 0.08b | 2.71 ± 0.03c | 5.87 ± 0.03c | 0.80 ± 0.14b | |
| 29° Brix 40 °C | 1.48 ± 0.29b | 0.48 ± 0.13b | 2.29 ± 0.57c | 4.88 ± 1.06c | 0.68 ± 0.23b | |
| 29° Brix 80 °C | 0.70 ± 0.01b | 0.19 ± 0.11b | 1.07 ± 0.00c | 2.58 ± 0.05d | 0.31 ± 0.00b | |
| 45° Brix 40 °C | 2.37 ± 0.04b | 0.71 ± 0.04b | 5.01 ± 0.32b | 10.66 ± 1.39b | 1.31 ± 0.10b | |
| 45° Brix 80 °C | 0.58 ± 0.04b | ND | 0.92 ± 0.02c | 2.074 ± 0.13d | 0.21 ± 0.02b | |
WP without pretreatment (R)
Different letters in the same column show significant difference (p ≤ 0.05)
In this work we find an exponential correlation (GOA = 2.743e9.8857·IP; r = 0.9) between the initial porosity (IP) of the sample and global oil absorption (GOA). Pinthus et al. (1995) reported a linear relation between oil uptake and sample porosity prior to frying. The difference can be explained because PS at 80 °C have high gelatinization, resulting in a very low porosity that does not agree with the trend of the rest of the pretreatments. Kawas and Moreira (2001) found that the formation of a tight barrier on the outer surface of tortilla chips due to severe starch gelatinization results in a lower final oil content. On the other hand, Ikoko and Kuri (2007) reported that more oil is absorbed when the frying time increases.
Generally, global fat uptake is measured in the food frying processes. However, the evaluation of the penetration depth of oil penetration is unknown (Clerjon et al. 2012).
Numerous reports claim that the highest oil absorption is located in the crust formed during deep-fat frying processes. The crust is formed by the physical changes and gelatinization of the product resulting from the temperatures of the fry.
The pretreatments modified the structure of both the surface and the interior of the plantain slices. The PS with lower oil absorption (45° Brix 80 °C and 29° Brix 80 °C) showed the surface completely gelatinized and in inner layers, the starch granules swelled up to 1.8 times more than in fresh ones (Fig. 1f–m).
Porosity prior to frying was a significant factor affecting oil uptake in deep fat frying. Samples pretreated at 40 °C at both concentrations of the osmotic solution (Table 1) showed the highest porosity, increasing the global oil uptake and individual fatty acid concentration (Table 2). Some authors have proposed interfacial tension between water vapor and oil as a mechanism to explain oil uptake. A relationship between a higher initial porosity and an increase in oil uptake results in a reduced porosity during frying which increases the interfacial tension (Pinthus and Saguy 1994).
The fatty acid profile at different depths allows us to evaluate the impact of structural changes prior to frying and the degradation of fatty acids, and also understand the fatty acid transfer phenomenon.
Significant differences in the fatty acid profile in cross-sectional slices were found (Table 2). However, at the same cross-section depth, most of the OD pretreatments did not affect the fatty acid profile significantly. Due to its greater presence in the oil, linoleic acid was found in greater quantity, followed by oleic acid. R and PS at 40 °C showed lower oil absorption at greater depth of the cross sections, while PS at 80° C did not show significant differences. As expected, a lower content of fatty acids was found at greater depth.
Porous pretreated samples show a reduction in fatty acid concentration at greater depths, probably due to the greater presence of oxygen that favors degradation reactions, particularly oxidation.
Previous work highlighted the importance of porosity in oil uptake, finding a linear relationship between porosity before frying and oil absorption (Dana and Saguy 2006). In this work we found a second order correlation (r > 0.84) between porosity and the uptake of fatty acids (Linoleic = 3476.2·IP2 − 753.98·IP + 35.984; Oleic = 1591.3·IP2 − 343.76·IP + 16.272; Palmitic = 403.67·IP2 − 82.98·IP + 4.2286; Linolenic = 421.12·IP2 − 91.153·IP + 4.3309; Stearic = 192.0·IP2 − 40.008·IP + 1.7899). This is probably because of a structural modification caused by the OD pretreatments that modify the volume, due to starch gelatinization, reducing the density and increasing oil absorption. Also, the frying time of the pre-treated samples was less than the reference (R) times. Ikoko and Kuri (2007) reported that more oil is absorbed when the frying time increases.
The microstructure, such as porosity, tortuosity and roughness, are important parameters in the absorption of oil during frying. However, the profile of absorbed fatty acids is also dependent on oxygen, food moisture and frying temperature.
Under these conditions the oil undergoes three deleterious reactions: hydrolysis caused by water, oxidation and thermal alteration caused by oxygen and heat (Frankel 1998). On the other hand, previous work reported the stability of oleic acid in prolonged periods of frying (Moulodi et al. 2015), which could explain its presence in the deeper sections.
Texture
A significant effect of pretreatment (α < 0.05) was found in crispness, crunchiness and hardness (Table 3). Crispness and hardness increase during frying in all samples.
Table 3.
Effect of pretreatment conditions on crispiness, crunchiness and hardness
| Frying time (s) | Without treatment (R) | 29° Brix 40 °C | 29° Brix 80 °C | 45° Brix 40 °C | 45° Brix 80 °C |
|---|---|---|---|---|---|
| Crispiness (adimensional) | |||||
| 30 | 0.07 ± 0.01ab | 0.05 ± 0.03b | 0.16 ± 0.08a | 0.13 ± 0.01ab | 0.04 ± 0.03b |
| 60 | 0.10 ± 0.03b | 0.10 ± 0.09b | 2.57 ± 0.41b | 35.35 ± 2.4a | 32.49 ± 1.8a |
| 90 | 0.12 ± 0.03c | 24.49 ± 6.4b | 17.82 ± 5.2b | 38.97 ± 6.8a | 37.18 ± 3.8a |
| 120 | 7.73 ± 0.42c | 25.31 ± 3.1a | 17.05 ± 1.8b | 24.83 ± 2.a | 28.88 ± 3.6a |
| 150 | 19.55 ± 1.5ab | 25.69 ± 4.1ab | 18.06 ± 5.6b | 26.40 ± 7.9ab | 31.55 ± 6.2a |
| 180 | 12.63 ± 0.65c | 27.59 ± 1.3a | 18.03 ± 2.8bc | 22.23 ± 4.7ab | 24.99 ± 3.9a |
| Crunchiness (N mm) | |||||
| 30 | 1.51 ± 0.29d | 7.42 ± 0.15a | 2.50 ± 0.25c | 3.41 ± 0.23b | 2.59 ± 0.35c |
| 60 | 1.82 ± 0.18c | 5.81 ± 1.2ab | 7.08 ± 0.71a | 7.44 ± 2.7a | 3.57 ± 0.81bc |
| 90 | 3.64 ± 0.51a | 3.59 ± 0.58a | 2.47 ± 0.58a | 4.45 ± 1.63a | 3.72 ± 0.85a |
| 120 | 6.38 ± 0.99a | 3.88 ± 0.65b | 2.91 ± 0.48b | 2.83 ± 0.77b | 3.19 ± 0.53b |
| 150 | 21.41 ± 2.88a | 3.63 ± 0.58b | 2.61 ± 0.75b | 2.85 ± 1.3b | 3.18 ± 0.75b |
| 180 | 26.38 ± 0.44a | 3.73 ± 0.35b | 2.27 ± 0.38c | 2.92 ± 0.79bc | 3.07 ± 0.85bc |
| Hardness (N) | |||||
| 30 | 0.79 ± 0.05b | 3.61 ± 0.30a | 0.81 ± 0.09b | 3.54 ± 0.43a | 0.93 ± 0.16b |
| 60 | 0.95 ± 0.02c | 2.38 ± 0.61b | 3.22 ± 0.79b | 9.72 ± 0.30a | 9.69 ± 0.37a |
| 90 | 1.52 ± 0.19c | 10.06 ± 1.18b | 7.84 ± 1.6b | 13.79 ± 0.78a | 14.04 ± 0.74a |
| 120 | 3.63 ± 0.42d | 10.11 ± 1.06bc | 9.01 ± 1.1c | 11.34 ± 0.09b | 15.55 ± 0.94a |
| 150 | 8.16 ± 0.07b | 9.62 ± 1.45ab | 8.41 ± 1.17b | 12.61 ± 3.78a | 10.88 ± 0.76ab |
| 180 | 8.88 ± 0.41b | 8.69 ± 0.84b | 7.66 ± 0.89b | 13.28 ± 2.44a | 13.69 ± 0.77a |
Different letters in the same column show significant difference (p ≤ 0.05)
The pretreatments with the highest concentration of sugar (45° Brix, 60 s) reached the crispness of the R more quickly (180 s). They also showed the same decreasing behavior at the end of the fry that can be explained by the increased plastic behavior of the crust, probably caused by the degradation of sugars and related to a decrease of the glass transition temperature (van Koerten et al. 2015) PS at 29° Brix at both temperatures increases crispness during the first 90 s of frying and remained the same until the end of the fry.
During frying a rapid increase in the breaking force was observed in the first 120 s for most PS, followed by a moderate decrease in the last 30 s. R samples maintained a constant increase in hardness until 180 s. The increase could be a result of starch gelatinization which softens the tissue. Elastic deformation of cell walls does not lead directly to fracture, is a plastic process resulting in higher hardness values at longer frying times. The loss of moisture at the end of the fry could explain the increase in hardness in the last seconds, caused by fractures at small deformations.
Furthermore, all the PS reached the same hardness in the first 90 s as that reached by the R samples after 180 s. Similar behavior was obtained in plantain chips subjected to OD with glucose (Ikoko and Kuri 2007).
The completely gelatinized PS produced more rigid structures during the first 30 s with lower values for texture, while the partially gelatinized PS showed higher values in the first 30 s. This indicates that the matrix has an elastic deformation which leads to a large plastic deformation of the cells requiring higher strength. There were significant differences between PS and R samples in terms of crunchiness, except at 90 s. A significant increase in crunchiness was observed in R samples, while PS showed little variation until the end of the fry.
Crispness and crunchiness are sensory attributes that are not fully understood but are related to the mechanical fracture of food. These descriptors express the sensations that are produced in the mouth on breaking under pressure, but crunchiness might also be applied to moist foods (Szczesniak and Kahn 1971). The strength, stiffness and size of the cell walls of the middle lamella, turgor pressure and fibrous tissues contribute to rigidity. However, processes such as drying affect the turgor of the cells; this leads to a decrease in turgidity and the tissue will be rubbery instead of crispy (Kilcast 2004). Compression or tension forces produce two failure modes in cellular tissue, depending on turgor pressure and resistance of the middle lamella, cell debonding and cell rupture (Mohsenin 1986). Although during frying the moisture content is reduced, the crunchiness value of R increases indicating that a greater total work is required to fracture the sample. This behavior has been explained by the degradation of cementing substances that keep the cells together, reducing cellular adhesion by the solubilization of pectin from the average lamella (Varela 1988) and this can be observed in our ESEM images (Fig. 1f–m). On the other hand, in PS osmotic drying weakens the forces that hold the cells together. If the forces that hold the cells together are weaker than the cell walls, the cells will separate. Osmotically dehydrated fruits have a low turgor pressure, so changes in mechanical response are more likely to arise due to cell debonding than cell rupture (Chiralt and Talens 2005). However, during frying, PS seem to suffer no major structural changes.
Conclusion
Pretreatment with OD modifies the microstructural and physical properties of plantain, such as porosity, density and moisture and solid content. Gelatinization was a remarkable microstructural change both in the surface and in deep cross-sections. These modifications have a strong impact on the properties of the chips. The content of solids influences the color, browning index and texture. These are quality properties of greater interest for the consumer because they are the first to be perceived by the senses. In this work, we found that all PSs achieved the best quality characteristics in shorter times than R. These results, and the microstructural changes, lead to a lower oil absorption. The total oil content in the food also has a great impact on the consumer’s perception. The PS showed a low oil absorption correlated with a low porosity, high surface gelatinization and short frying times. In this work we find an exponential correlation between the initial porosity and global oil absorption. These results confirm that the pretreatment OD allows one to control the amount of oil absorbed in the chips and suggests that the absorption occurs mostly on the surface rather than in the deeper layers of the crust. This result emphasizes the importance of product pretreatment as a vehicle for controlling porosity and the uptake of oil during deep-fat frying. In general, the best texture characteristics were reached in the PS at 29° Brix and frying times of 90 s, while the BI was better in samples subjected to lower pretreatment temperatures (40 °C) and 60 s of frying.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank to Ph.D. Mayahuel Ortega Aviles, from Centro de Nanociencias y Micro y Nanotecnología (CNMN-IPN) for his assistance in ESEM images and acknowledge the financial support from SIP-IPN for the Project SIP 20161487. Gallegos Marin would like to thank CONACyT for the Ph.D. fellowship 237890.
List of symbols
- a*
Chromaticity coordinate (+ red to − green component)
- As
Internal standard area
- Ax
Area of the compound of interest
- b*
Chromaticity coordinate (+ yellow to − blue component)
- Ci
Concentration of fatty acid in the final extract (mg/mL)
- Cs
Concentration of the internal standard in the extract (mg/mL)
- Cx
Concentration of the fatty acid in the sample (mg of fatty acid/100 mg of sample)
- L*
Color parameter of luminosity
- m
Mass (kg)
- V
Volume (m3)
- Vs
Volume of solvent (mL)
- W
Sample weight (mg)
Greek letters
- ε
Porosity (nondimensional)
- ρ
Density (kg/m3)
Subscripts
- a
Apparent
- ai
Air
- p
True
- ds
Dry solid
- b
Apparent
- p
Particle
- t
Value at any frying time
- 0
Value at initial time
- w
Water
- wb
Wet basis
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Official methods of analysis. 15. Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Bordin K, Tomihe Kunitake M, Kazue Aracava K, Silvia Favaro Trindade C. Changes in food caused by deep fat frying—a review. Arch Latinoam Nutr. 2013;63(1):5–13. [PubMed] [Google Scholar]
- Chiralt A, Talens P. Physical and chemical changes induced by osmotic dehydration in plant tissues. J Food Eng. 2005;67(1–2):167–177. doi: 10.1016/j.jfoodeng.2004.05.055. [DOI] [Google Scholar]
- Clerjon S, Kondjoyan A, Bonny JM, Portanguen S, Chevarin C, Thomas A, Bauchart D. Oil uptake by beef during pan frying: impact on fatty acid composition. Meat Sci. 2012;91(1):79–87. doi: 10.1016/j.meatsci.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Dana D, Saguy I. Mechanism of oil uptake during deep-fat frying and the surfactant effect-theory and myth. Adv Colloid Interface Sci. 2006;128–130:267–272. doi: 10.1016/j.cis.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Frankel EN. Lipid oxidation. Dundee: The Oily Press; 1998. [Google Scholar]
- Gallegos-Marin I, Mendez-Lagunas LL, Rodríguez-Ramírez J, Martínez-Sánchez CE. Structural properties changes during osmotic drying of plantain (Musa paradisiaca aab) and its role on mass transfer. Rev Mex Ing Quím. 2016;15(2):441–456. [Google Scholar]
- Gökçe D, Dilek K, Belma Ö. Color change kinetics of okra undergoing microwave. Dry Technol. 2007;25(5):925–936. doi: 10.1080/07373930701372296. [DOI] [Google Scholar]
- Gupta P, Shivhare US, Bawa AS. Studies on frying kinetics and quality of French fries. Dry Technol. 2000;18(1–2):311–321. doi: 10.1080/07373930008917706. [DOI] [Google Scholar]
- Happi ET, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103(2):590–600. doi: 10.1016/j.foodchem.2006.09.006. [DOI] [Google Scholar]
- Heredia A, Castelló ML, Argüelles A, Andrés A. Evolution of mechanical and optical properties of French fries obtained by hot air-frying. LWT Food Sci Technol. 2014;57(2):755–760. doi: 10.1016/j.lwt.2014.02.038. [DOI] [Google Scholar]
- Hernández-Jaimes C, Bello-Perez LA, Vernon-Carter EJ, Alvarez-Ramirez J. Plantain starch granules morphology, crystallinity, structure transition, and size evolution upon acid hydrolysis. Carbohydr Polym. 2013;95(1):207–213. doi: 10.1016/j.carbpol.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Ikoko J, Kuri V. Osmotic pre-treatment effect on fat intake reduction and eating quality of deep-fried plantain. Food Chem. 2007;102(2):523–531. doi: 10.1016/j.foodchem.2006.06.008. [DOI] [Google Scholar]
- Karizaki VM, Sahin S, Sumnu G, Mosavian MTH, Luca A. Effect of ultrasound-assisted osmotic dehydration as a pretreatment on deep fat frying of potatoes. Food Bioprocess Technol. 2013;6(12):3554–3563. doi: 10.1007/s11947-012-1012-5. [DOI] [Google Scholar]
- Kawas ML, Moreira RG. Effect of degree of starch gelatinization on quality attributes of fried tortilla chips. J Food Sci. 2001;66(2):300–306. doi: 10.1111/j.1365-2621.2001.tb11336.x. [DOI] [Google Scholar]
- Kilcast D. Texture in food. Cambridge: Woodhead Publishing Ltd; 2004. [Google Scholar]
- Krokida MK, Oreopoulou V, Maroulis ZB, Marinos-Kouris D. Effect of pre-drying on quality of French fries. J Food Eng. 2001;49(4):347–354. doi: 10.1016/S0260-8774(00)00233-8. [DOI] [Google Scholar]
- Mba OI, Dumont MJ, Ngadi M. Influence of palm oil, canola oil and blends on characteristics of fried plantain crisps. Br Food J. 2015;117(6):1793–1807. doi: 10.1108/BFJ-04-2014-0155. [DOI] [Google Scholar]
- Mellema M. Mechanism and reduction of fat uptake in deep-fat fried foods. Trends Food Sci Technol. 2003;14(9):364–373. doi: 10.1016/S0924-2244(03)00050-5. [DOI] [Google Scholar]
- Méndez-Lagunas LL, Rodríguez-Ramírez J, Reyes-Vazquez D, López-Ortíz A. Changes in physical properties and relations with allicin degradation during convective drying of garlic. J Food Meas Charact. 2017;11(3):1227–1232. doi: 10.1007/s11694-017-9499-0. [DOI] [Google Scholar]
- Mohsenin NM. Physical properties of plant and animal materials. New York: Gordon and Breach Science Publishers; 1986. [Google Scholar]
- Moreno MC, Bouchon P. A different perspective to study the effect of freeze, air, and osmotic drying on oil absorption during potato frying. J Food Sci. 2008;73(3):E122–E128. doi: 10.1111/j.1750-3841.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- Moulodi F, Qajarbeigi P, Rahmani K, Haj Hosseini Babaei A, Mohammadpoorasl A. Effect of fatty acid composition on thermal stability of extra virgin olive oil. J Food Qual Hazards Control. 2015;2(2):56–60. doi: 10.1111/j.1750-3841.2008.00669.x. [DOI] [Google Scholar]
- Pinthus EJ, Saguy I. Initial interfacial tension and oil uptake by deep-fat fried foods. J Food Sci. 1994;59(4):804–807. doi: 10.1111/j.1365-2621.1994.tb08132.x. [DOI] [Google Scholar]
- Pinthus EJ, Weinberg P, Saguy IS. Oil uptake in deep fat frying as affected by porosity. J Food Sci. 1995;60(4):767–769. doi: 10.1111/j.1365-2621.1995.tb06224.x. [DOI] [Google Scholar]
- Primo-Martin C, van Nieuwenhuijzen NH, Hamer RJ, van Vliet T. Crystallinity changes in wheat starch during the bread-making process: starch crystallinity in the bread crust. J Cereal Sci. 2007;45(2):219–226. doi: 10.1016/j.jcs.2006.08.009. [DOI] [Google Scholar]
- Primo-Martín C, van Dalen G, Meinders MBJ, Don A, Hamer RH, van Vliet T. Bread crispness and morphology can be controlled by proving conditions. Food Res Int. 2010;43(1):207–217. doi: 10.1016/j.foodres.2009.09.030. [DOI] [Google Scholar]
- Roe MA, Faulks RM. Color development in a model system during frying: role of individual amino acids and sugars. J Food Sci. 1991;56(6):1711–1713. doi: 10.1111/j.1365-2621.1991.tb08677.x. [DOI] [Google Scholar]
- Szczesniak AS, Kahn EL. Consumer awareness of and attitudes to food texture. I: adults. J Text Stud. 1971;2:280–295. doi: 10.1111/j.1745-4603.1971.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Lagarda JL, García-Armenta E, Pacheco-Aguilar R, Gutiérrez-Dorado R, Mazorra-Manzano MÁ, Lugo-Sánchez ME, Muy-Rangel MD. Relationships between morphometrical properties and the texture of an extrusion-expanded snack made from squid mantle (Dosidicus gigas) J Texture Stud. 2018;49(5):476–484. doi: 10.1111/jtxs.12321. [DOI] [PubMed] [Google Scholar]
- van Koerten KN, Schutyser MAI, Somsen D, Boom RM. Crust morphology and crispness development during deep-fat frying of potato. Food Res Int. 2015;78:336–342. doi: 10.1016/j.foodres.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Varela G. Current facts about the frying of food. Chichester: Ellis Horwood; 1988. [Google Scholar]
- Vitrac O (2000) Caractérisation expérimentale et modélisation de l’opération de friture. École Nationale Supérieure des Industries Agricoles et Alimentaires, Massy, France. Ph.D. thésis
- Wanna A, Che Man YB, Yusof S, Rahman RA. Effects of type of packaging material on physicochemical and sensory characteristics of deep-fat-fried banana chips. J Sci Food Agric. 2002;82(14):1621–1627. doi: 10.1002/jsfa.1233. [DOI] [Google Scholar]
- Yamsaengsung R, Ariyapuchai T, Prasertsit K. Effects of vacuum frying on structural changes of bananas. J Food Eng. 2011;106(4):298–305. doi: 10.1016/j.jfoodeng.2011.05.016. [DOI] [Google Scholar]
- Ziaiifar AM, Achir N, Courtois F, Trezzani I, Trystram G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int J Food Sci Technol. 2008;43(8):1410–1423. doi: 10.1111/j.1365-2621.2007.01664.x. [DOI] [Google Scholar]
- Zogzas NP, Maroulis ZB, Marinos-Kouris D. Densities, shrinkage and porosity of some vegetables during air drying. Dry Technol. 1994;12(7):1653–1666. doi: 10.1080/07373939408962191. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.