Abstract
Background
A complex community of microorganisms in the gastrointestinal (GI) tract, known as the gut microbiota, exerts major effects on gene expression and cytokine profile. Extracellular vesicles (EVs) which are produced by bacteria could be sensed by Toll like receptors (TLRs). The interaction between gut microbiota and TLRs affects homeostasis and immune responses. In this study, we evaluated TLR9 gene expression and cytokines level in Caco-2 cell line treated with Lactobacillus casei as one of the gut microbiota and its EVs.
Methods
In the present study, L. casei derived EVs was extracted via ultracentrifugation. The quality control assessment included the evaluation of physicochemical characteristics of EVs. For the treatment of Caco-2 cell line, L. casei and its EVs (100 and 150 μg/mL) were used. In addition, qRT-PCR assay was carried out to evaluate the mRNA expression of TLR9 gene. ELISA assay was also performed to determine the levels of IFNγ, TNF-α, GM-CSF, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12, IL-17A, and IL-10 cytokines.
Results
The results showed that L. casei slightly increased TLR9 gene expression in the Caco-2 cell line. It was also found that EVs at concentrations of 100 and 150 μg/mL could significantly decrease TLR9 gene expression. Furthermore, L. casei significantly increased IL-10 and IFNγ levels. Based on the findings, the level of IL-17A, as a proinflammatory cytokine, decreased by L. casei. Both concentrations of EVs decreased the level of IFNγ, while increasing the concentrations of IL-4 and IL-10. EVs from L. casei could modulate immune responses in the Caco-2 cell line. Both EVs and L. casei activated the expression and secretion of several cytokines.
Conclusions
L. casei and its EVs have pivotal role in the cross talk between gut microbiota and the host especially in the modulation of the immune system. This study shows for the first time the increasing level of anti-inflammatory cytokines by EVs released by L. casei. Based on the last studies on immunomodulatory effect of EVs on immune cells and our results in cell line level, we postulate that L. casei derived EVs could be possible candidates for the reduction of immune responses.
Keywords: Lactobacillus casei, Extracellular vesicles, Toll-like receptors, Gut microbiota
Introduction
The gastrointestinal (GI) tract contains a large community of microorganisms. Colonization of these microorganisms has important implications for the immune system function, digestion, and other host activities. Many microorganisms can be found in the digestive tract. Microbiota is a microbial community in the digestive tract, which increases the host metabolic capacity for processing food and drug compounds and regulates various signaling pathways. Therefore, human health is majorly affected by the digestive tract microbiota, considering the gastrointestinal hemostasis, immune system regulation and development, and protection against pathogens. Besides digestion, microbiota also has various other essential functions, including vitamin production, immune system modulation, and gastrointestinal communication [1]. Gut microbiota is recognized as a regulatory immune system, given the presence of a variety of immunogenic molecules, such as extracellular vesicles (EVs), lipopolysaccharides, and peptidoglycans [2, 3].
The gut barrier, as the first line of defense in the gut innate immunity, is composed of intestinal epithelial cells (IEPCs), mucus membranes produced by goblet cells, and innate and adaptive immune factors (e.g., immunoglobulins and antimicrobial peptides). The gut barrier characterizes the gut microbiota and its interactions with the host [4, 5]. The function of GI tract is monitored by pattern recognition receptors (PRRs) [6], including toll-like receptors (TLRs). PRRs comprise a group of proteins, which respond to small molecular sequences in the microorganism. These sequences are known as microbe-associated molecular patterns (MAMPs) [7].
Different cells, such as IEPCs, express TLR genes [8]. TLRs are expressed by IEPCs which has regulating effect on the immune system [9]. On the other hand, dysbiosis is caused by an imbalance between the intestinal microbiota and the immune system. In fact, changes in the gut microbial ecosystem result in many metabolic disorders, such as leaky gut syndrome, where excessive TLR activation occurs in IEPCs [10]. This situation that is characterized by impaired permeability of gut barrier, known as leaky gut syndrome, result in a great activation of TLRs in intestinal epithelial cells (IEPCs). As a result, increased cytokines and chemokines lead to low grade inflammation. Increased inflammatory cytokines disrupt insulin signaling cascade and may cause insulin resistance (IR), ultimately stimulate metabolic syndrome and obesity [11].
A promising therapeutic strategy is to use probiotics. Bacteria from the genus Lactobacillus are among the most common probiotics. Lactobacillus casei, as one of the most common Lactobacillus species, is a probiotic with different industrial applications. The beneficial effects of this species on immunity [12], allergies [13], and cholesterol level [13] have been confirmed in previous studies. Also, according to some studies, L. casei improves the gut microbiota pattern [14].
The food industry has widely used lactic acid bacteria in its products. Therefore, it is important to identify the advantages of these bacteria in order to propose new bacterial components. EVs are identified as molecular components, which are produced by bacteria. They are spherical bilayer lipid structures (diameter, 20–500 nm), which normally have a varied cargo including nucleic acids, lipoproteins, and toxins; they also play important roles in metabolic and immune systems [15].
No study has yet evaluated the characteristics of EVs, which are derived from L. casei and trigger innate inflammatory responses. In this study, we selected L. casei, as an outstanding probiotic strain in the GI tract, due to its therapeutic advantages, such as modulation of immune responses [16]. Since EVs also contain bacterial MAMPs, it was assumed that they play a significant role in modulating innate immune responses. The current study aimed at evaluating the effects of these nanostructures on TLR9 gene expression and cytokine profile in the Caco-2 cell line as an IEPC model.
Materials and methods
Strains and cultures
After L. casei (ATCC 393 strain) was prepared, a single colony of L. casei was inoculated and cultured on the MRS medium (Sigma-Aldrich, USA). Bacteria were inoculated in MRS broth for 24 h at 37 °C to collect more biomasses [17].
Isolation of EVs
EVs were extracted from culture supernatants. The supernatants were extracted from cultures, which were centrifuged (Beckman, USA) at 20000×g for one hour at 4 °C. Next, ultracentrifugation (Beckman, USA) was conducted for two hours at 125000×g (4 °C) in duplicate. Particle-free sucrose 3% was also used to resuspend the samples. To remove large particles and possible contaminants, the supernatants were passed through a filter (0.22 μm). For further analyses, the sample subsets were stored in a freezer (−80 °C) for long-term storage [18]. The absorbance of EVs was determined using a NanoDrop technique, which could indicate the protein concentration after ultracentrifugation. Also, protein bands of EVs were identified using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Scanning electron microscopy (SEM)
After fixing and washing the EVs in PBS, consisting of 2% paraformaldehyde and 2.5% glutaraldehyde, the samples were air-dried and gold-coated with using a sputter coating system (KYKY Technology, China), based on physical vapor deposition. Finally, the samples were evaluated in a SEM system (KYKY Technology, China) [19].
Measurement of EV size via dynamic light scattering (DLS) and zeta potential measurements
The size distribution and diameter of the isolated EVs, which were resuspended in a sucrose solution, were determined using DLS in a particle size analyzer (Nano-ZS, ZEN3600, Malvern Instruments, UK). The L. casei-derived EVs were prepared via sonication at 35 kHz for 3 min in a Bandelin ultrasonic bath. The zeta potential of EVs in 3% sucrose solution was evaluated using the Malvern Zetasizer (Nano ZEN3600, Malvern Instrument, Malvern, UK) [20].
Zeta potential measurements
A zeta potential analyzer (Nano-ZS, ZEN3600, Malvern Instruments, UK) was used to determine the zeta potential of EVs in 3% sucrose solution [20].
Cell culture and EV treatment
The human colon carcinoma cell line, IBRC C10094 Caco-2, was provided by the Biological Resource Center (Tehran, Iran). It was then cultured in DMEM/high glucose medium (Gibco, USA), which was supplemented with 1% penicillin/streptomycin (Gibco, USA) and 10% fetal bovine serum (FBS, Gibco, USA) at 37 °C in a 5% CO2 atmosphere [21]. The confluent cells were harvested and seeded into the wells of six-well plates. EVs (100 and 150 μg/mL), L. casei with a multiplicity of infection (MOI) of ~100:1 (100 bacteria:cells) [22], and control samples including sucrose and PBS were added to each well, followed by overnight incubation until further assays.
RNA extraction and cDNA preparation
The RNX-Plus kit (CinnaGen Co., Tehran, Iran) was used to extract total RNA from studied samples, according to manufacturer’s protocols. The quality and quantity of extracted RNA was evaluated by using electrophoresis on 1% agaros gel and spectrophotometery (NanoDrop 2000, Thermo Fisher Scientific, USA), respectively. For reverse-transcribing RNA to cDNA, oligo (dT) primers were used in a RevertAid first-strand cDNA synthesis kit (Thermo Scientific, USA) in accordance with the manufacturer’s protocols.
Gene expression analysis
In order to characterize the effect of L. casei and its EVs on the expression level of TLR9 gene and the housekeeping gene, GAPDH, quantitative reverse transcription-PCR (qRT-PCR) was carried out in a LightCycler® 96 SW 1.1 system (Roche, Germany). The reaction mixture contained 1 μL of DNA template (50 ng), 8 μL of nuclease-free water, 10 μL of SYBR Green PCR Master Mix (Takara, Japan), and 0.5 μL of each primer (10 pmoL). Specific primers for TLR9 gene were applied to amplify TLR9 and GAPDH genes, based on the qRT-PCR assay. The used primers and their sequences were as follows: TLR9 gene (F, 5’-CTGCCACATGACCATCGAG and R, 5’GGACAGGGATATGAGGGATTTGG) and GAPDH gene (F, 5’-GGAGCGAGATCCCTCCAAAAT and R, 5′: GGCTGTTGTCATACTTCTCATGG) [23, 24]. The qPCR thermal cycling was first incubation at 95 °C for 60 s, followed by 40 cycles of including 95 °C for 5 s, 55 °C (annealing) for 30 s, 72 °C for 30 s, and melting curve was drawn by gradual increasing of temperature from 55 to 95 °C. To draw the standard curve and calculating the amplification efficiency, a 10-fold serial dilution was prepared from template cDNA, so that the 6th sample had concentration of 10−6.
Each sample was run in triplicate to ensure the reliability of the results. Also, a non-template control (NTC) was included in each run. Gene expression was normalized to GAPDH transcript in each sample, and fold changs was calculated using the 2-∆∆Ct method [25].
ELISA assay
After 24 h of incubating the cells with L. casei and its EVs, the supernatants were stored at −80 °C. Cytokine production (IFNγ, TNF-α, GM-CSF, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12, IL-17A, and IL-10) was analyzed using an ELISA kit (Multi-Analyte ELISArray Kit; Qiagen, Germany). The Multi-Analyte ELISArray Kit allows us to obtain relative qualitative profiling results from the samples. The levels of 12 cytokines in the samples were determined by comparing their optical densities. All analyses were performed in duplicate.
Data analysis
For comparisons between the test and control groups, independent sample t test and one- way ANOVA was performed in GraphPad Prism 7.04 (GraphPad, La Jolla, CA, USA). The significance levels were set as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Characteristics of L. casei-derived EVs
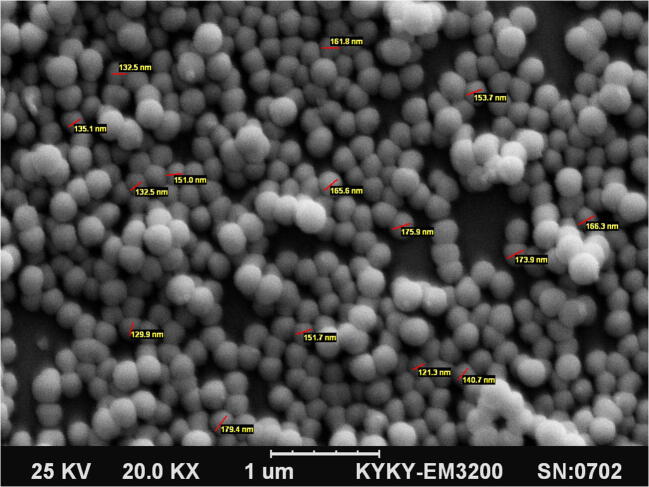
L. casei produced EVs in the MRS broth. SEM was used to determine the morphology and size of EVs. Based on the findings, the spherical EVs diameter ranged from 100 nm to 200 nm (Fig. 1). Mean dimension of EVs was 140.7 ± 20.3 nm.
Fig. 1.
L. case-derived EVs with an average diameter of 100–200 nm (magnification, ×20 K)
SDS-PAGE assay
The SDS-PAGE assay on 12% gel was applied to determine the protein patterns. The protein content was measured to be 2.4 mg/mL. Also, the EVs protein bands were in the range of 10–180 kD, based on this technique (Fig. 2).
Fig. 2.
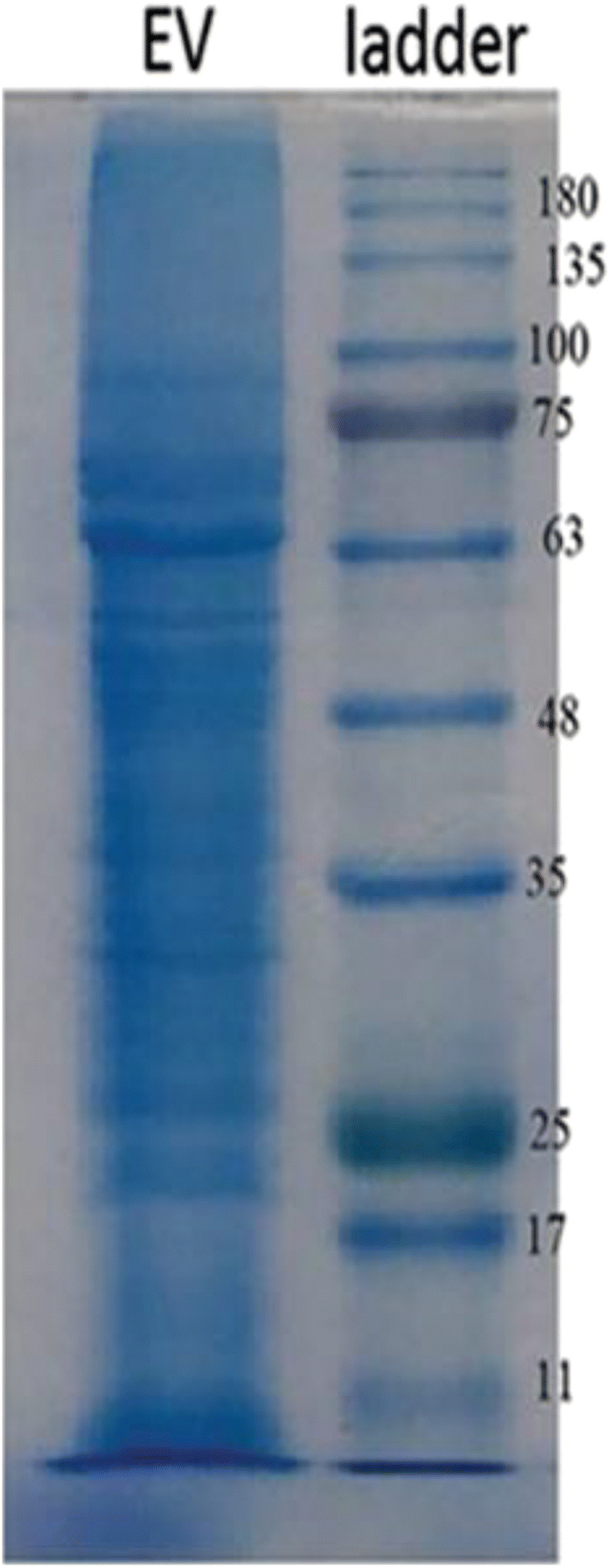
Protein profiles of L. casei-derived EVs (EVs were isolated from the supernatant via ultracentrifugation)
Physical characterization of L. casei-derived EVs and zeta potential measurements
EVs from L. casei cultures were studied using dynamic light scattering (DLS) to determine the approximate size of the vesicles. DLS revealed two populations of approximately 227.3 and 38.19 nm from L. casei collected via ultracentrifugation (Fig. 3). In this study, the zeta potential of the isolated EVs was determined. Based on the findings, the zeta potential measurements were negative. The zeta potential of EVs, extracted via ultracentrifugation, was −0.5 mV, as shown in Fig. 4.
Fig. 3.

Physicochemical characteristics of L. casei-derived EVs based on DLS. Size distribution is determined based on the intensity of EVs in the ultracentrifugation technique. The DLS histogram indicates the bimodal distribution of nanovesicles
Fig. 4.

The zeta potential measurements of EVs extracted via ultracentrifugation
TLR9 gene expression
The effects of L. casei and its EVs on TLR9 gene expression in the human Caco-2 cell line were investigated using the qRT-PCR assay. L. casei slightly increased the gene expression in comparison with the control group (PBS) (Fig. 5a). The cell line was treated with L. casei-derived EVs at two concentrations of 100 and 150 μg/mL. The mRNA level of TLR9 gene significantly decreased at both concentrations of EVs (Fig. 5b).
Fig. 5.

The qRT-PCR analysis of L. casei and its EVs regarding TLR9 gene expression. A) The cells were serum-deprived and treated with L. casei (MOI ∼ 100:1) or PBS overnight. B) Under similar conditions, the other group of cells was treated with L. casei-derived EVs (100 and 150 μg/mL) or sucrose overnight. *P < 0.05 was statistically significant
Cytokine profiles
In order to investigate the effects of L. casei and its EVs on cytokine profile in the Caco-2 cell line, the Multi-Analyte ELISArray Kit was used. After overnight stimulation of the cell line with L. casei, the levels of IL-10, IFNγ, and GM-CSF cytokines increased significantly, whereas the concentrations of IL-17a and IL-2 decreased. However, the level of other cytokines did not change significantly (Fig. 6a).
Fig. 6.
The ELISA assay of L. casei and its EVs regarding the cytokine profile. A) The cells were serum-deprived and treated with either L. casei at a MOI ∼ 100:1 or PBS overnight. B) Under similar conditions, the other group was treated with L. casei-derived EVs (100 μg/mL) or sucrose overnight. C) Under similar conditions, the final group of cells was treated with either L. casei-derived EVs (150 μg/mL) or sucrose. *P < 0.05 and **P < 0.01 were considered statistically significant. All analyses were performed in duplicate
The levels of IL-10 and IL-4 significantly increased by using 100 and 150 μg/mL of EVs (Fig. 6b, c). On the other hand, the level of IFNγ decreased at both concentrations of EVs. Following treatment with EVs at 100 μg/mL, the levels of IL-6 and GM-CSF slightly increased; however, other cytokines did not change significantly. On the other hand, insignificant changes occurred in the levels of IL-1b and IL-2 following treatment with EVs at 150 μg/mL.
Discussion
No study has yet evaluated the characteristics of EVs, which are derived from L. casei and trigger innate inflammatory responses so this experimental study aimed to determine the physicochemical characteristics of EVs derived from L. casei, and to compare the effects of L. casei and its EVs on TLR9 gene expression and cytokine profile in the Caco-2 cell line.
Based on the findings, L. casei could secrete EVs with specific properties. L. casei-derived EVs, which were isolated via ultracentrifugation, indicated two peaks. The results showed the nanoscale size of L. casei EVs, as 90% of particles exhibited an average size below 400 nm. This method is considered suitable for studying particle size in suspensions and calculating the differential size distribution of EV populations. Differences in the average size of L. casei-derived EVs may suggest the potential cargo-sorting effect of molecules, resulting in the nano-structure of EVs. The results of DLS analysis were confirmed via electron microscopy.
In order to determine the stability of EVs in terms of aggregation, flocculation, or dispersion, the zeta potential of nanoparticles was measured. EVs, extracted via ultracentrifugation, were negatively charged, based on the zeta potential measurements. The zeta potential in this study resembled that of EVs reported by Dean et al. [26]. The small zeta potential of EVs represents their instability in solutions and indicates the need for caution in storing and handling EVs at temperatures other than −80 °C.
In addition, TLR9 gene was evaluated in this experimental study. Generally, TLRs contribute to immune responses, and L. casei is known to affect the immune system and homeostasis [12]. Evidence suggests that administration of L. casei (CRL 431) increases the expression of TLR9 genes and improves TNF-α, IFN-γ, and IL-10 production [27]. In addition, maintenance of intestinal homeostasis is dependent on TLR signaling [28]. Therefore, identification of compounds released by bacteria and their receptors is very important.
TLR signaling is involved in the proliferation and differentiation of intestinal epithelial cells and can induce the production of inflammatory and anti-inflammatory cytokines. In this study, it was shown that bacteria can stimulate TLR9 genes, while TLR9 mRNA level decreased significantly by L. casei-derived EVs. A remarkable finding of this experiment was that EVs might contain cargos, which reduce the expression of this gene. EVs at a concentration of 150 μg/mL showed the greatest reducing effect on TLR9 gene expression versus L. casei and EVs at 100 μg/mL. Considering the importance of appropriate immune responses, our findings revealed a new mechanism regulating TLR9 expression by L. casei through EVs.
GI bacteria, such as L. casei, can mediate cytokine secretion. Chiba et al. found that L. casei, as an important probiotic, can induce a high concentration of IL-12 and stimulate the immune system. It was also found that L. casei could have modulating effects on the immune system by inhibiting IL-17 production [29]. Wang et al. demonstrated that probiotic L. casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure [30]. Nunez et al. showed that L. casei CRL 431 administration decreases inflammatory cytokines in mouse model [31]. The present results showed that L. casei and its EVs could alter the cytokine profile in the Caco-2 cell line. Also, the effect of L. casei on IL-10 and IFNγ production was particularly significant in the Caco-2 cell line. Overall, the main characteristic of L. casei is to induce the production of IL-10 and IFNγ. It is known that IL-10 and IFNγ production is an important immunoregulatory effect of probiotics. Therefore, L. casei can be characterized as a probiotic with an immunoregulatory potential via reduction of IL-17a and induction of IL-10 and IFNγ production.
Ahmadi Badi S et al. demonstrated that outer membrane vesicles (OMVs) of Bacteroides fragilis, had stimulatory effect on anti-inflammatory cytokines (IL-4 and IL-10) while it decreased IFNγ concentration as a pro-inflammatory cytokine in Caco-2 cell line [32]. Shen Y et al. showed that OMVs of a human commensal mediate immune regulation and disease protection [33]. Maerz J.K et al. proved that OMVs blebbing contributes to B. vulgatus mpk-mediated immune response silencing [34]. Fábrega MJ et al. showed anti-inflammatory Effects of OMVs from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice [35]. Kang C.-S et al. showed that EVs derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis [36]. Seo M.K et al. showed Therapeutic effects of kefir grain Lactobacillus derived EVs in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease [37]. Al-Nedawi K et al. showed that Lactobacillus rhamnosus increase the gut DC content and enhanced IL-10 secretion [38]. Kim JH proved that EVs derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression [18]. Aghasadeghi MR et al. showed that OMVs of Neisseria meningitidis serogroup B induce strongly Th1-oriented responses [39].
In this study, the Caco-2 cell line, treated with EVs, showed significant upregulation of IL-4 and IL-10. On the other hand, the level of IFNγ, as the main proinflammatory cytokine secreted by Th1 cells, significantly decreased by EVs. It can be concluded that EVs exert beneficial effects by increasing the production of IL-4 and IL-10 and decreasing the production of IFNγ. Overall, EVs seem to be more effective in modulating immune responses, compared to L. casei. In the present study, after extracting and evaluating the physicochemical characteristics of L. casei-derived EVs, their effects on TLR9 gene expression, as well as proinflammatory and anti-inflammatory cytokines, were evaluated. Based on the present findings, EVs may be proper suitable for modulation of inflammatory responses. It is speculated that EVs carry a small amount of microorganism cargo. For this reason, it has been more effective in modulating the immune system than the bacteria themselves.
We believe that this is the first study reporting the effects of L.casei derived EVs on TLR9 gene expression, as well as the concentration of IFNγ, IL-10 and IL-4 on Caco-2 cell line. Interestingly, L.casei derived EVs had stimulatory effect on anti-inflammatory cytokines (IL-4 and IL-10) while it decreased IFNγ concentration as a pro-inflammatory cytokine.
Given the protein diversity of EVs, it is suggested that further studies of the exact protein composition of this nanostructure should be determined. The small zeta potential indicates the instability of vesicles in solution. Therefore, the extracted EVs require storage at −80 °C and further studies on stability should be carried out on the stability of EVs.
Contrary to the roles described for the application of EVs in numerous articles, however, the production pathway and mechanism of regulation and selection of materials within EVs and what factors are involved in the production and secretion of these nanostructures not fully understood. Successful separation and purification and proper production of these nanostructures and other signals which regulate the pathway for the production or biogenesis of EVs is a critical step for further analysis.
Acknowledgements
We thank Prof. Andrea Masotti for his comments and editorial support. We are also grateful to our colleagues at Mycobacteriology and Pulmonary Research Department and Microbiology Research Center of Pasteur Institute of Iran. This research received funding from the National Institute for Medical Research Development by project no.942995 and Pasteur Institute of Iran.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 2.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MurAcA M, Putignani L, FIerAbrAccI A, Teti A, Perilongo G. Gut microbiota-derived outer membrane vesicles: under-recognized major players in health and disease. Discov Med. 2015;19(106):343–348. [PubMed] [Google Scholar]
- 4.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10):e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vereecke L, Beyaert R, van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17(10):584–593. doi: 10.1016/j.molmed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 7.Akhter N, Wu B, Memon AM, Mohsin M. Probiotics and prebiotics associated with aquaculture: a review. Fish & shellfish immunology. 2015;45(2):733–741. doi: 10.1016/j.fsi.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Yu S, Gao N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci. 2015;72(17):3343–3353. doi: 10.1007/s00018-015-1931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6(3):451. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome medicine. 2016;8(1):42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73(1):147–162. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdeano CM, Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clinical and Vaccine Immunology. 2006;13(2):219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Kumar M, Ghosh M, Ganguli A. Modeling in vitro cholesterol reduction in relation to growth of probiotic Lactobacillus casei. Microbiol Immunol. 2013;57(2):100–110. doi: 10.1111/1348-0421.12008. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Devi M. Probiotics: a comprehensive approach toward health foods. Crit Rev Food Sci Nutr. 2014;54(4):537–552. doi: 10.1080/10408398.2011.594185. [DOI] [PubMed] [Google Scholar]
- 15.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13(10):620. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitvogel L, Daillère R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017;15(8):465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- 17.Xu M, Gagné-Bourque F, Dumont M-J, Jabaji S. Encapsulation of Lactobacillus casei ATCC 393 cells and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J Food Eng. 2016;168:52–59. doi: 10.1016/j.jfoodeng.2015.07.021. [DOI] [Google Scholar]
- 18.Kim J-H, Jeun E-J, Hong C-P, Kim S-H, Jang MS, Lee E-J, et al. Extracellular vesicle–derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. Journal of Allergy and Clinical Immunology. 2016;137(2):507–16. e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, Watanabe H, Senpuku H. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One. 2011;6(10):e26163. doi: 10.1371/journal.pone.0026163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunath K, von Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89(1):113–125. doi: 10.1016/S0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrov Z, Gotova I, Chorbadjiyska E. In vitro characterization of the adhesive factors of selected probiotics to Caco-2 epithelium cell line. Biotechnology & Biotechnological Equipment. 2014;28(6):1079–1083. doi: 10.1080/13102818.2014.969948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tien M-T, Girardin SE, Regnault B, Le Bourhis L, Dillies M-A, Coppée J-Y, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176(2):1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 23.He Y-z, Liang Z, Wu M-r, Wen Q, Deng L, C-y S, et al. Overexpression of EPS8 is associated with poor prognosis in patients with acute lymphoblastic leukemia. Leuk Res. 2015;39(6):575–581. doi: 10.1016/j.leukres.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Lin A, Zhang C, Tian Z, Zhang J. Phosphorothioate-modified CpG oligodeoxynucleotide (CpG ODN) induces apoptosis of human hepatocellular carcinoma cells independent of TLR9. Cancer Immunol Immunother. 2014;63(4):357–367. doi: 10.1007/s00262-014-1518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–8. [DOI] [PubMed]
- 26.Dean SN, Leary DH, Sullivan CJ, Oh E, Walper SA. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci Rep. 2019;9(1):877. doi: 10.1038/s41598-018-37120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo NA, Perdigón G, de LeBlanc ADM. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC microbiology. 2011;11(1):177. doi: 10.1186/1471-2180-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, Shimizu T, Yamashiro Y, Kiyoshima-Shibata J, Nanno M, Nomoto K. Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology. 2010;130(3):352–362. doi: 10.1111/j.1365-2567.2009.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Xie J, Li Y, Dong S, Liu H, Chen J, Wang Y, Zhao S, Zhang Y, Zhang H. Probiotic Lactobacillus casei Zhang reduces pro-inflammatory cytokine production and hepatic inflammation in a rat model of acute liver failure. Eur J Nutr. 2016;55(2):821–831. doi: 10.1007/s00394-015-0904-3. [DOI] [PubMed] [Google Scholar]
- 31.Núñez IN, Galdeano CM. de LeBlanc AdM, Perdigón G. Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition. 2015;31(7–8):1000–1007. doi: 10.1016/j.nut.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Badi SA, Khatami S, Irani S, Siadat SD. Induction Effects of Bacteroides fragilis Derived Outer Membrane Vesicles on Toll Like Receptor 2, Toll Like Receptor 4 Genes Expression and Cytokines Concentration in Human Intestinal Epithelial Cells. Cell Journal (Yakhteh). 2019;12(1). [DOI] [PMC free article] [PubMed]
- 33.Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12(4):509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maerz JK, Steimle A, Lange A, Bender A, Fehrenbacher B, Frick J-S. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes. 2018;9(1):1–12. doi: 10.1080/19490976.2017.1344810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fábrega M-J, Rodríguez-Nogales A, Garrido-Mesa J, Algieri F, Badía J, Giménez R, et al. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.C-s K, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS one. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo M, Park E, Ko S, Choi E, Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4, 6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J Dairy Sci. 2018;101(10):8662–8671. doi: 10.3168/jds.2018-15014. [DOI] [PubMed] [Google Scholar]
- 38.Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao Y-K, Forsythe P, et al. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2014;29(2):684–695. doi: 10.1096/fj.14-259721. [DOI] [PubMed] [Google Scholar]
- 39.Reza Aghasadeghi M, Sharifat Salmani A, Mehdi Sadat S, Javadi F, Memarnejadian A, Vahabpour R, et al. Application of outer membrane vesicle of Neisseria meningitidis serogroup B as a new adjuvant to induce strongly Th1-oriented responses against HIV-1. Curr HIV Res. 2011;9(8):630–635. doi: 10.2174/157016211798998772. [DOI] [PubMed] [Google Scholar]