Abstract
Background and aims
сomparative animal study of effectiveness of intermittent administration of lyophilized single-, three- and alive multistrain probiotic in short courses on insulin resistance (IR) in rats with experimental obesity.
Methods
70 rats were divided into 7 groups (n = 10 in each). Rats of group I were left intact. Newborn rats in groups II-VII were administered monosodium glutamate (MSG) (4 mg/g) by injection. Rats in group II (MSG-obesity group) were left untreated. The rats in groups III-V received lyophilized mono-probiotics B.animalis VKL, B.animalis VKB, L.casei IMVB-7280 respectively. The rats in group VI received all three of these probiotic strains mixed together. Group VII was treated with multi-probiotic “Symbiter”, containing 14 different live probiotic strains (Lactobacillus, Bifidobacterium, Propionibacterium, Acetobacter genera).
Results
Treatment of newborn rats with MSG lead to the development of obesity in all MSG-obesity rats and up to 20–70% after probiotic administration. Additions to probiotic composition, with preference to alive strains (group VII), led to significantly lower rates of obesity, decrease in HOMA-IR (p < 0.001), proinflammatory cytokines levels – IL-1β (p = 0.003), IL-12Bp40 (p < 0.001) and elevation of adiponectin (p = 0.003), TGF-β (p = 0.010) in comparison with MSG-obesity group. Analysis of results in groups treated with single-strain probiotics (groups III-V) shows significant decrease in HOMA-IR, but changes were less pronounced as compared to mixture groups and did not achieve intact rats level. Other metabolic parameters were not affected significantly by single strains.
Conclusion
Our findings provide major clues for how to design and use probiotics with more efficient compositions in obesity and IR management and may bring new insights into how host-microbe interactions contribute to such protective effects.
Keywords: Obesity, Insulin resistance, Lyophilized and alive probiotic strains, Lactobacillus, Bifidobacterium, Multistrain probiotics
Introduction
As reported by The World Health Organization (WHO) in 2014, over 1.9 billion adults are overweight, and more than one-third of them are obese [1]. Obesity and insulin resistance (IR) are the major predisposing factors that increase risks of comorbidities, such as type 2 diabetes (T2D), metabolic syndrome, hypertension, hyperlipidemia, nonalcoholic fatty liver disease (NAFLD), cardiovascular diseases, and several types of cancer [2], which inevitably brings a heavy financial burden including direct and indirect costs [3].
IR may be defined as a subnormal glucose response to insulin and most commonly occurs in association with obesity [4]. Over the past 10 years, an increasing number of sources have suggested other components of the mechanisms of these obesity/IR interactions that lie between the genetic and environmental factors, where the gut microbiota are now considered to make an important contribution to these mechanisms [5, 6]. Data from previous studies present several mechanisms that could help explain the link between changes in gut microbiota and pathogenesis of IR and are related to lipopolysaccharide (LPS), short chain fatty acids (SCFA) and bile acids metabolism [5, 7]. By increasing production of SCFAs such as acetate, propionate and butyrate, gut microbiota activates free fatty acids receptors (FFAR) in intestine enteroendocrine cells can stimulate the secretion of incretin hormones such as glucagon-like peptide (GLP)-1 and GLP-2. Incretins directly stimulated insulin releasing and adiponectin gene expression, which contribute to pancreatic ß-cells proliferation and improvement of insulin sensitivity [8, 9]. Another mechanism by which the microbiome may contribute to IR is significant alterations in the intestinal barrier which lead to increased intestinal permeability, with subsequent translocation of microbiome-derived LPS to the bloodflow [6]. Up to threefold increase in serum LPS concentrations named metabolic endotoxemia which interacts with CD14 TLR-4 (toll-like receptor-4) receptor complex and acts as a trigger factor that induces low-grade systemic inflammation and initiates pro-oxidative stress status [10]. Finally, gut microbiota via modulation of bile salt hydrolase enzyme (BSH) activity, can directly increase the levels of primary bile acid which in turn binds and activates the farnesoid X receptor (FXR) [11]. Activation of FXR leads to increased storage of glucose, decreased production of glucose from non-glucose nutrients, increases synthesis and secretion of insulin [11, 12].
Probiotics are defined as live microorganisms that are beneficial to the health of the host when administered in adequate amounts [13]. Strains of lactic acid bacteria belonging to the genera Lactobacillus and Bifidobacterium are commonly used as probiotics and are the most studied strains in the treatment and prevention of obesity-associated disorders [14–16]. Moreover, several potential bacterial candidates, such as Akkermansia muciniphila, Parabacteroides goldsteinii, Enterobacter halii or Saccharomyces cerevisiae var. boulardii, have been identified and novel mechanisms of action intervening their positive effects for IR/obesity were discovered [17–19].
The background of current study, were previously reported by our group findings that probiotic composition, with preference to alive strains, led to a significantly lower prevalence of obesity, reduction of visceral adipose tissue weight and serum lipid levels as compared to single-strain probiotic in rats with monosodium glutamate (MSG)-induced obesity [20]. Based on this suggestion and according to well known fact that obesity contributes to IR and associated with low-grade systemic chronic inflammation it was interesting to investigate strain-specific impact of probiotic on these parameters.
So, aim of the current study was to conduct сomparative effectiveness analysis of intermittent administration of lyophilized single-, three- and live multi-strain probiotic in short courses on IR and chronic inflammatory markers in rats with experimental MSG-obesity.
Methods
Ethics statement
All procedures performed in animals were in accordance with general ethical principles of animal experiments, approved by the First National Congress on Bioethics Ukraine (September 2001). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Taras Shevchenko National University of Kyiv (Protocol number: 2/2017).
Study design
The study was conducted using 70 newborn Wistar male rats, which were divided into 7 groups of 10 animals each. In group I (intact animals) saline was administered subcutaneously (s/c) at 2nd, 4th, 6th, 8th and 10th postnatal days. All newborn rats of the II-VIII groups for experimental obesity induction were injected with MSG at the same timeslot as previously described [21–25]. The volume of both saline and MSG for injection was the same for all groups (I-VIII) and calculated as follow – 4 mg/g body weight per day.
All animals selected for the experiment were standardized six pups per mother to ensure better lactation and subjected to veterinary examination, acclimatization for five days, and then randomly divided into one of 6 treatment groups, numbered and appropriately labeled. We started administration of probiotic strains to rats at the age of 30 days and continued for 3 months in 2 two-week courses (1 course per month) [23, 24]. From weaning (1 month) to 4 month of age, rats had unrestricted access to standard rodent chow (PurinaW) and water during the entire experimental period [20].
Group II (MSG-obesity) received 2.5 ml / kg of water (intragastrically). Group III-VII were administrated with different probiotic mixtures. The groups III-V were monocomponent and received single-strain lyophilized probiotics Bifidobacterium animalis VKL (group III), Bifidobacterium animalis VKB (group IV) and Lactobacillus casei IMVB-7280 (group V) respectively. The probiotics were administrated at an average total daily dose of 50 mg/kg (5 × 109 CFU/kg).
The group VI-VII were classified as poliprobiotic groups as rats received mixture of alived or lyophilized strains. The group VI received 2.5 ml/kg of an aqueous solution of a mixture of the three probiotic strains (2:1:1 Lactobacillus casei IMVB-7280, Bifidobacterium animalis VKL, Bifidobacterium animalis VKB) at a dose of 50 mg/kg (5 × 109 CFU/kg) (g) (intragastrically) [20]. The group VII was treated with “Symbiter” at a dose of 140 mg/kg (1.4 × 1010 CFU/kg), which was supplied by Scientific and Production Company “O.D. Prolisok” (Ukraine). This multiprobiotic mixture contains biomass of 14 alive strains – Lactobacillus + Lactococcus (6 × 1010 CFU/g), Bifidobacterium (1 × 1010/g), Propionibacterium (3 × 1010/g), Acetobacter (1 × 106/g) [20].
Anthropometric measurements and obesity parameter assessment
After 3 months, rats of all groups were analyzed for changes in body weight. One day before the experiment, the animals did not receive food but had free access to water. To determine presence of obesity Lee index was used, which was calculated by the following formula: cube root of body weight (g) / naso-anal size (cm). Animals with Lee index greater than 0.300 were classified as obese, equal to or less than 0.300 as normal [26].
Sample collection and blood biochemistry analysis
All animals were fasted for about 12 h prior sacrifice which was performed by cervical dislocation under urethane anesthesia. Blood was gathered into a microtube which contained a mixture of EDTA with NaF in a 1:2 ratios. Then blood fasting glucose (FPG) was determined using the Trinder’s glucose oxidase method. The rest of the blood from the sample was transferred into a sterile tube, centrifuged at 3500 rpm for 15 min and stored at −80 °C. For further analysis, serum supernatant aliquoted into microcentrifuge tubes was used. Fasting insulin (FPI) was measured with the electrochemiluminescence immunoassay (ECLIA) method using the Rat/Mouse Insulin Kit (Linco Research, USA). For assessment of IR widespread HOMA equation (FPG × FPI/22.5) was used [27]. ELISA was used to determine serum adiponectin by commercial kits «BioVendor» (Czech Republic).
Cytokines measurement
The level of serum cytokines such as IL-1β, IL-4, IL-10, IL-12B p40, transforming growth factor (TGF)-β and interferon (INF)–γ were measured using enzyme-linked immunosorbent assay (ELISA) with specific mice-produced (for IL-4, IL-10, TGF-β) monoclonal antibodies (Sigma). For IL-1β and INF-γ determination specific goat origin polyclonal antibodies were used. The IL-12B p40 polyclonal antibodies were of rabbit origin. Cytokines were immobilized in 96-well plates with a sorption surface. For this purpose, 100 μl of standards were added to the corresponding wells to construct the calibration curve. Other wells were filled with 100 μl of serum. To each well 50 μl of appropriate antibodies was added. Then mixture was incubated at room temperature for 2 h, washed 5 times with buffer and fluid was removed. In next step, 100 μl of the conjugate (streptavidin peroxidase) was added to each well, including a null sample. The samples were then incubated at room temperature for 30 min. After incubation, the enzyme-substrate reaction was stopped by adding 100 μl of H2SO4 to each well. At the end of the reaction, the optical density of the standards and samples of the test serum with a wavelength of 450 nm was determined. The content was expressed as absorbance units of optical density.
Statistical analysis
The SPSS statistical package, version 21.0 (SPSS, Inc., Chicago, Illinois) and GraphPad Prism version 6.0 were used for all statistical analyses and a P value less than 0.05 was considered statistically significant. All data were expressed as mean ± SD. Data distribution was analyzed using the Kolmogorov-Smirnov normality test. Variables with parametric distribution were then analyzed using one-way Analysis of Variance (ANOVA) and if the results were significant, a Tuckey Post Hoc test was performed. Data with non-parametric distribution was analyzed using Kruskall-Wallis test. Designations of significant inter-group differences on diagrams were made using letters a, b, c, d. The same letter over two diagram columns indicates the absence of differences between two corresponding data groups. Different letters over two diagram columns reflect significant differences between two corresponding data groups [7].
Results
In Table 1 presented weight gain dynamics of different experimental groups. Initials assessment was done at 30-days of life before intervention started. Baseline measurement stated significantly lowest body weight in intact rats as compared to all other groups. At day 60 and after 1-month of probiotic administration there were no significant changes in body weight between intact and both lyophilized (VI) and alive (VII) poliprobiotic groups. However, we observed significantly lower weight in these 3 groups as compared to MSG-obesity but not against single-strain (III-V) groups respectively. Later in terms of 90 and 120 days body weight flatten and at the end of experiment the weight of all rats did not differ significantly, however was highest in MSG-obesity rats (Table 1).
Table 1.
Weight gain dynamics in experimental animals
| Weight, g | Intact rats (n = 10) | MSG-induced obesity (n = 10) | B. animalis VKL (n = 10) |
B. animalis VKB (n = 10) |
L. casei IMVB-7280 (n = 10) | Poliprobiotics (n = 10) |
Symbiter (n = 10) |
p |
|---|---|---|---|---|---|---|---|---|
| 30 days | 59.5 ± 8.92a | 76.4 ± 10.57b | 72.0 ± 5.61b | 70.9 ± 8.42b | 72.7 ± 6.63b | 72.6 ± 6.29b | 70.5 ± 7.39b | 0.001 |
| 60 days | 148.0 ± 13.16a | 171.7 ± 15.78b | 156.0 ± 16.25ab | 155.6 ± 17.66ab | 150.5 ± 18.62ab | 145.9 ± 15.93a | 148.8 ± 14.67a | 0.013 |
| 90 days | 201.2 ± 11.41 | 217.4 ± 24.48 | 203.3 ± 27.08 | 196.7 ± 27.68 | 195.3 ± 25.64 | 194.8 ± 25.52 | 200.0 ± 25.85 | 0.427 |
| 120 days | 245.5 ± 12.7 | 274.5 ± 38.61 | 253.6 ± 32.76 | 244.8 ± 33.67c | 244.1 ± 38.95 | 235.5 ± 34.48 | 237.5 ± 32.57 | 0.168 |
Data are presented as the M ± SD. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis. a, b, c, d Values at the same row with different superscript letters shows significant differences in p < 0.05
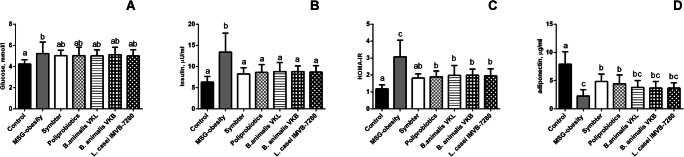
The analysis of the HOMA-IR and serum insulin level demonstrates that under condition of MSG-induced obesity rats became IR. In MSG-obesity rats we marked 3-fold significant increase of HOMA-IR (3.07 ± 0.3 versus 1.18 ± 0.07; p < 0.001) and 2-fold higher insulin concentration (13.4 ± 1.14 versus 6.32 ± 0.42 μIU/ml; p < 0.001) as compared to intact animals (Fig. 1B, C). Probiotic therapy lead to significant decrease of serum insulin in all intervention groups (III-VII) as compared to MSG-obesity with achievement of intact rats level (Fig. 1B). However, the HOMA-IR analysis, in terms of viability and strain dependence of probiotic composition, demonstrates that only multi-component mixture with preference on live strains more pronounced improves IR as compared to other intervention groups, since only the VII group has not shown a significant difference compared to intact animals (p = 0.098) (Fig. 1C).
Fig. 1.
Glucose metabolism parameters in 4-month old rats (n = 10 in each group) in the condition of MSG-induced obesity and after probiotic administration (A – glucose; B – insulin; C – HOMA-IR; D – adiponectin). Data are presented as the M ± SD. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis. a, b, c Values on the same row with different superscript letters show significant differences in p < 0.05
Changes of IR markers in agreement with adiponectin levels, which is known to be one of the major regulators, that improves peripheral tissue sensitivity to insulin. In rats with MSG-obesity significant reduction of adiponectin by 3.5 times (2.27 ± 0.36 versus 7.92 ± 0.69 ng/ml, p < 0.001) as compared to intact animals were observed (Fig. 1D). On the other hand, we stated that both alive or lyophilized policomponent probiotic mixtures (group VI-VII) at least partially increase insulin sensitivity, which is manifested by a 2-fold significant restoration of serum adiponectin in contrast to MSG-obesity rats (Fig. 1D). However, it should be noted that its level does not increase to the level of intact rats. For mono-strain groups (III-V), there were insignificant differences unlike to MSG-obesity rats (Fig. 1D).
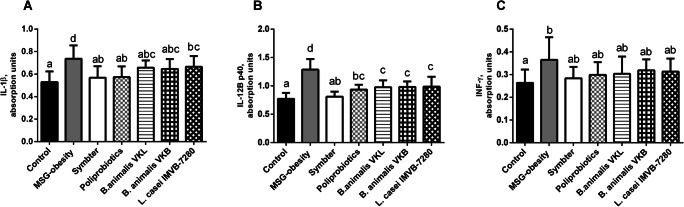
Cytokines profile analysis has shown the development of low-grade chronic systemic inflammation under condition of MSG-induced obesity. We noted significant increase of proinflammatory cytokines IL-1β (0.73 ± 0.11 vs 0.53 ± 0.09; p < 0.001), IL-12B p40 (1.28 ± 0.18 vs 0.77 ± 0.10; p < 0.001) and INF-γ (0.36 ± 0.09 vs 0.26 ± 0.05; p = 0.017) as compared with intact rats (Fig. 2A-C). Probiotic therapy is associated with significant decrease of IL-1β and IL-12B p40 levels, but certain features were observed depending on the composition. The most pronounced anti-inflammatory effect in relation to IL-1β was exhibited at the same extent for both poliprobiotic mixtures (groups VI-VII). Monostrain probiotic therapy, characterized by less pronounced effect. In general, the IL-1β level after the mono-strain (groups III-V) probiotic administration changes insignificant, but L. casei strain IMVB-7280 in comparison with B. animalis VKL and VKB was associated with a minimal effect (Fig. 2A). The most pronounced decrease of IL-12B p40 was mentioned for alive multiprobiotic mixture in comparison to other interventional groups, since only for group VII we did not find significant difference matched to intact rats (p = 0.993). In monotherapy with probiotics (group III-V), the decrease of IL-12B p40 was almost identical (Fig. 2B). The INF-γ level after probiotics administration, regardless of the viability and activity of the composition, was significantly comparable to intact and MSG-obesity rats (Fig. 2C).
Fig. 2.
Serum pro-inflammatory cytokines levels in 4-month old rats (n = 10 in each group) in the condition of MSG-induced obesity and after probiotic administration (A – IL-1β; B – IL-12B p40; C – INF-γ). Data are presented as the M ± SD. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis. a, b, c, d Values on the same row with different superscript letters show significant differences in p < 0.05
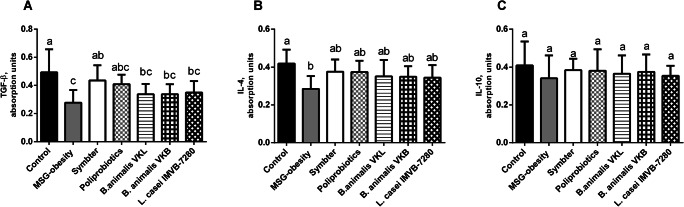
We also demonstrated significant reduction of anti-inflammatory cytokines such as IL-4 (0.28 ± 0.06 vs 0.41 ± 0.07; p = 0.001) and TGF-β (0.27 ± 0.08 vs 0.49 ± 0.16; p < 0.001) in MSG-obesity rats matched to intact animals (Fig. 3A, B). Interestingly, that concentration of IL-10, one of the most important anti-inflammatory cytokines, changes insignificant among all experimental groups (Fig. 3C). The increase of IL-4 was most pronounced in both poliprobiotic groups, but significantly not differ from monostrain therapy (groups III-V) (Fig. 3B). The TGF-β significantly increased as compared to MSG-obesity and restored up to intact animal level only after multiprobiotic (group VII) administration (Fig. 3A).
Fig. 3.
Serum anti-inflammatory cytokines levels in 4-month old rats (n = 10 in each group) in the condition of MSG-induced obesity and after probiotic administration (A – TGF-β; B – IL-4; C – IL-10). Data are presented as the M ± SD. One-way ANOVA with post hoc Tukeys test for multiple comparisons were performed for data analysis. a, b, c Values on the same row with different superscript letters show significant differences in p < 0.05
Discussion
Regulation of gut microbiota with pro- and/or prebiotics supplementation against obesity has attracted attention, although results were inconsistent. It has been shown that certain probiotic strains, including Bifidobacteria and Lactobacilli or mixtures of the two reduce IR in different animal models in obesity or T2D, whereas other probiotics have had no effect. We established a well-controlled animal model which is an early-onset obesity resulting from MSG induced lesions in arcuate nucleus to neonatal animals [28, 29]. This model characterized with increased fat to body weight ratio according to accumulation of visceral adipose tissue, severe hyperleptinemia, hyperinsulinemia and an extremely high HOMA index that pointed to development of IR [30]. The strain-specific probiotic impact on these metabolic parameters was the main aim of our study. In our previous study, we noted the development of obesity in all MSG-obesity rats and up to 20–70% after probiotics. Moreover, supplementation of probiotic composition, with preference to alive strains, led to a significantly lower prevalence of obesity, reduction of VAT weight and serum lipid levels as compared to single-strain probiotic [20].
Li et al. studied the anti-diabetic effects of L. casei CCFM419 in mice with high-fat diet (HFD) and low dose streptozotocin-induced T2D. In 4 weeks, the probiotic group improved insulin sensitivity by insulin tolerance test, lowered fasting insulin level and decreased HOMA-IR value compared to the diabetic control. The positive effects on IR may be due to amelioration of systemic inflammation as indicated by improved TNF-α, IL-6 and IL-10 level and SCFA/gut microbiota pathways [31]. Another study on high fructose-induced T2D in Wistar rats, demonstrated that low-fat (2.5%) yogurt dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei after 8 weeks significantly delayed the onset of glucose intolerance, hyperglycemia, hyperinsulinemia, dyslipidemia, and oxidative stress [32]. Diet-induced obese C57BL/6 J mice treated with probiotics (Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032) gained less in body weight, showed lowered plasma insulin, leptin, total-cholesterol and in adipose tissue down-regulated pro-inflammatory genes (TNFα, IL6, IL1β and MCP1) [33]. Similar to our results, oral administration of Bifidobacterium animalis subsp. lactis CECT 8145 strain (1010 CFU/day) suspended in skim milk in Zücker fatty rats significantly increased plasma adiponectin (an insulin-sensitising adipokine), however did not result in significant changes in triglyceride levels [34].
Several recent studies, in agreement with our findings, demonstrated that probiotic multistrain mixture can produce better results than single strain in obesity and related disorders management. Roselli et al., investigated that mixture of Bifidobacterium lactis Bi1, B. breve Bbr8 and B. breve BL10 (B. mix) when administered before and together with HFD, as well as after for 12 weeks of HFD was able to both prevent and ameliorate already existing obesity by reducing weight gain, adipose tissue fat accumulation, adipocyte size, and macrophage and CD4+ T cell infiltration, improving lipid profile and regulating leptin and cytokine secretion. From the other side mixture containing Lactobacillus rhamnosus GG, L. acidophilus LA1/K8, or a mixture of L. bulgaricus Lb2 and S. termophilus Z57 were not effective [35]. Alard et al., reported that multi-strain mixture (L. rhamnosus LMG S-28148 and B. animalis subsp. lactis LMG P-28149) improve adiposity, insulin resistance and dyslipidemia through adipose tissue immune cell-remodelling HFD-induced obese mice. At the gut level, the mixture modified the uptake of fatty acids and restored the expression level of the SCFA receptor GPR43, abundance of Akkermansia muciniphila and Rikenellaceae [36]. A multispecies Lactobacillus- and Bifidobacterium-containing probiotic mixture (B. lactis LA 303, B. lactis LA 304, L. acidophilus LA 201, L. plantarum LA 301 and L. salivarius LA 302) significantly reduced the increase in body weight, serum glucose concentration and insulin resistance induced by the HFD in C57/BL6J mice [37]. We suggested that when in mixtures multi-strain probiotics formed mutualistic relationships and, therefore, were able to share with different metabolites, affect different receptors, and, as a result, synergistically produce the enhanced overall effect greater than the sum of the single effects. In contrast to our data, recent study [38] reported effects of single species versus dual species on markers of obesity in HFD-induced obese rats. Intervention groups were HFD supplemented with Lactobacillus casei strain Shirota, HFD supplemented with Bifidobacterium longum and HFD supplemented with a mixture of these two bacterial species. After 15 weeks of supplementation, B. longum showed better results in terms of modulating leptin level, fat mass, adipocyte size and lipoprotein lipase expression, as well as increasing adiponectin and PPAR-γ expression compared to dual species [38].
Sometimes it is unknown whether the ingested microbial cells are viable- or killed. Our study demonstrates that from the two poliprobiotic mixtures that were investigated, the more effective one contained alive strains. Recent study compared the effects of heat-killed (HK) and live Lactobacillus reuteri GMNL-263 (Lr263) on insulin resistance in high-fat diet (HFD)-induced rats. It was suggested that similar to live Lr263, HK Lr263 caused significant decrease in the weight gain, serum glucose, insulin, and lipid profiles in the serum and liver [39]. However, even sterilized bacterial cells are functional. Current study demonstrates that in mice on a HFD, sterilized Bifidobacteria suppressed fat accumulation, improved IR, and lowered blood glucose levels [40].
Conclusion
Additions to probiotic composition, with preference to alive strains, led to significantly lower levels of obesity, decrease in HOMA-IR, proinflammatory cytokines levels (IL-1β, IL-12Bp40) and elevation of adiponectin and TGF-β in comparison with MSG-obesity. Single-strain analysis (group III-V) shows significant decreasing of HOMA-IR, but changes were less pronounced as compared to mixture groups and did not achieved intact rats level. Other metabolic parameters were not affected significantly by single strains.
The insulin sensitizing effect of probiotics thus appears to be dependent on strain or dose, though underlying mechanisms are still largely unknown. Our findings provide major clues for how to design and use probiotics with more efficient compositions in obesity and IR management and may bring new insights into how host-microbe interactions contribute to such protective effects.
Acknowledgments
The authors express their sincere thanks to Dr. Yankovsky Dmitro Stanislavovych for the help, advice and financial support of this work.
Compliance with ethical standards
Conflict of interest
None to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Obesity and overweight. Fact sheet N 311, http://www.who.int/mediacentre/factsheets/fs311/en, 2015.
- 2.Dee A, Kearns K, O’Neill C, Sharp L, Staines A, O’Dwyer V, et al. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes. 2014;7:242. doi: 10.1186/1756-0500-7-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melvin A, O'Rahilly S, Savage DB. Genetic syndromes of severe insulin resistance. Curr Opin Genet Dev. 2018;50:60–67. doi: 10.1016/j.gde.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Mykhalchyshyn G, Kobyliak N, Bodnar P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Metab Disord. 2015;14:44. doi: 10.1186/s40200-015-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyriachenko Y, Falalyeyeva T, Korotkyi O, Molochek N, Kobyliak N. Crosstalk between gut microbiota and antidiabetic drug action. World J Diabetes. 2019;10:154–168. doi: 10.4239/wjd.v10.i3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 7.Kobyliak N, Falalyeyeva T, Boyko N, Tsyryuk O, Beregova T, Ostapchenko L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res Clin Pract. 2018;141:190–199. doi: 10.1016/j.diabres.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterol. 2013;145:396–406. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat Inflamm. 2014;2014:162021. doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 1802;2010:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–144. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 14.Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto L, Kononenko L, et al. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: a randomized clinical study. Minerva Med. 2018;109:418–428. doi: 10.23736/S0026-4806.18.05845-7. [DOI] [PubMed] [Google Scholar]
- 15.Kobyliak N, Abenavoli L, Mykhalchyshyn G, Falalyeyeva T, Tsyryuk O, Kononenko L, et al. Probiotics and smectite absorbent gel formulation reduce liver stiffness, transaminases and cytokine levels in NAFLD associated with type 2 diabetes: a randomized clinical study. Clinical Diabetology. 2019;8:205–214. doi: 10.5603/DK.2019.0016. [DOI] [Google Scholar]
- 16.Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. 2018;12:617–624. doi: 10.1016/j.dsx.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Van Hul M. Novel opportunities for next-generation probiotics targeting metabolic syndrome. Curr Opin Biotechnol. 2015;32:21–27. doi: 10.1016/j.copbio.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 19.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobyliak N, Falalyeyeva T, Beregova T, Spivak M. Probiotics for experimental obesity prevention: focus on strain dependence and viability of composition. Endokrynol Pol. 2017;68:659–667. doi: 10.5603/EP.a2017.0055. [DOI] [PubMed] [Google Scholar]
- 21.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 22.Kobyliak N, Falalyeyeva T, Bodnar P, Tl B. Probiotics supplemented with Omega-3 fatty acids are more effective for hepatic Steatosis reduction in an animal model of obesity. Probiotics Antimicrob Proteins. 2017;9:123–130. doi: 10.1007/s12602-016-9230-1. [DOI] [PubMed] [Google Scholar]
- 23.Kondro M, Mykhalchyshyn G, Bodnar P, Kobyliak N, Falalyeyeva T. Metabolic profile and morpho-functional state of the liver in rats with glutamate-induced obesity. Curr. Issues Pharm. Med Sci. 2013;26:379–381. doi: 10.12923/j.2084-980X/26.4/a.05. [DOI] [Google Scholar]
- 24.Kobyliak N, Abenavoli L, Falalyeyeva T, Virchenko O, Natalia B, Beregova T, et al. Prevention of NAFLD development in rats with obesity via the improvement of pro/antioxidant state by cerium dioxide nanoparticles. Clujul Med. 2016;89:229–235. doi: 10.15386/cjmed-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobyliak N, Abenavoli L, Falalyeyeva T, Beregova T. Efficacy of probiotics and Smectite in rats with non-alcoholic fatty liver disease. Ann Hepatol. 2018;17:153–161. doi: 10.5604/01.3001.0010.7547. [DOI] [PubMed] [Google Scholar]
- 26.Novelli ELB, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, Novelli Filho JL. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 27.Vogeser M, Konig D, Frey I, Predel HG, Parhofer KG, Berg A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin Biochem. 2007;40:964–968. doi: 10.1016/j.clinbiochem.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti generated protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson R, Pelleymounter MA, Millard WJ, Liu S, Eppler B. Attenuation of leptin-mediated effects by monosodium glutamate-induced arcuate nucleus damage. Am J Phys. 1997;273:202–206. doi: 10.1152/ajpendo.1997.273.1.E202. [DOI] [PubMed] [Google Scholar]
- 30.Matysková R, Maletínská L, Maixnerová J, Pirník Z, Kiss A, Zelezná B. Comparison of the obesity phenotypes related to monosodium glutamate effect on arcuate nucleus and/or the high fat diet feeding in C57BL/6 and NMRI mice. Physiol Res. 2008;57:727–734. doi: 10.33549/physiolres.931274. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang E, Yin B, Fang D, Chen P, Wang G, Zhao J et al. Effects of lactobacillus casei ccfm419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef. Microbes 2017; 8: 421–432. doi: 10.3920/BM2016.0167. doi: 10.5603/EP.a2017.0055. [DOI] [PubMed]
- 32.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Park DY, Ahn YT, Park SH, Huh CS, Yoo SRYR, et al. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carreras NL, Martorell P, Chenoll E, Genovés S, Ramón D, Aleixandre A. Anti-obesity properties of the strain Bifidobacterium animalis subsp. lactis CECT 8145 in Zücker fatty rats. Benef Microbes. 2018;9:629–641. doi: 10.3920/BM2017.0141. [DOI] [PubMed] [Google Scholar]
- 35.Roselli M, Finamore A, Brasili E, Rami R, Nobili F, Orsi C, et al. Beneficial effects of a selected probiotic mixture administered to high fat-fed mice before and after the development of obesity. J Funct Foods. 2018;45:321–329. doi: 10.1016/j.jff.2018.03.039. [DOI] [Google Scholar]
- 36.Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham AL, Mariadassou M, Maguin E, Waligora-Dupriet AJ, Pot B, Wolowczuk I, Grangette C. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. 2016;18:1484–1497. doi: 10.1111/1462-2920.13181. [DOI] [PubMed] [Google Scholar]
- 37.Holowacz S, Guigné C, Chêne G, Mouysset S, Guilbot A, Seyrig C, Dubourdeau M. A multispecies Lactobacillus- and Bifidobacterium-containing probiotic mixture attenuates body weight gain and insulin resistance after a short-term challenge with a high-fat diet. Pharma Nutrition. 2015;3:101–107. doi: 10.1016/j.phanu.2015.03.003. [DOI] [Google Scholar]
- 38.Karimi G, Jamaluddin R, Mohtarrudin N, Ahmad Z, Khazaai H, Parvaneh M. Single-species versus dual-species probiotic supplementation as an emerging therapeutic strategy for obesity. Nutr Metab Cardiovasc Dis. 2017;27:910–918. doi: 10.1016/j.numecd.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh FC, Lan CC, Huang TY, Chen KW, Chai CY, Chen WT, Fang AH, Chen YH, Wu CS. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016;7:2374–2388. doi: 10.1039/c5fo01396h. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi K, Ben Othman M, Sakamoto K. Sterilized bifidobacteria suppressed fat accumulation and blood glucose level. Biochem Biophys Res Commun. 2018;501:1041–1047. doi: 10.1016/j.bbrc.2018.05.105. [DOI] [PubMed] [Google Scholar]