Abstract
Purpose
As epigenetic modifications like chromatin histone modifications have been suggested to play a role in the pathophysiology of Diabetic Nephropathy (DN) and are also found to be regulated by microRNAs. Our main purpose was to explore the role of microRNA in histone modulations associated with DN. There is downregulation of miR-29b due to advanced glycation end products in diabetes. Histone Deacetylase-4 (HDAC4) is amongst the histone modulators which promotes podocytes’ impairment and upregulates transforming growth factor-1 (TGF-β1) leading to renal fibrosis. Moreover, macrophage infiltration causes podocytes’ apoptosis and IL-6 mediated inflammation. As miR-29b is downregulated in diabetes and HDAC4, TGF-β1 and IL-6 could be the possible therapeutic targets in DN, our study was focussed on unveiling the role of miR-29b in modulation of HDAC4 and hence, in podocyte dysfunction and renal fibrosis in DN.
Methods
In silico analysis and luciferase assay were done to study the interaction between miR-29b and HDAC4. In-vitro DN model was developed in podocytes and miR-29b mimics were transfected. Also, podocytes were co-cultured with macrophage and miR-29b mimics were transfected. At the end, in-vivo DN model was generated in C57BL/6 J male mice and the effect of miR-29b mimics was reconfirmed.
Results
It was found that miR-29b targets the 3′ untranslated region of HDAC4. In both in-vitro and in-vivo DN model, downregulation of miR-29b and subsequent increase in HDAC4 expression was observed. The miR-29b mimics suppressed podocytes’ inflammation mediated through macrophages and attenuated HDAC4 expression, glomerular damage and renal fibrosis.
Conclusion
This study concludes that miR-29b regulates the expression of HDAC4 which plays a role in controlling renal fibrosis and podocytes’ impairment in DN.
Electronic supplementary material
The online version of this article (10.1007/s40200-019-00469-0) contains supplementary material, which is available to authorized users.
Keywords: miR-29b, HDAC4, TGF-β1, Histone modifications, Diabetic nephropathy, Renal fibrosis
Introduction
Podocytes are the kidney cells possessing a visceral epithelial cell lining in the region of the glomerulus. It represents three-dimensional morphology with interdigitating foot processes that are associated with the slit diaphragm (a regulator of blood filtration in the kidney). Any molecular events that result into morphological changes in the podocytes viz. podocytopenia or effacement of podocytes’ foot processes, confer to chronic kidney disease leading to an End-stage renal disorder [1]. Podocytes regulate the process of glomerular filtration barrier and are also involved in the synthesis of components of the glomerular basement membrane (GBM) as well as in the formation of slit diaphragm proteins and maintenance of endothelial cell viability. All these functions of podocytes are adversely affected under stress condition [2]. Podocytopathy is a major pathological incidence involved in diabetic nephropathy (DN) that contributes to proteinuria and other renal complications. Under hyperglycaemic conditions, several alterations in podocytes’ occur including their de-differentiation, apoptosis, an imbalance in angiogenic responses and fission of mitochondria in their microenvironment [3].
Epigenetics play a vital role during the emergence as well as the progression of pathogenesis in DN. During the progression of DN, the epigenetic modifications like DNA methylation and chromatin histone modifications occur owing to the engagement of cytokines and growth factors [4]. Histone deacetylases (HDACs) are one of the major histone modulators and there are numerous studies which have highlighted the role played by HDACs in the enhancement of kidney diseases [5, 6]. Autophagy is an essential mechanism involved in biological processes and pathological conditions which regulates the tubular injuries, genetic alterations in the kidney, development of kidney and aging process as well as pathogenesis of DN [7, 8]. Amongst several HDACs, HDAC4 specifically targets podocytes and causes their impairment by inducing the secretion of inflammatory cytokines through the suppression of essential autophagy mechanisms [9].
MicroRNAs are the small, non-coding RNAs which act as regulators of epigenetic alterations in several diseased conditions [4]. It has been widely reported that there is a downregulation of miR-29 family in hyperglycaemic conditions by transforming growth factor-β1 (TGF-β1) which further complicates the pathogenesis of renal fibrosis [10]. It is reported that overexpression of miR-29a is beneficial in podocytopathy because it ameliorates high glucose induced podocyte dysfunction via disruption of HDAC4 signalling [3]. Literature suggests that miR-29b suppressed renal inflammation mediated via nuclear factor-κβ (NF-κβ) and T-bet-/IFN-γ mediated immune response in db/db mice [11]. Regulation of HDACs’ activity by the miR-29 family has also been described in hepatic stellate cells [12], in myogenesis [13] and in myeloid leukemia [14]. Further, the epigenetic loop of miR-29b/HDAC4 has also been testified in multiple myeloma [15]. Apart from its role in histone modification, miR-29b plays a pivotal role in DNA methylation in DN [16].
Infiltration of macrophages is also one of the major reasons involved in renal damage. It has been studied that activation of M2 macrophages causes loss of podocytes’ integrity [17]. It is also reported that macrophages cause podocytes’ apoptosis [18]. Further, HDACs have been found to be involved in development and activation of macrophages and hence, research needs to be done to explore the interplay between HDACs and macrophages in diseased conditions [19]. Moreover, HDAC4 is reported to regulate inflammatory cytokine production in macrophages [20]. As miR-29b is well reported in fibrosis, a study was carried out to check its role in silicosis. It was found that there was downregulation of miR-29b due to inflammatory mediators released from silica treated macrophages which upon overexpression of miR-29b lead to inhibition of extracellular matrix synthesis [21].
This investigation expounds the interaction between miR-29b and HDAC4 in DN associated podocyte dysfunction. This study aims to establish that miR-29b is a propitious therapeutic approach towards managing the pathogenesis of DN. In addition to this, the effect of upregulation of miR-29b in renal fibrosis is also explored and reported herein. The effect of miR-29b on expression levels of HDAC4 in macrophages and podocytes co-culture is being studied as a crosstalk with other inflammatory and apoptotic markers (Fig. 1).
Fig. 1.
Graphical abstract. The figure shows the graphical representation of the results and conclusion drawn from this study
Materials and methods
In-silico studies
The markers which are targeted by miRNAs and are involved in epigenetic modifications in DN were identified through in-silico analysis. The target prediction of miRNA was performed using databases like FindTar and TargetScan. Secondary structure was predicted by RNA hybrid tool. These are the target prediction tools used for miRNAs to predict their seed sequence within the human epigenetic regulatory gene.
Podocyte cell culture
A conditionally human immortalized podocyte cell line was obtained as a generous gift from Dr. Jeffrey Kopp (National Institute of Health, USA). It was maintained in 25 cm2 type-I collagen-coated flask (0.2 mg/ml). Initially, the cells were cultured at 330 C for 10 d and after attainment of 80% confluency, the cells were cultured at 37 °C for 10–14 d to achieve their maturation. The cells were supplemented with RPMI medium along with 10% heat-inactivated Fetal Bovine Serum (10% FBS), 1x Insulin-Transferrin-Selenium (ITS) G Supplement (Invitrogen; USA, Cat. No. 41400–045) solution and 100 U/ml Penicillin and 100 μg/ml Streptomycin. The medium was changed 3 times in a week. After achieving the desired expansion, podocytes were seeded on a 6-well plate at a density of 3 × 105 cells per well with a culture media. To induce maturation of podocytes, they were maintained at 37 °C for 10 d. After achieving maturation of podocytes, a medium devoid of ITS solution was used with 1% FBS and the cells were treated with high glucose (30 mM) and Human Recombinant Protein TGF-β1 (10 ng/ml) and incubated in these conditions for 48 h.
Co-culture of podocytes with macrophages
In a trans-well system of co-culture, RAW 264.7 cells (4 × 105) were seeded on a 0.4 μm trans-well insert (Millipore) in the presence of podocytes (4 × 105) with or without high glucose (30 mM) for 48 h. In the co-culture experiments, podocytes were plated on 12-well plates and cultured overnight in normal RPMI 1640 media with 10% FBS and 100 U/ml of Penicillin and 100 μg/ml of Streptomycin. Then, the cells were washed for 3 times with PBS. After that, normal RPMI 1640 media along with high glucose (HG) was added to podocytes and co-cultured cells containing media with and without high glucose were kept for 24–72 h and the cells were maintained at 37 °C in 5% CO2 environment.
Extraction of RNA
Total mRNA was extracted from podocytes and kidney tissue of mice using Trizol reagent (Invitrogen) and its quantification was performed using NanoDrop. For analysis of mRNA expression profiles, initially cDNA (complementary DNA) was synthesized from 1 μg of RNA using iScript cDNA synthesis kit (BioRad). Along with it, miRNA isolation was performed using the miReasy® kit (Qiagen) and cDNA synthesis was performed using TaqMan microRNA reverse transcription kit (Applied Biosystems).
Quantitative real time-PCR (qRT-PCR)
cDNA synthesized from extracted RNA was further processed for Reverse Transcriptase PCR (RT-PCR) to assess mRNA expression profiles of Synaptopodin, CD2 associated protein (CD2AP), NF-κβ, Caspase-3, Interleukin-6 (IL-6) and HDAC4 which were normalized using 18 s ribosomal RNA (rRNA) as an endogenous control (primer sequences are listed in S1Table). A quantitative Real-time PCR (qRT-PCR) of HDAC4 was performed in triplicate with a dilution of 1:10 of cDNA using iQ Syber Green Supermix (BioRad) following Syber Green Assay for HDAC4 which was normalised using 18 s rRNA as an endogenous control whereas the qRT-PCR for miR-29b was performed using 2X TaqMan Universal PCR Master Mix (Applied Biosystems) and probes of miR-29b and U6snRNA (U6 small nuclear RNA) in which the U6snRNA was used as an endogenous control. Quantitative expression profile analysis for qRT-PCR was performed using StepOne Real-time PCR machine (Applied Biosystems).
Western blot
Initially, protein lysates were extracted from podocytes and kidney tissue of mice using RIPA buffer containing Protease-cocktail inhibitor. The total concentration of protein was quantified by BCA assay using bicinchoninic acid protein estimation kit (Thermo Scientific). SDS-PAGE was performed to achieve separation of proteins and the acrylamide gel was transferred on PVDF membrane using RTA Trans Turbo Kit (BioRad). Further, western blotting was carried out to detect specific proteins using primary antibodies of Podocin (NPHS2) (1:1000, ab50339, Abcam), CD2AP (1:250, HPA003326, Sigma), TGF-β1 (1:4000, ab92486, Abcam), Collagen type-IV (1:1000, ab6586, Abcam), HDAC4 (1:1000, ab12172, Abcam) and β-actin (1:5000, SC2005, Santacruz) and the blots were kept for incubation at 4 °C overnight. Wash with Tris-buffered Saline (TBS) was given and incubated for 1 h with secondary antibodies (Goat anti-mouse IgG-HRP 1:20000, ab97046, Abcam and Goat anti-rabbit IgG-HRP 1:20000, ab6721, Abcam). Again, the wash was given and enhanced chemiluminescence enzyme substrate (BioRad) was added for detection of bands. Quantification of band intensity was performed using ImageJ software.
Transfection with miR-29b mimics
miR-29b mimics were purchased from Ambion (Thermo Scientific) and were transfected according to manufacturer’s instructions in podocytes and co-culture of podocytes and macrophages at a final concentration of 25–30 pmol using a Lipofectamine RNAiMax® (Invitrogen) transfection reagent and the transfection efficiency was analyzed by studying the miRNA expression profiles of miR-29b. The medium was changed after 24 h and the cells were incubated for 48 h. After 48 h, the cells were harvested for analysis. The transfection efficiency of transfection reagent was determined by quantitative analysis of expression profiles of miR-29b through Real-time PCR.
Enzyme-linked Immunosorbent assay (ELISA)
Initially, cell lysates and cell culture supernatant were collected from cell culture and stored at −80 °C. ELISA was performed for Collagen-IV and IL-6 using Human Collagen type-IV ELISA Kit (Bioassay Technology Laboratory) and IL-6 ELISA (Sigma) Kit. In order to measure podocyte associated Collagen-IV and IL-6, cell lysate and cell culture supernatant samples were applied to their respective pre-coated 96- well plates and incubated for 3 h at room temperature. The wells were then washed with wash buffer (3 times for 5 min each) and horseradish peroxidase (HRP)-conjugated anti-Collagen-IV antibodies and anti-IL-6 antibodies were added. The wells were again incubated for 1.5 h at room temperature. After that, it was again washed with wash buffer (3 times for 5 min each) and HRP substrate was added (50 μl). The resulting chromogenic solution was stopped using a stop solution and the absorbance was determined at 450 nm using a microplate reader.
Luciferase reporter assay
The 3’ UTR sequence of HDAC4 targeted by miR-29b along with its mismatch sequences was synthesized using IDT, USA (S2Table). The pmirGLO plasmid vector was procured from Promega (S1Fig). The sequence was cloned into PmeI and Xbal Restriction endonuclease (RE) sites present in the pmirGLO vector. The confirmation of cloned vector of pmirGLO-HDAC4 through RE digestion was performed (S2Fig). Podocytes were transfected with 1 pmol of mimics of miR-29b and subsequent co-transfection with 1 μg of pmirGLO-HDAC4 was performed using Fugene® HD transfection reagent in 24-well plate. After 48 h of transfection with miR-29b-pmirGLO vector construct, podocytes were analyzed for luciferase activity using Dual-Glo® Luciferase Assay System (Promega, USA). The normalized firefly luciferase activity (firefly luciferase activity/Renilla luciferase activity) for each construct was compared with the pmirGLO vector without insert (Control group).
In-vivo studies
The in-vivo DN model in C57/BL6J male mice was developed by intraperitoneal administration of 150 mg/kg Streptozotocin (STZ) in 6–8 weeks old mice. The C57/BL6J mice were then divided into four groups: Control group, negative control group, diabetic group and miR-29b mimics treated group. Each group comprised of six mice for assessment of potential mortality. The blood glucose levels were checked every week and the mice having blood glucose levels of 250 mg/dl or more were considered as diabetic. The miR-29b mimics (2 ng/mm3) were administered every alternate day to diabetic mice and simultaneous administration of a scrambled sequence of miR-29b mimics to another diabetic group was given for 12 weeks. In order to confirm DN model generation in mice after 12 weeks of diabetes, parameters like blood glucose, HbA1c, urinary albumin excretion rate (UAER) estimation using albumin ELISA kit (Abcam) in urine samples, N-acetylglucosaminidase (NAG) activity using NAG activity kit (Abcam) in urine samples, blood urea nitrogen (BUN) and serum creatinine were assessed using Stat fax 3300 bioanalyzer.
Histological evaluation of kidney tissue
The tissue samples of interest were harvested and immediately stored in 10% w/v formalin solution. The histological evaluation was performed by hematoxylin and eosin staining as well as trichome staining as per the standard manufacturer’s procedure (Trichome stain Abcam).
Statistical analysis
All the data were expressed as the mean ± SD. The data for different groups were analyzed using one-way ANOVA and Dunnett’s test. The p-values less than 0.05 were considered as statistically significant. The statistical analysis was done using GraphPad Prism Version 5.01 software.
Results
Bioinformatics analysis and luciferase reporter assay suggests that miR-29b directly targets the 3’ UTR of HDAC4
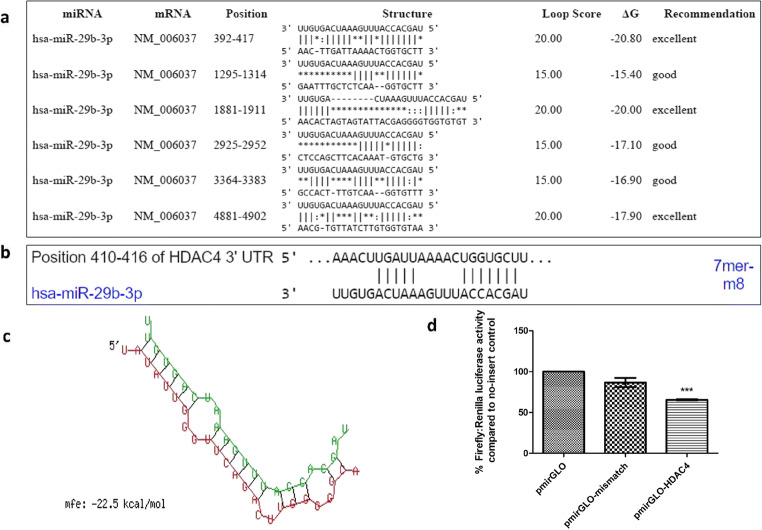
The target prediction by miR-29b was done through softwares namely FindTar and TargetScan (Fig. 2). The in-silico analysis inferred that miR-29b targets the 3’UTR of HDAC4. The secondary structure prediction of miR-29b with HDAC4 has been shown in Fig. 2 as obtained by RNAhybrid tool. To validate the interaction between miR-29b and HDAC4, Luciferase Reporter Assay was carried out. All cloned constructs were sequenced by Sanger sequencing (S3Fig). Luciferase activity measurement showed that upon transfection with miR-29b mimics, normalized Luciferase activity was significantly reduced in comparison with the control group and cells transfected with mismatch sequence (Fig. 2).
Fig. 2.
Bioinformatics analysis of miR29b-HDAC4 and Luciferase assay for confirmation of target prediction. a. The low binding energy between miR-29b and HDAC4 shows high stability of interaction as per the FindTar analysis. b. Binding position prediction of miR29b with HDAC4 by TargetScan. c. Secondary structure prediction of miR29b with HDAC4 by RNAhybrid. Red sequence: HDAC4; Green sequence: miR-29b-3p. d. In comparison to pmirGLO vector without insert, there was a significant increase in luciferase activity of pmirGLO vector with HDAC4 insert transfected with miR-29b mimics. Data are expressed as the mean ± SD calculated from three experiments. *** depicts P < 0.001 in comparison to the control group (n = 3)
Development and characterization of in-vitro DN model in podocytes treated with high glucose & TGF- β1
The establishment of DN model was confirmed by comparing the expression profiles of apoptotic and inflammatory markers in high glucose (30 mM) treated podocytes with high glucose (30 mM) and TGF- β1 (10 ng/ml) treated podocytes. It is already reported in the previous literature that high glucose is responsible for apoptosis of podocytes by increased levels of reactive oxygen species [22]. Hence, the in-vitro DN model was characterised by checking the expression of Caspase-3, NF-κβ, and IL-6 with the help of RT-PCR. The expression levels of Caspase-3, NF-κβ, and IL-6 were found to be elevated under high glucose and TGF-β1 as compared to only high glucose-treated podocytes (Fig. 3). The expression levels of CD2AP and Synaptopodin were analysed by RT-PCR while Podocin was analysed by western blotting. However, the expression profiles of slit diaphragm proteins like Synaptopodin, Podocin and CD2AP which contribute in maintenance of podocytes’ integrity and proper functioning of GBM were reduced (Fig. 3). This outcome suggests that high glucose and TGF-β1 cause inflammation and apoptosis in podocytes and disrupt the podocytes’ integrity and physiological functioning.
Fig. 3.
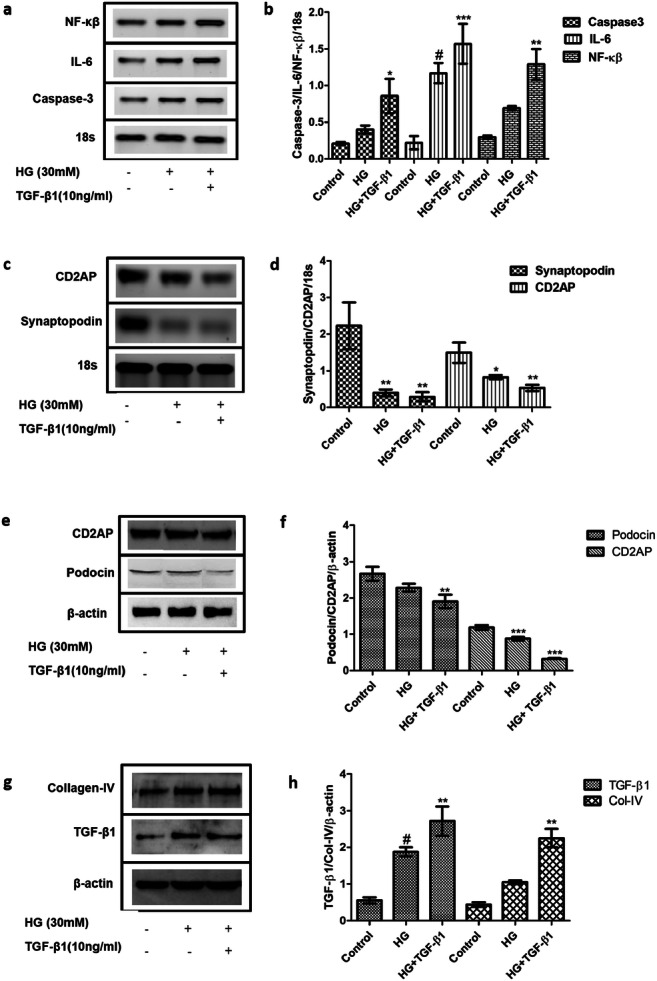
Confirmation of in-vitro DN model generation. a & b. RT-PCR analysis showed increased relative mRNA expression levels of IL-6, NF-κβ and Caspase3 in the HG and TGF-β1 treated group as compared to the control group. c & d. mRNA expression levels of Synaptopodin and CD2AP were decreased in the HG and TGF-β1 treated group as compared to the control group as per RT-PCR analysis. e & f. Relative protein expression levels of Podocin and CD2AP were decreased in the HG and TGF-β1 treated group as compared to the control group as per western blotting analysis. g & h. The expression levels of Collagen type-IV and TGF-β1 were increased in the high glucose and TGF- β1 treated group as compared to only high glucose treated group as per western blotting analysis. Data are expressed as the mean ± SD calculated from three experiments. * depicts P < 0.05, ** depicts P < 0.01, and *** depicts P < 0.001 in comparison to the control group. HG, High Glucose; TGF-β1, Transforming growth factor- β1; NF- κβ, Nuclear factor- κβ; TNF-α, Tumor necrosis factor- α; CD2AP, CD2 associated protein; IL-6, Interleukin-6 (n = 3)
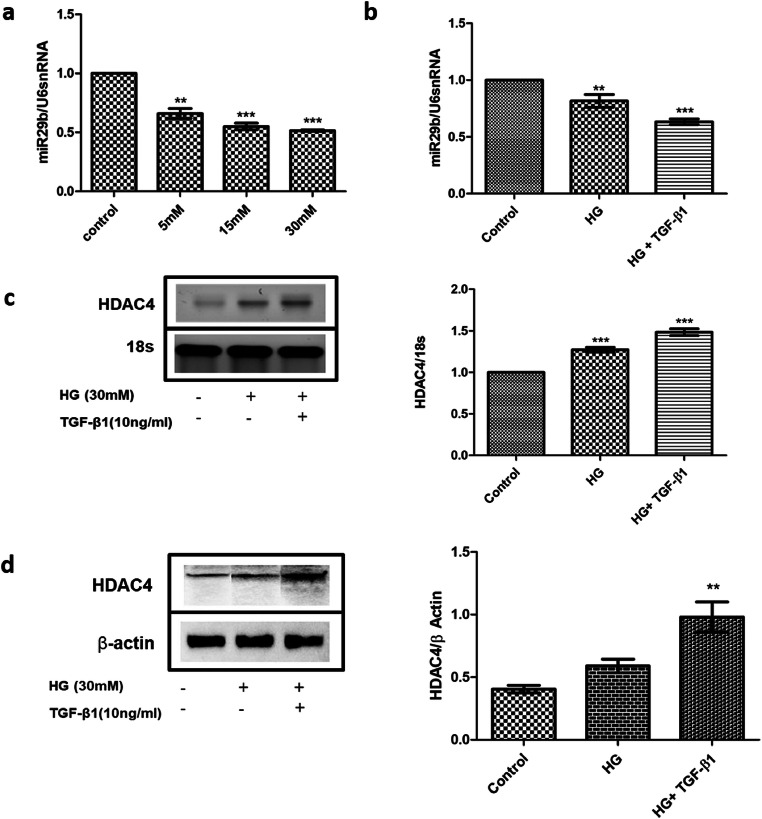
High glucose and TGF-β1 promoted progression of renal fibrosis in podocytes by downregulation of miR-29b expression and elevation of HDAC4 expression
Although miR-29b regulates the expression levels of Collagen type-I, III and IV in euglycemic conditions, in hyperglycaemic conditions there is upregulation of TGF-β1 which consequently elevates the expression levels of Collagen type-I, III and IV while downregulating the miR-29b expression which ultimately promotes renal fibrosis in podocytes [10]. The expression levels of Collagen type-IV (COL4A1) and TGF-β1 were analyzed and it was noted that the elevation in expression levels of these biomarkers are involved in the pathogenesis of renal fibrosis as observed in the podocytes treated with high glucose and TGF- β1 as compared to only high glucose-treated podocytes (Fig. 4). In addition, when the expression profiles of miR-29b were analyzed under different glucose concentrations (5 mM, 15 mM, and 30 mM of high glucose concentration and in TGF-β1 (10 ng/ml) and high glucose (30 mM) concentration), it was observed that miR-29b expression levels were significantly downregulated in podocytes treated with high glucose (30 mM) and podocytes treated with high glucose (30 mM) and TGF-β1 (10 ng/ml) (Fig. 4). This suggests that TGF-β1 is an additional contributor in downregulation of miR-29b under hyperglycaemic conditions. The TGF-β1 was characterized in high glucose DN model in podocytes for the expression of HDAC4 by RT-PCR and western blotting. It was found that under high glucose and TGF-β1 conditions, HDAC4 expression levels were significantly elevated as compared to podocytes treated with only high glucose (Fig. 4).
Fig. 4.
Effect of HG and TGF-β1 on the expression of miR-29b and HDAC4. a. miR-29b expression was significantly downregulated in glucose concentration of 5 mM, 15 mM and 30 mM in a dose dependent manner. b. Relative miR-29b expression levels were decreased in the HG and TGF-β1 treated group when compared to the control group as per quantitative real-time PCR analysis. c. Relative mRNA expression levels of HDAC4 were increased in the HG and TGF-β1 treated group as per real-time PCR analysis. d. Relative protein expression patterns of HDAC4 were increased in the HG and TGF-β1 treated group as per western blotting analysis. Data are expressed as the mean ± SD calculated from three experiments. ** depicts P < 0.01 and *** depicts P < 0.001 in comparison to the control group. HG, high glucose; TGF-β1, Transforming growth factor- β1 (n = 3)
Overexpression of miR-29b alleviated the progression of renal fibrosis in podocytes by modulation of HDAC4 expression
The transfection of miR-29b mimics in the DN model generated in podocytes with high glucose and TGF-β1 was found to be associated with alterations in expression levels of HDAC4. Upon transfection with miR-29b mimics, an overexpression (p < 0.01) of miR-29b was observed (Fig. 5) that induced downregulation of HDAC4 (Fig. 5). The transfection of miR-29b mimics elevated the expression of miR-29b and elevated level was found to be associated with the alleviation in the expression levels of TGF-β1 and Collagen type-IV (Fig. 5) with a marked decrease in expression levels of HDAC4 (Fig. 5). This is suggested to control the progression of renal fibrosis via regulating the Collagen type-I, II and IV expression levels which may consequently suppress the accumulation of ECM. Moreover, HDAC4 is also reported to be involved in upregulation of TGF-β1 and hence, in promoting fibrosis. As miR-29b mimics modulate the expression levels of HDAC4 (Fig. 5), it could further cause downregulation of TGF-β1 and hence, renal fibrosis could be controlled.
Fig. 5.
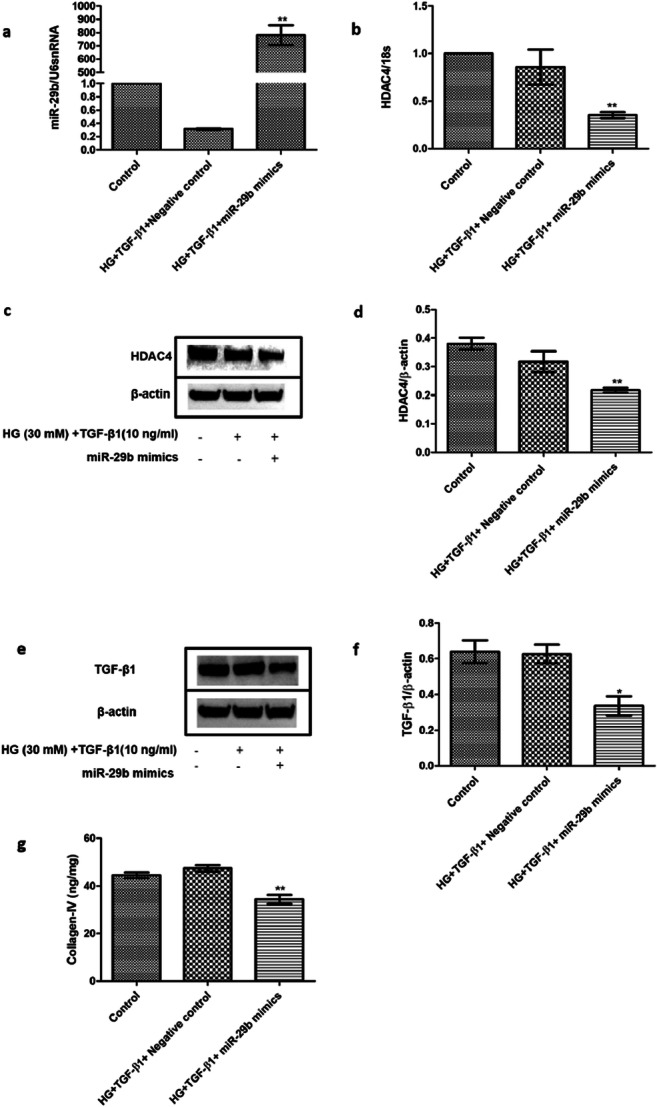
Effect of miR-29b mimics on expression patterns of miR-29b, HDAC4, TGF-β1, and COL4A1. a. Relative expression levels of miR29b were increased on transfection with miR29b mimics. b. Relative mRNA expression levels of HDAC4 were significantly downregulated in the miR-29b mimics treated group when compared with the control group as per real-time PCR analysis. c & d. Protein expression levels of HDAC4 were decreased in the miR-29b mimics treated group when compared with the control group as per western blotting analysis. e & f. Protein expression levels of TGF-β1 were decreased in the miR29b mimics treated group as per western blotting analysis. g. Protein expression levels of collagen type-IV were decreased in the miR29b mimics treated group when analyzed through ELISA. Data are expressed as the mean ± SD calculated from three experiments. * depicts P < 0.05 and ** depicts P < 0.01 in comparison to the control group (n = 3). HG, High glucose; TGF-β1, Transforming growth factor-β1
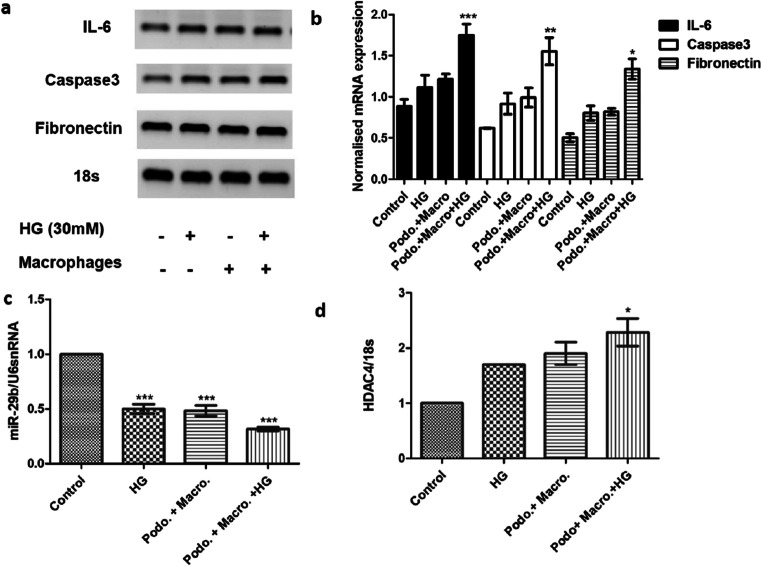
miR-29b mimics decreased inflammation and renal fibrosis in macrophage-mediated podocytes’ impairment via modulation of HDAC4
In order to check the role of the macrophage in inflammation, apoptosis, and fibrosis under hyperglycemic condition, we co-cultured podocytes with macrophages and treated them with high glucose (30 mM). In the presence of high glucose, there was an increase in mRNA level of IL-6, Caspase- 3 and fibronectin which infers inflammation, apoptosis, and fibrosis in podocytes due to the presence of macrophage. Macrophages under hyperglycemic condition decreased miR-29b expression whereas increased HDAC4 expression in podocytes as analyzed by qRT-PCR (Fig. 6). Initially, the expression profiles of miR-29b, HDAC4, IL-6, caspase-3 and Fibronectin in podocytes were compared with podocytes treated with high glucose, coculture of podocytes and macrophages, coculture of podocytes and macrophages treated with high glucose. Thereafter, expression of miR29b and HDAC4 in coculture of podocytes and macrophages treated with high glucose and miR-29b mimics was checked. Under hyperglycaemic condition in presence of macrophages, elevation in HDAC4 expression levels was observed while miR-29b expression was decreased significantly (p < 0.001) (Fig. 6). HDAC4 could be one of the contributors of macrophage-mediated podocytes’ inflammation as it has been already reported to be involved in podocytes’ impairment [3, 23, 24]. Upon transfection with miR-29b mimics in podocytes treated with macrophages and high glucose, downregulation of HDAC4 was observed (Fig. 7). However, expression levels of miR-29b were found to be upregulated (Fig. 7). In the co-cultured study of podocytes with macrophage under hyperglycaemic condition, there was upregulation of IL-6 (Fig. 6). However, miR-29b upregulation was shown to alleviate the expression of IL-6 in podocytes (Fig. 7) which suggests that miR-29b could show a protective role in suppression of podocytes’ inflammation mediated through macrophages. It is known that COL4A1 and TGF-β1 are amongst the major biomarkers of renal fibrosis. Upon transfection with miR-29b mimics, it was observed that downregulation in expression patterns of COL4A1 and TGF-β1 occurred. This suggests the protective role of miR-29b mimics in the suppression of renal fibrosis mediated through macrophages (Fig. 7).
Fig. 6.
Effect of macrophage on the expression of miR-29b, HDAC4, IL-6, Caspase-3, and Fibronectin. a & b. Relative mRNA expression levels of IL-6, caspase-3 and fibronectin were increased in the co-culture of podocytes and macrophages under hyperglycaemic conditions as compared to the control group as per the real-time PCR analysis. c. Relative miR-29b expression levels were downregulated in the co-culture of podocytes and macrophages under hyperglycaemic conditions as compared to the control group when analyzed through real-time PCR. d. Relative mRNA expression levels of HDAC4 were increased in the co-culture of podocytes and macrophages under hyperglycaemic conditions as compared to the control group when analyzed through real-time PCR. Data are expressed as the mean ± SD calculated from three experiments. * depicts P < 0.05, ** depicts P < 0.01 and *** depicts P < 0.001 in comparison to the control group. HG, High glucose; IL-6, Interleukin-6 (n = 3)
Fig. 7.
Effect of miR-29b mimics on macrophage-mediated podocyte impairment. a. Relative miR-29b expression levels were increased in the miR-29b mimics treated as compared to the untreated groups under hyperglycaemic condition as per the real-time PCR analysis. b. Relative mRNA expression levels of HDAC4 were downregulated in the miR-29b mimics treated co-culture of podocytes and macrophages under hyperglycaemic conditions as compared to the untreated groups under hyperglycaemic condition when analyzed through real-time PCR. c. Expression patterns of HDAC4, COL4A1 & TGF-β were increased in the co-culture of podocytes and macrophages under hyperglycaemic conditions as compared to the control group. d. The IL-6 levels were reduced in the miR-29b mimics treated group when analyzed through ELISA. Data are expressed as mean ± SD calculated from three experiments. * depicts P < 0.05, ** depicts P < 0.01 and *** depicts P < 0.001 in comparison to the control group. HG, High glucose; TGF-β1, Transforming growth factor- β1; HG, High glucose; IL-6, Interleukin-6; COL4A1, Collagen type-IV (n = 3)
miR-29b mimics treatment attenuated glomerular damage and renal fibrosis by decreasing the expression of HDAC4, COL4A1 and TGF-β1 in diabetic mice
DN model generation was confirmed after 12 weeks of diabetes by several parameters using urine and blood serum sample (Table 1). In order to check the effects of miR-29b in the in-vivo diabetic mice, expression of miR-29b in serum sample was checked. As reported earlier in the literature, downregulation of miR-29b in case of diabetes was observed. After treatment with miR-29b mimics, a significant upregulation in the levels of miR-29b was observed in the mimics treated group (Fig. 8). It was observed in the diabetic mice model that there is a decrease in the levels of miR-29b which was found to be elevated after giving miR-29b mimics treatment. It was confirmed by the luciferase assay that miR-29b effectively targeted HDAC4. After treatment with miR-29b mimics in diabetic mice, there was a decrease in the expression levels of HDAC4. As downregulation of miR-29b is associated with renal fibrosis, decrease in expression levels of COL4A1 and TGF-β1 was observed (Fig. 8). This suggests a role of miR-29b in attenuation of renal fibrosis by decreasing HDAC4, COL4A1, and TGF-β1 which are found to play major roles in case of renal fibrosis in DN. In order to check the role of miR-29b mimics in attenuating kidney damage, the kidney tissue was stained to check % area fibrosis and glomerular area. It was observed through trichome staining that deposition of collagen fibers is significantly reduced after treatment with miR-29b mimics as compared to untreated diabetic mice. As per the results of Hematoxylin and Eosin staining, it was observed that the basement membrane of the glomerulus is highly detached in case of untreated diabetic mice. Treatment with miR-29b mimics significantly reduced the glomerular damage via a reduction in the glomerular area as compared to diabetic mice (Fig. 8). Hence, miR-29b mimics were effective in decreasing glomerular damage and renal fibrosis as per the histological evaluation.
Table 1.
Parameters assessed for in-vivo DN model generation
| Parameters | Control (n = 6, ±SD) | Diabetic (n = 6, ±SD) | Diabetic + miR-29b mimics (n = 6, ±SD) | Negative control (scramble) (n = 6, ±SD) |
|---|---|---|---|---|
| Blood glucose (mmol) | 9.94 ± 0.515 | 29.7 ± 2.531*** | 22.94 ± 3.688*** | 33.33 ± 3.157 |
| Creatinine clearance (ml/min) | 0.105 ± 0.033 | 0.0699 ± 0.027 | 0.0786 ± 0.034 ns | 0.066 ± 0.016* |
| Serum BUN | 26.36 ± 1.976 | 33.43 ± 1.97* | 29.88 ± 1.04 ns | 31.087 ± 4.77* |
| Serum creatinine (μmol/L) | 69.246 ± 11.749 | 91.3466 ± 12.07* | 76.613 ± 17.382 ns | 92.82 ± 7.396* |
| NAG activity (U/L) | 0.004 ± 0.001 | 0.032 ± 0.010*** | 0.0206 ± 0.004* | 0.029 ± 0.008*** |
| HbA1c (%) | 0.74 ± 0.440 | 9.041 ± 0.252*** | 8.60 ± 1.096*** | 11.036 ± 1.812*** |
| UAER (μg/min) | 0.00750 ± 0.0011 | 0.583 ± 0.098*** | 0.452 ± 0.232*** | 0.667 ± 0.180*** |
Fig. 8.
Effect of miR-29b mimics on renal fibrosis and expression patterns of HDAC4, COL4A1 & TGF-β in the in-vivo DN model. a. Decreased expression of miR-29b in diabetic condition was found to be elevated in the miR-29b mimics treated group (n = 5). b. Protein expression levels of HDAC4, COL4A1 and TGF-β1 were downregulated in the miR-29b mimics treated group in comparison to control group when analyzed through western blotting. c. Trichome staining showed that miR-29b mimics treatment decreased renal fibrosis as compared to the control group as per the analysis by ImageJ. d. Hematoxylin and Eosin staining showed that miR-29b mimics treatment decreased glomerular damage as compared to control as per the analysis by ImageJ. In c & d, 1 to 4 is assigned as- 1: Control, 2: Diabetic, 3: Negative control, 4: Diabetic + miR-29b mimics treated group. Data are expressed as the mean ± SD calculated from three experiments. * depicts P < 0.05 and ** depicts P < 0.01 in comparison to the control group (n = 6). TGF-β1, Transforming growth factor- β1; COL4A1, Collagen type-IV
Discussion
Podocyte loss and their impairment are the major features involved in the pathogenesis of DN [25]. Podocytes possess the ability to live under various stress conditions and to regulate the homeostasis process. However, due to extreme stress in diseased conditions there is a loss of podocytes’ integrity and the cellular metabolism is disturbed. Under extreme stress conditions, podocytes lose their contact with GBM that forms the final barrier to avoid protein loss which ultimately leads to proteinuria [2, 26]. Various molecular events are involved in podocytes’ impairment and histone acetylation is one amongst them. There are reports which suggest involvement of HDAC isoforms in pathophysiology of DN via targeting different molecular pathways [27]. Histone acetylation regulates the homeostasis and autophagy of podocytes via inhibition of HDACs [28]. Although several HDACs’ inhibitors have been shown to play a defensive role in the pathogenesis of DN. Studies which are particularly related to the subtypes of HDACs have not been explored much in DN pathogenesis. It has been reported that HDAC4 expression levels get upregulated in the pathogenesis of DN and it is one of the major contributors of podocytes’ injury. The miRNAs have shown to act as regulators of acetylation events occurring in DN pathogenesis. Literature suggests that there are several microRNAs which are modulated by epigenetic modifications while there are several other microRNAs which modulate the enzymes like DNA methyltransferases and histone deacetylases which are vital in epigenetic modifications. It is reported that overexpression of miR-93 prevented podocyte injury by decreasing hyperglycemia induced acetylation of H3 (H3K14Ac). Natarajan et al. found that miR-125b is involved in epigenetic regulation of inflammatory gene expression. It is reported that microRNAs like miR-1 and miR-449 modulates histone deacetylase HDAC4 and HDAC1 respectively [29]. There are many studies which are focused on exploring the role played by microRNAs in DN related epigenetic modulations. Although there are several reports on microRNA associated epigenetic modifications in DN, the role of ‘miR-29b’ in modulation of ‘HDAC4’ was not explored. In our study, we investigated miR-29b/HDAC4 circuitry and we found that due to upregulation of miR-29b, HDAC4 expression levels were alleviated and due to this, the biomarkers involved in inflammation and renal fibrosis were also decreased. Hence, our study suggests that miR-29b is involved in the pathophysiology of DN by modulation of HDAC4. These findings would help in understanding the role of miR-29b in DN and its effect on HDAC4 which would consequently aid in unveiling the role played by epigenetic modulations in pathophysiology of DN. Future studies in this direction can explore the effect of miR-29b on several targets other than HDAC4, TGF-β1, COL4A1 and IL-6 which would be involved in the role play between miR-29b and epigenetic modulations in DN.
The main focus of this study is on the role of miR-29b in histone modifications occurring in the pathogenesis of DN. In some reports, the interaction between miR-29b and HDAC4 in multiple myeloma was mentioned. However, this correlation has not yet been established in the pathogenesis of DN. Our in-silico analysis showed that miR-29b effectually targets the 3’ UTR region of HDAC4. It has been already reported that HDAC4 is one of the major epigenetic factors that contributes to renal injury by suppressing essential autophagy process and exacerbating inflammation in podocytes [24]. In addition to this, it has been also found that HDAC4 causes upregulation of TGF-β1 which promotes renal fibrosis and also confers to proteinuria in podocytes [3]. It has been already reported that under hyperglycaemic conditions, HDAC4 expression levels get upregulated. HDAC4 causes proteinuria, podocytes’ integrity loss and their apoptosis and inflammation [3, 9]. Moreover, it has been already reported that TGF-β1 downregulates miR-29b in podocytes and promotes renal fibrosis via upregulation of Collagen type-I, III and IV expressions [10].
Literature reports that upon administration of high glucose (30 mM) and TGF-β1 (10 ng/ml), there is elevation in expression levels of TGF-β1 that causes upregulation of Collagen type-I, III and IV. This causes excessive deposition of extracellular matrix (ECM) and ultimately results in renal fibrosis [10]. Hence, in-vitro DN model in human podocytes epithelial cells was generated by inducing stress with high glucose (30 mM) and TGF-β1 (10 ng/ml). To characterize the in-vitro DN model, expression profiles of biomarkers involved in the pathogenesis of DN and the biomarkers NF-κβ, IL-6 and Caspase-3 that are responsible for inflammation and apoptosis respectively were analysed in podocytes [4]. An elevated expression of these markers suggests that due to stress of high glucose and TGF-β1, there is inflammation and apoptosis in podocytes.
Additionally, Synaptopodin-a podocyte-specific marker was found to be downregulated in enhanced DN conditions [30]. In the in-vitro DN model developed in podocytes, Synaptopodin expression was found to be decreased. CD2AP acts as an adapter molecule to bind nephrin carboxy-terminal domain which serves to link the slit diaphragm to the podocyte cytoskeleton. Hence, in DN conditions, downregulation of CD2AP causes loss of podocytes integrity [31]. Podocin is a podocyte-specific marker which is another slit diaphragm protein found to be downregulated in DN conditions [32]. In the generated model, downregulation of Synaptopodin, CD2AP as well as Podocin has been achieved. This suggests that due to the high glucose stress and TGF-β1, loss in slit diaphragm proteins occurs that results in loss of podocytes’ integrity and filtration capacity of GBM.
The manifestations of renal fibrosis is a major phenomenon involved in the pathogenesis of DN. TGF-β1 also gives rise to renal fibrosis by decreasing the expression level of miR-29b and elevating Collagen type-I and IV expressions [10]. Hence, in order to characterize the DN model developed in podocytes, expression profile analysis of TGF-β1 and Collagen-IV was performed showing their upregulation and suggesting that renal fibrosis has been successfully generated in in-vitro DN model developed in podocytes. As the expression levels of miR-29b get downregulated by TGF-β1, expression profile analysis of miR-29b in hyperglycaemic conditions (high glucose concentration in different gradations) and in DN model generated with high glucose and TGF-β1 was also performed showing its downregulation in both the conditions. It has been reported that Histone Deacetylase 4 (HDAC4) was found to be involved in podocyte injury and Renal Fibrosis in DN [9] [3]. In the DN model generated in podocytes, upregulation of HDAC4 was observed suggesting that due to enhanced stress of high glucose and TGF-β1, elevation in HDAC4 was occurred conferring to podocytes’ impairment.
When the miR-29b mimics were transfected in in-vitro DN model generated in podocytes, expression profiles of miR-29b were found to be significantly higher as compared to the control group. This signifies that miR-29b mimics successfully transfected in podocytes. Moreover, upon transfection of miR-29b mimics, downregulation in expression patterns of HDAC4 was observed which indicates that miR-29b targets HDAC4 in the pathogenesis of DN. Upon transfection with miR-29b mimics, downregulation in expression patterns of HDAC4 followed by downregulation in TGF-β1 and Collagen-IV was observed indicating that as HDAC4 downregulation occurs there is subsequent downregulation in expression levels of TGF-β1. In DN pathogenesis, HDAC4 raises the expression levels of TGF-β1 that elevate the expression levels of Collagen-IV causing extracellular matrix deposition and ultimately renal fibrosis. Upon transfection with miR-29b mimics, HDAC4, TGF- β1 and Collagen-IV downregulation occurred, which signifies that miR-29b targets HDAC4 and biomarkers involved in renal fibrosis and could suppress the biological events occurring in podocytes including their integrity loss, apoptosis, inflammation, and proteinuria via inhibition of HDAC4 [24].
It has been already found that due to activation of macrophages under hyperglycemia, release of apoptotic and inflammatory markers occurred [17, 18] and this could be one of the contributors for upregulation of HDAC4 because it has been already reported that HDAC4 is involved in apoptosis and inflammation of podocytes [3]. In our study, we studied expression profiles of miR-29b and HDAC4 in podocytes upon activation of macrophages under hyperglycaemic conditions. For this purpose, we co-cultured podocytes with macrophages and treated with high glucose in which we found downregulation of miR-29b and upregulation of HDAC4.
It has been reported that macrophages release several apoptotic and inflammatory cytokines under stress conditions. Hence, for characterization of this podocytes’ model generated with macrophages and high glucose, we analyzed the expression profiles of the apoptotic marker, Caspase-3 and inflammatory markers, IL-6 which showed a significant elevation in their expressions. Additionally, it has been also reported that activation of macrophages under hyperglycaemic conditions confers to fibrosis [33] and hence, expression profiling of fibronectin, a pro-fibrotic cytokine was performed which showed a significant elevation in its expression suggesting that activation of macrophages under hyperglycaemic conditions might be one of the major factors of podocytes’ injury. Further, we transfected miR-29b mimics in co-culture of podocytes and macrophages treated with high glucose. Herein, we found that there was a reduction in expression levels of HDAC4, TGF-β1, and COL4A1 which showed the effect of miR-29b in decreasing fibrosis in inflammed condition. Amongst several inflammatory markers, we found specific downregulation of IL-6 in co-culture of podocytes and macrophages transfected with miR-29b mimics showing the defensive role of miR-29b podocytes’ inflammation induced specifically due to IL-6.
It has been also reported that IL-6 induces TGF-β1/Smad3 signaling pathway and confers to fibrosis [34, 35]. In this line, when we analyzed the expression profiles of TGF-β1, we found a significant rise in its expression as well in co-culture of podocytes and macrophages when treated with high glucose. It has been reported in a study, upon administration of IL-6 collagen deposition was upregulated via TGF-β1 and miR-29b expression was suppressed. However, knockdown of IL-6 resulted in enhanced expression of miR-29b and mitigation of cardiac fibrosis [36]. This correlation could be established in renal fibrosis because due to overexpression of miR-29b, downregulation in IL-6 occurred that conferred to TGF-β1 downregulation. Surprisingly, we also found that in co-culture of podocytes and macrophages when treated with high glucose, a significant elevation in HDAC4 expression occurred. It has been also reported that HDAC4 promotes the upregulation of TGF-β1 and inflammatory maker, IL-6 and accelerates renal fibrosis and podocytes’ inflammation. However, when co-culture of podocytes and macrophages was treated with high glucose along with miR-29b mimics, expression profiles of HDAC4 were significantly downregulated. This suggests that under hyperglycaemic conditions, due to activation of macrophages, HDAC4 upregulation occurs and this promotes TGF-β1 mediated renal fibrosis. Additionally, upregulation of IL-6 also causes TGF-β1/Smad3 signaling pathway conferring to fibrosis and IL-6 itself is responsible for podocytes’ inflammation. Luciferase assay results suggested that miR-29b effectively targeted HDAC4 due to which overexpression of miR-29b lead to reduced HDAC4 expression levels.
In order to reconfirm the effect of miR-29b mimics on renal fibrosis in DN, in-vivo DN model was generated which showed downregulation in renal tissue levels of miR-29b. In the STZ induced diabetic model, there is an increase in collagen deposition, fibronectin levels, and TGF-β which suggests renal fibrosis and development of DN [37]. After treatment with miR-29b mimics to the diabetic mice, an upregulation in its levels was observed. As miR-29b targeted 3’ UTR of HDAC4, upregulation of miR-29b mimics in the mimics treated group showed decreased levels of HDAC4 in the renal tissue.
The miR-29 family is known to regulate genes of extracellular matrix and regulate fibrosis. Loss of miR-29b is found to increase TGF-β1 mediated renal fibrosis [38]. It was also found that miR-29b lowered collagen deposition via suppression of PI3K/Akt/Sp1 pathway. Also, levels of TGF-β1 and COL4A1 were found to be attenuated after treatment with mimics. It was observed in C57BL/6 J mice that accumulation of macrophages is also amongst the factors responsible for renal fibrosis [39]. Histological analysis also showed that miR-29b mimics were effective in ameliorating renal fibrosis and glomerular damage in DN. This signifies that miR-29b could provide a crucial therapy in histone modifications occurring in the pathogenesis of renal fibrosis and podocytes’ impairment. Reports suggest that microRNAs may have therapeutic applications and they can be used as biomarkers to detect DN at its early stage as well as during disease progression. Disease and tissue specific microRNAs as well as circulatory microRNAs could be approached for DN diagnosis [40]. Apart from this, urinary microRNAs hold a value in non-invasive way of biomarker detection in kidney disorders like DN. In a study carried by Wang et al. suggested that urinary microRNAs like miR-21, miR-29 and miR-93 are novel biomarkers of renal fibrosis [41]. Apart from the use of microRNAs as biomarker in renal damage, microRNAs can also be used as therapeutic targets. Zhong et al. reported that miR-21 inhibition would be an effective therapy for DN. The overexpression as well as knockdown effects of miR-21 in kidney cells suggested that miR-21 plays a role in hyperglycaemia induced fibrotic and inflammatory condition. Moreover, the therapeutic potential of miR-21 in diabetic kidney injury was examined by an ultrasound microbubble mediated transfer of miR-21 in diabetic mice [42]. Hence, microRNAs can be used as biomarkers as well as therapeutic agents in DN and provides hope of novel therapeutic approaches to diagnose and treat DN. Our research will aid future research in this direction to explore miR-29b as a potential biomarker for DN as well develop therapeutic strategies using miR-29b for the treatment of DN.
Conclusion
In conclusion, miR-29b downregulation in DN due to subsequent increase in HDAC4 levels leads to increased severity. After treatment with miR-29b mimics, a decrease in HDAC4 expression, inflammation, apoptosis, podocyte impairment, glomerular damage and renal fibrosis were observed. Hence, exploring the role of miR-29b in histone modifications associated with DN would help in understanding the pathophysiology behind DN in future.
Electronic supplementary material
(PDF 498 kb)
Acknowledgments
The research was carried out at National Institute of Pharmaceutical Education and Research- Ahmedabad with the financial support from the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animals studies
All applicable institutional guidelines for the care and use of animals were followed. All procedures performed in this study involving animals were in accordance with the ethical standards of the institution at which the study was conducted (NIPER-A institutional animal ethics committee, NIPER-A/IAEC/2017/032).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Piyush Gondaliya and Aishwarya Dasare contributed equally and can be interchangeably written as first authors.
Contributor Information
Rakesh Kumar Tekade, Email: rakeshtekade@niperahm.ac.in.
Akshay Srivastava, Email: akshay.srivastava@niperahm.ac.in.
Kiran Kalia, Email: director@niperahm.ac.in.
References
- 1.Siegerist F, et al. Acute podocyte injury is not a stimulus for podocytes to migrate along the glomerular basement membrane in zebrafish larvae. Sci Rep. 2017;7:43655. doi: 10.1038/srep43655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata M. Podocyte injury and its consequences. Kidney Int. 2016;89(6):1221–1230. doi: 10.1016/j.kint.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Lin C-L, et al. MicroRNA-29a promotion of Nephrin acetylation ameliorates hyperglycemia-induced Podocyte dysfunction. J Am Soc Nephrol. 2014;25(8):1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato M, Natarajan R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert RE, et al. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int. 2011;79(12):1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 6.de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, Kitchens CA, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21(5):794–802. [DOI] [PMC free article] [PubMed]
- 7.Jiang M, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82(12):1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang L, Zhou Y, Cao H, Wen P, Jiang L, He W, et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One. 2013;8(4):e60546. [DOI] [PMC free article] [PubMed]
- 9.Wang X, et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014;86(4):712–725. doi: 10.1038/ki.2014.111. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23(2):252–65. [DOI] [PMC free article] [PubMed]
- 11.Chen H-Y, Zhong X, Huang XR, Meng XM, You Y, Chung AC, et al. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther. 2014;22(4):842–53. [DOI] [PMC free article] [PubMed]
- 12.Mannaerts I, Eysackers N, Onyema OO, van Beneden K, Valente S, Mai A, et al. Class II HDAC inhibition hampers hepatic stellate cell activation by induction of microRNA-29. PLoS One. 2013;8(1):e55786. [DOI] [PMC free article] [PubMed]
- 13.Zhou L, Wang L, Lu L, Jiang P, Sun H, Wang H. A novel target of microRNA-29, Ring1 and YY1-binding protein (Rybp), negatively regulates skeletal myogenesis. J Biol Chem. 2012;287(30):25255–25265. doi: 10.1074/jbc.M112.357053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol. 2013;24(5):727–43. [DOI] [PMC free article] [PubMed]
- 15.Amodio N, Stamato MA, Gullà AM, Morelli E, Romeo E, Raimondi L, et al. Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in multiple myeloma. Mol Cancer Ther. 2016;15(6):1364–75. [DOI] [PubMed]
- 16.Gondaliya P, et al. miR29b regulates aberrant methylation in In-Vitro diabetic nephropathy model of renal proximal tubular cells. PLOS ONE. 2018;13(11):e0208044. doi: 10.1371/journal.pone.0208044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. American Journal of Physiology-Renal Physiology. 2013;305(12):F1719–F1727. doi: 10.1152/ajprenal.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, Song Z, Zhou M, Yang Y, Zhao Y, Liu B, et al. Infiltrating macrophages in diabetic nephropathy promote podocytes apoptosis via TNF-α-ROS-p38MAPK pathway. Oncotarget. 2017;8(32):53276–87. [DOI] [PMC free article] [PubMed]
- 19.Das Gupta K, Shakespear MR, Iyer A, Fairlie DP, Sweet MJ. Histone deacetylases in monocyte/macrophage development, activation and metabolism: refining HDAC targets for inflammatory and infectious diseases. Clinical & translational immunology. 2016;5(1):e62–2. [DOI] [PMC free article] [PubMed]
- 20.Wang B, Liu TY, Lai CH, Rao YH, Choi MC, Chi JT, et al. Glycolysis-dependent histone deacetylase 4 degradation regulates inflammatory cytokine production. Mol Biol Cell. 2014;25(21):3300–7. [DOI] [PMC free article] [PubMed]
- 21.Lian X, Chen X, Sun J, An G, Li X, Wang Y, et al. MicroRNA-29b inhibits supernatants from silica-treated macrophages from inducing extracellular matrix synthesis in lung fibroblasts. Toxicology Research. 2017;6(6):878–88. [DOI] [PMC free article] [PubMed]
- 22.Liu BC, Song X, Lu XY, Li DT, Eaton DC, Shen BZ, et al. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta. 2013;1833(6):1434–42. [DOI] [PMC free article] [PubMed]
- 23.Wing MR, et al. DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrology Dialysis Transplantation. 2014;29(4):864–872. doi: 10.1093/ndt/gft537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Q, Dong Z. HDAC4 blocks autophagy to trigger podocyte injury: non-epigenetic action in diabetic nephropathy. Kidney Int. 2014;86(4):666–668. doi: 10.1038/ki.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes. 2005;54(6):1626–1634. doi: 10.2337/diabetes.54.6.1626. [DOI] [PubMed] [Google Scholar]
- 26.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13(12):3005–3015. doi: 10.1097/01.ASN.0000039661.06947.FD. [DOI] [PubMed] [Google Scholar]
- 27.Khullar, M., B.S. Cheema, and S.K. Raut, Emerging Evidence of Epigenetic Modifications in Vascular Complication of Diabetes. Front Endocrinol, 2017. 8(237). [DOI] [PMC free article] [PubMed]
- 28.Yi C, Yu L. How does acetylation regulate autophagy? Autophagy. 2012;8(10):1529–1530. doi: 10.4161/auto.21156. [DOI] [PubMed] [Google Scholar]
- 29.Sankrityayan H, Kulkarni YA, Gaikwad AB. Diabetic nephropathy: the regulatory interplay between epigenetics and microRNAs. Pharmacol Res. 2019;141:574–585. doi: 10.1016/j.phrs.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11(9):1656–66. [DOI] [PubMed]
- 31.Benigni A, Gagliardini E, Tomasoni S, Abbate M, Ruggenenti P, Kalluri R, et al. Selective impairment of gene expression and assembly of nephrin in human diabetic nephropathy. Kidney Int. 2004;65(6):2193–200. [DOI] [PubMed]
- 32.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–96. [DOI] [PMC free article] [PubMed]
- 33.Schoeler D, Grützkau A, Henz BM, Küchler J, Krüger-Krasagakis S. Interleukin-6 enhances whereas tumor necrosis factor α and interferons inhibit integrin expression and adhesion of human mast cells to extracellular matrix proteins. J Investig Dermatol. 2003;120(5):795–801. doi: 10.1046/j.1523-1747.2003.12126.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-β receptor compartmentalization and turnover enhances TGF-β1 signaling. J Biol Chem. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 35.Villiger P, et al. IL-1 beta and IL-6 selectively induce transforming growth factor-beta isoforms in human articular chondrocytes. J Immunol. 1993;151(6):3337–3344. [PubMed] [Google Scholar]
- 36.Zhang Y, et al. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFβ1 and miR-29 pathways. Sci Rep. 2016;6:23010. doi: 10.1038/srep23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira, A., M.J. Pires, and P.A. Oliveira, Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In vivo (Athens, Greece), 2017. 31(1): p. 1–22. [DOI] [PMC free article] [PubMed]
- 38.Hsu Y-C, et al. Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling. Sci Rep. 2016;6:30575. doi: 10.1038/srep30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow FY, et al. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrology Dialysis Transplantation. 2004;19(12):2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 40.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Current diabetes reports. 2016;16(3):35–5. [DOI] [PMC free article] [PubMed]
- 41.Wang G, et al. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol. 2012;36(5):412–418. doi: 10.1159/000343452. [DOI] [PubMed] [Google Scholar]
- 42.Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56(3):663–74. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 498 kb)