Abstract
Purpose
The aim of this study was to investigate the effect of probiotic bacteria of Lactobacillus acidophilus, cinnamon powder and their combinations on the glycemic and antioxidant indices in patients with type 2 diabetes.
Methods
A total of 136 patients randomized with type 2 diabetes entered the study and were randomly divided into four groups who were matched for age and gender. Thereafter, alongside their routine pharmacotherapy, each group followed one of the following diets: Group A: Lactobacillus acidophilus 108 cfu and 0.5 g of powdered cinnamon (synbiotic). Group B: Lactobacillus acidophilus (probiotic), Group C: powdered cinnamon. Group D: rice flour powder as placebo. At the beginning and end of the intervention, fasting blood sugar (FBS), HbA1c, advance glycation end products (AGE), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and antioxidant enzymes of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) were measured.
Results
Following 3 months of treatment, the mean FBS level was decreased significantly in probiotic, cinnamon, and synbiotic supplementation groups compared with control (P < 0.01). FBS levels in probiotic, cinnamon, and synbiotic groups were significantly decreased compared with the control group (P = 0.001, P = 0.063 and P = 0.001, respectively). The mean HbA1C in probiotic, cinnamon, and synbiotic groups were also decreased (P = 0.001, P = 0.001 and P = 0.04, respectively). The mean AGE in synbiotic group was significantly decreased (P = 0.037). Probiotic, cinnamon and synbiotic all could improve antioxidant enzyme activity modestly. However, the most significant effect was seen in probiotic group.
Conclusions
According to the current results, the use of probiotic supplements (individually or in combination with cinnamon) leads to a reduction in blood glucose and an increase in antioxidant enzymes in people with type 2 diabetes.
Keywords: Diabetes mellitus, Probiotic, Cinnamon, Synbiotic, Antioxidant enzymes
Background
Diabetes mellitus is one of the most common chronic endocrine disorders worldwide. It has been estimated that a total of 425 million people suffered from diabetes globally which is predicted to rise by 50% over the next 30 years [1]. Approximately one in every ten Iranian adults suffers from diabetes [2]. Because of its high prevalence, most countries spend 5% to 20% of their health budget on the control and treatment of diabetes [3]. Various studies have shown that a change in lifestyle can improve blood glucose in type 2 diabetes. However, lifestyle changes should be integrated with pharmacological agents for most patients. Because of the progressive deterioration of pancreatic beta cells, patients may need multiple drugs as well as insulin therapy [4]. Recent studies on nutritional supplements have suggested that some compounds, such as dietary fiber, vitamins, flavonoids, sterols, and antioxidants, may be involved in controlling blood glucose and preventing complications of diabetes and can be applied together with pharmacotherapy [5]. Probiotics are non-pathogenic microorganisms that favorably influence the health of the host by changing the balance of gut microbiota. When consumed in a sufficient amount and in a viable state, they can be considered as functional foods. However, their effects on diabetes have been controversial [6]. Lactic acid-producing bacteria, especially Lactobacillus and Bifidobacterium, are normally part of the digestive ecosystem and are known as probiotics. Oral administration of these probiotics may improve metabolic disorders such as diabetes by modifying the normal flora of the intestine [7, 8].
Prebiotics refer to special vegetative fibers that are fermented by the bacteria in the intestine and contribute to the growth, proliferation, and function of the digestive microbial community [9]. The combination of probiotic bacteria and a prebiotic compounds form synbiotics. Cinnamon is one of the most famous and widely-used spices that have prebiotic properties and therapeutic applications. Some studies have shown that cinnamon can improve the blood glucose and lipid profiles in people with diabetes [10, 11]. The advantages of cinnamon may be due to its prebiotic properties, phytochemicals, antioxidants, and essential oils [10, 11]. In keeping with the high beneficial effects of cinnamon, scarce data are available about its effects on glycemia and oxidative stress in diabetic patients. Additionally, rare surveys have been conducted to apprise the effects of probiotic bacteria and symbiotic products on diabetic patients. Thus, the present study was carried out to investigate the effects of Lactobacillus acidophilus probiotic bacteria, cinnamon powder, and their combinations on the glycemic and antioxidant indices in patients with type 2 diabetes.
Methods
Preparation of the supplements and placebo capsules
The symbiotic, probiotic, cinnamon and placebo capsules were prepared by Institute of Medicinal plants, Karaj, Iran. Lactobacillus acidophilus PTCC 1643 was obtained and used as probiotic treatment (Zist-yar Sina Co, Iran). Cinnamon (Cinnamomum zeylanicum) from the Lauracea family and the Laurales order was purchased, and the plant species was approved by an expert in medicinal plants. Rice flour was used as placebo in this study. Following treatments were prepared for groups: Synbiotic; Lactobacillus acidophilus 108 cfu mixed with 0.5 g of powdered cinnamon in hard gelatin capsules, Probiotic; Lactobacillus acidophilus 108 cfu in hard gelatin capsules, Cinnamon; 0.5 g of powdered cinnamon in hard gelatin capsules and placebo; 0.5 g of rice flour powder in hard gelatin capsules.
Participants
The inclusion criteria consisted of type 2 diabetes, no insulin use, 40 to 60 years of age, fasting blood glucose levels of 125–250 mg/dl, and HbA1c of 7–8%. The exclusion criteria included pregnancy, lactation, consumption of specific medicines (effective on blood glucose such as corticosteroids, diuretics, and antibiotics), use of vitamin supplements and medicinal plants, changes in anti-diabetic medications during the previous 4 months, history of lactose intolerance or allergies, chronic and uncontrolled hepatic, renal, psychological, or cardiac disease, cigarette or alcohol addiction. Additionally, people with a specific diet or heavy exercise were not included in the study. Patients were asked to bring their medications at each visit to determine how many medications they had used according to assigned plan. Additionally, during the visits, telephone calls were made to ask about the use of the drug. Additionally, patients were asked not to change their diet and exercise during the study.
Protocol
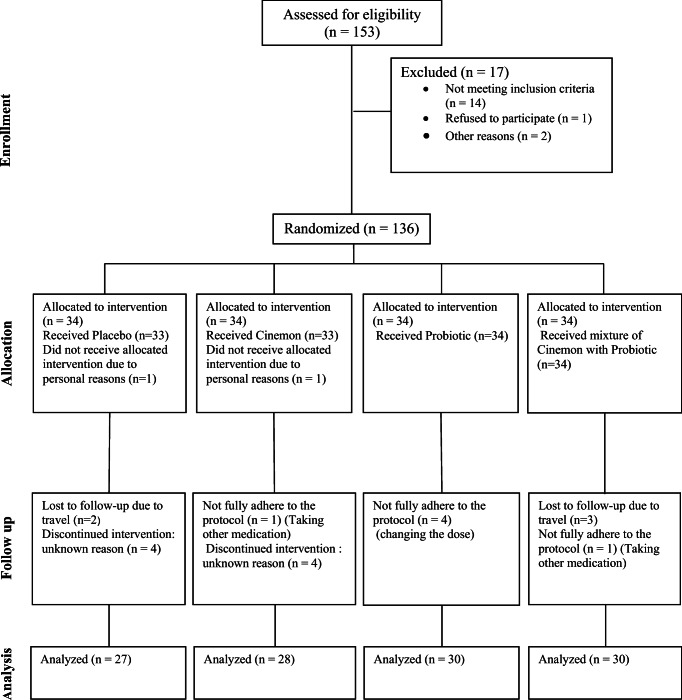
A total of 136 volunteers who met the inclusion criteria entered the study and were randomly divided into four groups of synbiotic, probiotic, cinnamon, and control. Groups were matched based on age and gender. This sample size was calculated to be sufficient to estimate a 20 mg/dl difference in fasting glucose between the groups, considering type I error = 0.05 and 80% power. The CONSORT diagram of patient recruitment is depicted in Fig. 1. Block randomization with computer-generated random-numbers table and sequentially numbered containers each representing a block consisting of 4 patients were used for treatment allocation.
Fig. 1.
CONSORT flow diagram for participants
The following supplement regimens were added to the patients’ routine pharmacotherapy:
Group A received a capsule of Lactobacillus acidophilus 108 cfu and 0.5 g of powdered cinnamon per day;
Group B received a capsule of Lactobacillus acidophilus 108 cfu per day;
Group C received a capsule containing 0.5 g of powdered cinnamon per day; Group D received a capsule containing 0.5 g of rice flour powder per day.
Initially, patients were provided with complete information regarding the implementation of the project and the potential side effects of the supplements. All participants signed written consent forms and were told they could leave the study at any time, after informing their physician of their decision to do so. Patients were instructed to take their treatment every morning with food for 3 months. The incidence of any side effects caused by the use of the supplements was to be reported by the patients. At the beginning and end of the interventional period, 12-h–fasting venous blood samples were collected and HbA1c was determined. The sera were kept at −70 °C until used in biochemical tests. A human ELISA kit (Diaclone, France) was used to measure the antioxidant enzymes of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). Standard kits (Pars Azmoon Co, Iran) were obtained for the FBS, AST, and ALT tests, and the Nycocard instrument kit was used to determine HbA1c. The study was registered in the Iranian Registry of Clinical Trials (IRCT20170305032883N2), and the protocol was approved by the ethics committee (IR.ABZUMS.REC.1394.92) of the Alborz University of Medical Sciences, Alborz province, Iran.
Assessment of adverse effects
All the patients were asked to report any adverse effects by phone to physician.
Statistical analysis
The sample size was calculated with 80% power using a two-sided test at the 5% significance level and based on the effect size of 0.5 (0.5% decrease in HbA1c). Randomization was done based on sex with stratified permuted blocked randomization method using “sealed envelope” online software with a block size of 4 (AABB, ABAB, ABBA, BAAB, BABA, BBAA). Researchers, subjects and data analysts were blinded to the treatment. The Statistical Package for the Social Sciences version 24.0 (SPSS Inc., Chicago, IL, USA) was used for statistical evaluation of data. Quantitative data are expressed as mean ± standard deviation. Within-group differences are compared using the paired t-test. Between- group differences are analyzed using one-way ANOVA following Tukey’s post hoc test. P value less than 0.05 is considered statistically significant.
Results
Demographic characters of studied patients
A total of 136 patients in 4 different groups were entered to the study. Two patients were initially refused from treatment due to their personal reasons. Additionally, five patients due to travel, 8 patients due to anonymity, 4 patients due to changes in treatment protocol, and 2 patients due to delivery of other medications were excluded from the study. Finally, 115 patients were completed the study in the probiotic (n = 30), symbiotic (n = 30), cinnamon (n = 28), and placebo (n = 27) groups (Fig. 1). Table 1 reveals demographic characteristics of diabetic patients before intervention. Age of studied patients had the ranges of 58.2 ± 11.8 to 59.7 ± 12.2 years. Majority of studied patients in all examined groups were female. Weight of studied patients had the ranges of 79.3 ± 13.3 to 83.5 ± 13.6 Kg.
Table 1.
Demographic characteristic of diabetic patients before intervention
| Characters | Groups | |||
|---|---|---|---|---|
| Control | Probiotic | Cinnamon | Synbiotic | |
| N = 27 | N = 30 | N = 28 | N = 30 | |
| Age (year) | 58.2 ± 11.8 | 59.7 ± 12.2 | 58.8 ± 12.8 | 58.4 ± 11.4 |
| Sex (male/female) | 12/15 | 10/20 | 14/16 | 13/15 |
| Weight (Kg) | 83.5 ± 13.6 | 79.3 ± 13.3 | 81.6 ± 12.6 | 79.5 ± 11.8 |
Data are presented as the mean ± SD. * P < 0.05 is considered as significant
Effects of probiotic, cinnamon and synbiotic on FBS, HbA1c and AGE
Table 2 signifies the effects of probiotic, cinnamon and synbiotic on FBS, HbA1c and AGE factors in diabetic patients. At the beginning of this study, no significant difference was observed between groups regarding mean age, body mass index and gender. Following 3 months of treatment, the mean fasting blood suger (FBS) level in probiotic, cinnamon, and synbiotic groups were significantly lower compared with the control group (P = 0.001, P = 0.063 and P = 0.001 respectively). Furthermore, HbA1c levels declined moderately corresponding to blood glucose. The mean HbA1C percent in probiotic, cinnamon, and synbiotic groups were lower compared with the control group (P = 0.001, P = 0.001 and P = 0.04, respectively). Advanced glycation end products (AGE) in synbiotic group was significantly lower compared with the control group during this study (P = 0.037). Otherwise the lowest amounts of FBS, HbA1c and AGE factors were found in the symbiotic (147.70 ± 3.71 mg/dL), probiotic (7.42 ± 1.23%) and symbiotic (62.78 ± 9.14%) groups.
Table 2.
The effects of probiotic, cinnamon and synbiotic on FBS, HbA1c and AGE in diabetic patients
| Biochemical factors | Groups | ||||
|---|---|---|---|---|---|
| Control | Probiotic | Cinnamon | Synbiotic | P value | |
| N = 27 | N = 30 | N = 28 | N = 30 | ||
| FBS (mg/dL) | 177.3 ± 23.02 | 150.43 ± 43.36*** | 152.50 ± 48.1* | 147.70 ± 3.71*** | 0.001 |
| HbA1c (%) | 8.48 ± 0.59 | 7.42 ± 1.23*** | 7.68 ± 0.83*** | 7.66 ± 1.11* | 0.001 |
| AGE (%) | 68.12 ± 5.60 | 67.06 ± 8.6 | 67.12 ± 6.9 | 62.78 ± 9.14* | 0.03 |
Data are presented as the mean ± SD. FBS Fasting blood sugar, HbA1c Hemoglobin A1c, AGE Advanced glycation end-products. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the control group
Effects of probiotic, cinnamon and synbiotic on biochemical factors
Table 3 signifies the effect of probiotic, cinnamon and synbiotic on biochemical factors in diabetic patients. The highest amounts of CAT, GPx, SOD, and OxLDL were found in probiotic (2.44 ± 0.50 U/mL) and cinnamon (2.44 ± 0.26 U/mL), probiotic (92.15 ± 8.41 U/ mL), probiotic (4.58 ± 0.42 U/mL) and control (17.07 ± 1.01 mU/L) groups. However, the highest amounts of CAT, GPx, SOD, and OxLDL were found in control (1.95 ± 0.34 U/mL), cinnamon (84.61 ± 13.43 U/ mL), control (3.99 ± 0.27 U/mL) and synbiotic (15.88 ± 1.98 mU/L) groups. Probiotic, cinnamon, and synbiotic supplementation improved antioxidant enzyme activity modestly; however, the most significant effect was seen in the probiotic group.
Table 3.
The effect of probiotic, cinnamon and synbiotic on biochemical factors in diabetic patients
| Biochemical factors | Groups | ||||
|---|---|---|---|---|---|
| Control | Probiotic | Cinnamon | Synbiotic | P value | |
| N = 27 | N = 30 | N = 28 | N = 30 | ||
| CAT (U/mL) | 1.95 ± 0.34 | 2.44 ± 0.50 * | 2.44 ± 0.26 * | 2.20 ± 0.31 | < 0.001 |
| GPx (U/mL) | 84.89 ± 6.52 | 92.15 ± 8.41* | 84.61 ± 13.43 | 89.71 ± 9.04 | 0.01 |
| SOD (U/mL) | 3.99 ± 0.27 | 4.58 ± 0.42 * | 4.16 ± 0.60 | 4.13 ± 0.64 | < 0.001 |
| OxLDL (mU/L) | 17.07 ± 1.01 | 16.85 ± 1.53 | 16.32 ± 1.21 | 15.88 ± 1.98 * | 0.03 |
Data are presented as the mean ± SD. CAT Catalase, GPx Glutathione peroxidase, SOD Superoxide Dismutase, OxLDL Oxidized low-density lipoprotein. Statistical differences were determined using ANOVA followed by a Tukey post-hoc test; *, P < 0.05 compared with the control group
Generally, probiotic and cinnamon could increase CAT level (P < 0.001 and P = 0.04 respectively). Probiotic was the only treatment that changed GPx and SOD (P = 0.01 and P < 0.001 respectively). OxLDL was affected mostly by synbiotic (P = 0.03). No side effects were reported.
Discussion
The results of this study showed that probiotics, cinnamon, and synbiotics (a combination of probiotic and cinnamon) all can reduce the levels of fasting blood glucose and HbA1c in diabetic patients, and none of the treatments had a significant superiority over the others. In the case of antioxidant enzymes activity, however, probiotic intake had the highest impact compared with the other groups. Previous studies have reported controversial results on the effects of cinnamon and probiotics for the treatment of type 2 diabetes. Some experimental studies have shown the effect of probiotics on HbA1c. It has been shown that Lactobacillus acidophilus significantly reduced glycosylated hemoglobin in streptozotocin-induced diabetic mice after 14 days [12]. Yadav found that supplementation with probiotics in diabetic rats for 6 weeks significantly reduced fasting glucose and HbA1c [13]. On the other hand, the findings of Al-Salam et al. showed that probiotics were not effective on blood glucose in healthy rats [14]. The results of some clinical studies have been consistent with the present study reporting probiotics to be beneficial in reducing HbA1c. Ejtahed et al. found a significant reduction in glycemic indices and insulin resistance in people with diabetes following the consumption of probiotic yogurt compared with plain yogurt [15]. The precise mechanism of probiotics in reducing blood glucose is not clear, but several possible mechanisms have been proposed in this regard. The accumulation of lactic acid in the intestinal epithelium may reduce glucose uptake by the intestine [16]. In addition, probiotics can prevent and delay pancreatic degradation by inhibiting the production of pro-inflammatory cytokines [17, 18]. The positive effect of cinnamon on glycemic indices was observed in the current study. Cinnamon contains amidone, mucilage, tannin, calcium oxalate, sugar, cinnamomin, resin, and essential oils. The main component of cinnamon essential oil is cinnamaldehyde, which can determine the commercial value of cinnamon products [19]. The anti-microbial, anti-oxidant, anti-inflammatory, and anti-spasmodic properties which have been reported for cinnamon seem to be related to cinnamaldehyde. This plant has an anti-Helicobacter pylori effect and also inhibits the growth of Escherichia coli, Listeria monocytogenes, Salmonella typhimurium, Staphylococcus aureus, and Clostridium species [20, 21]. It has been shown that cinnamon extract inhibits tumor necrosis factor, cyclooxygenase 2, and nitric oxide synthase enzymes [22]. The terpene compounds of cinnamon have inhibitory effects on the metabolism of arachidonic acid. Polyphenols in the cinnamon prevent the formation of advanced glycated end products [23]. It has been reported that cinnamon strengthens insulin function in patients with diabetes and improves blood glucose, triglycerides, and cholesterol [24, 25]. In patients with type 2 diabetes, phosphorylation of the insulin receptor decreases. Moreover, cinnamon compounds stimulate insulin-receptor autophosphorylation and inhibit phosphotyrosine phosphatase, which is an enzyme responsible for de-phosphorylation of insulin-receptor and increases sensitivity to insulin. Studies suggest that cinnamon increases glucose uptake by activating the insulin receptor and also increases glycogen synthesis [24]. Khadem Haghighian et al. studied the effect of cinnamon on blood glucose levels in diabetic patients and reported that the average levels of fasting blood glucose, HbA1c, and insulin resistance decreased significantly in the intervention group compared with the control group, which is consistent with the results of the present study [26, 27].
Another study, however, showed that the use of 1 g/d cinnamon for 8 weeks had no effect on the level of blood glucose in patients with diabetes [28].
The results of the present study showed that the level of antioxidant enzymes increased moderately in all cinnamon, synbiotic, and probiotic groups with the most significant change seen in the probiotic group. Various animal studies have shown that probiotics inhibit oxidative stress by reducing inflammatory damage and increasing levels of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase [29].
The hepatic enzymes (AST, ALT) were investigated in the present study to monitor drug side effects and hepatic toxicity. Hepatic enzyme levels decreased significantly in all three groups. It can be concluded that drugs did not induce hepatic toxicity in the study’s patients. The results of other clinical surveys indicated that probiotics prescribed for diabetic patients reduced hepatic enzyme levels [30–32].
Although some recent studies have shown that consuming synbiotic foods may have a beneficial effect on biochemical factors, inflammatory factors, and oxidative stress biomarkers, such effects have been found mostly in animal models or non-diabetic patients [33–35]. In the present study, however, synbiotic supplementation was not superior to probiotics or cinnamon alone in improving glycemic and antioxidant indices. Put together, the study strengths were classification of patients into 4 different groups, presence of single drug, combination and control groups and finally adequate time of the study. However, lack of insulin measurement and lack of patient height for measurement of BMI index were the weakness points of the present study.
Diverse surveys revealed boost incidence of some kinds of foodborne pathogens in different kinds of food samples [36–53]. Besides the clinical portion of using of natural medicinal plants, they also can control the growth and proliferation of diverse kinds of foodborne pathogens in different types of food. Thus, they also can use as a natural preservatives in food industry.
Conclusion
According to the current results, the use of probiotic supplements (individually or in combination with cinnamon) leads to a reduction in blood glucose and an increase in antioxidant enzymes in people with type 2 diabetes. The results of this study showed that probiotics, cinnamon, and synbiotics (a combination of probiotic and cinnamon) all can reduce the levels of fasting blood glucose and HbA1c in diabetic patients, and none of the treatments had a significant superiority over the others. The level of antioxidant enzymes increased moderately in all cinnamon, synbiotic, and probiotic groups with the most significant change seen in the probiotic group. According to all achieved results, probiotic had better results in improving blood glucose profile and antioxidant enzymes in type 2 diabetic patients.
Acknowledgements
The authors would like to thank the vice-chancellor for research of Alborz University of Medical Sciences for financial support of the project. We also thank to Medicinal Plants Research Center.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho N, Shaw J, Karuranga S, Huang Y, da Rocha FJ, Ohlrogge A, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed]
- 2.Ramezankhani A, Harati H, Bozorgmanesh M, Tohidi M, Khalili D, Azizi F, et al. Diabetes mellitus: findings from 20 years of the Tehran lipid and glucose study. Int J Endocrinol Metab. 16(4 Suppl):e84784. [DOI] [PMC free article] [PubMed]
- 3.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabete Endocrinol. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 4.Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4(2):287–302. doi: 10.1007/s13679-015-0155-x. [DOI] [PubMed] [Google Scholar]
- 5.Mirmiran P, Bahadoran Z, Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: a review. World J Diabetes. 2014;5(3):267. doi: 10.4239/wjd.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes AC, Bueno AA, de Souza RGM, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13(1):60. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr Clin Res Rev. 2018;12(5):617–624. doi: 10.1016/j.dsx.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Motevaseli E, Khorramizadeh MR, Hadjati J, Bonab SF, Eslami S, Ghafouri-Fard S. Investigation of antitumor effects of Lactobacillus crispatus in experimental model of breast cancer in BALB/c mice. Immunotherapy. 2018;10(2):119–129. doi: 10.2217/imt-2017-0088. [DOI] [PubMed] [Google Scholar]
- 9.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrient. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maierean SM, Serban M-C, Sahebkar A, Ursoniu S, Serban A, Penson P, et al. The effects of cinnamon supplementation on blood lipid concentrations: a systematic review and meta-analysis. J Clin Lipidol. 2017;11(6):1393–1406. doi: 10.1016/j.jacl.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Costello RB, Dwyer JT, Saldanha L, Bailey RL, Merkel J, Wambogo E. Do cinnamon supplements have a role in glycemic control in type 2 diabetes? A narrative review. J Acad Nutr Diet. 2016;116(11):1794–1802. doi: 10.1016/j.jand.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit. 2017;23:3044–3053. doi: 10.12659/MSM.902600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav H, Jain S, Sinha P. Effect of Dahi containing Lactococcus lactis on the progression of diabetes induced by a high-fructose diet in rats. Biosci Biotechnol Biochem. 2006;70(5):1255–1258. doi: 10.1271/bbb.70.1255. [DOI] [PubMed] [Google Scholar]
- 14.Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metabol Pharmacokinet. 2008;33(2):101–106. doi: 10.1007/BF03191026. [DOI] [PubMed] [Google Scholar]
- 15.Ejtahed HS, Mohtadi Nia J, Homayouni Rad A, Niafar M, Asghari Jafarabadi M, Mofid V. The effects of probiotic and conventional yoghurt on diabetes markers and insulin resistance in type 2 diabetic patients: a randomized controlled clinical trial. Iranian J Endo Metabol. 2011;13(1):112. [Google Scholar]
- 16.Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients. 2018;10(5):E643. doi: 10.3390/nu10050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javanmard A, Ashtari S, Sabet B, Davoodi SH, Rostami-Nejad M, Esmaeil Akbari M, et al. Probiotics and their role in gastrointestinal cancers prevention and treatment; an overview. Gastroenterol Hepatol Bed Bench. 2018;11(4):284–295. [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal B, Mukherjee A, Srivastav S. Role of probiotics in pancreatic cancer prevention: the prospects and challenges. Adv Biosci Biotechnol. 2016;7:468–500. [Google Scholar]
- 19.Liang Y, Li Y, Sun A, Liu X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci Nutr. 2019;7(6):2186–2193. doi: 10.1002/fsn3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhammad JS, Zaidi SF, Shaharyar S, Refaat A, Usmanghani K, Saiki I, et al. Antiinflammatory effect of cinnamaldehyde in Helicobacter pylori induced gastric inflammation. Biol Pharm Bull. 2015;38:109–115. doi: 10.1248/bpb.b14-00609. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Meng X, Li Y, Zhao CN, Tang GY, Li HB. Antibacterial and antifungal activities of spices. Int J Mol Sci. 2017;18(6):E1283. doi: 10.3390/ijms18061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, et al. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:429320. doi: 10.1155/2012/429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starowicz M, Zieliński H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in European cuisine. Antioxidants (Basel) 2019;8(4):E100. doi: 10.3390/antiox8040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medagama AB. The glycaemic outcomes of cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108. doi: 10.1186/s12937-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson RA, Zhan Z, Luo R, Guo X, Guo Q, Zhou J, et al. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med. 2015;6(4):332–336. doi: 10.1016/j.jtcme.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DK, Jang S, Baek EH, Kim MJ, Lee KS, Shin HS, et al. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009;8(1):21. doi: 10.1186/1476-511X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth D, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 28.Zahmatkesh M, Fallah Huseini H, Hajiaghaee R, Heidari M, Mehrafarin A, Tavakoli-far B. The effects of Cinnamomum zeylanicum J. Presl on blood glucose level in patients with type 2 diabetes, a double-blind clinical trial. J Med Plants. 2012;1(41):258–263. [Google Scholar]
- 29.Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9(5):E521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, et al. The effects of Lactobacillus casei on glycemic response, serum Sirtuin1 and Fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2019;23(1):68–77. doi: 10.29252/.23.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raygan F, Rezavandi Z, Bahmani F, Ostadmohammadi V, Mansournia MA, Tajabadi-Ebrahimi M, et al. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51. doi: 10.1186/s13098-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barengolts E, Smith ED, Reutrakul S, Tonucci L, Anothaisintawee T. The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: a meta-analysis of nine randomized controlled trials. Nutrients. 2019;11(3):E671. doi: 10.3390/nu11030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahboobi S, Rahimi F, Jafarnejad S. Effects of prebiotic and Synbiotic supplementation on glycaemia and lipid profile in type 2 diabetes: a meta-analysis of randomized controlled trials. Adv Pharm Bull. 2018;8(4):565–574. doi: 10.15171/apb.2018.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerdó T, García-Santos JA, Bermúdez MG, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11(3):E635. doi: 10.3390/nu11030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasri K, Jamilian M, Rahmani E, Bahmani F, Tajabadi-Ebrahimi M, Asemi Z. The effects of synbiotic supplementation on hormonal status, biomarkers of inflammation and oxidative stress in subjects with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. BMC Endocr Disord. 2018;18(1):21. doi: 10.1186/s12902-018-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranjbar R, Yadollahi Farsani F, Safarpoor DF. Phenotypic analysis of antibiotic resistance and genotypic study of the vacA, cagA, iceA, oipA and babA genotypes of the Helicobacter pylori strains isolated from raw milk. Antimicrob Res Infect Control. 2018;7:115. doi: 10.1186/s13756-018-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjbar R, Safarpoor Dehkordi F, Sakhaei Shahreza MH, Rahimi E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob Res Infect Control. 2018;7:53. doi: 10.1186/s13756-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safarpoor FD, Gandomi H, Basti AA, Misaghi A, Rahimi E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob Res Infect Control. 2017;6:104–4. [DOI] [PMC free article] [PubMed]
- 39.Ranjbar R, Masoudimanesh M, Dehkordi FS, Jonaidi-Jafari N, Rahimi E. Shiga (Vero)-toxin producing Escherichia coli isolated from the hospital foods; virulence factors, o-serogroups and antimicrobial resistance properties. Antimicrob Res Infect Control. 2017;6:4. doi: 10.1186/s13756-016-0163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranjbar R, Seif A, Safarpoor DF. Prevalence of antibiotic resistance and distribution of virulence factors in the shiga toxigenic Escherichia coli recovered from hospital food. Jundishapur J Microbiol. 2019;12(5):e82659. [Google Scholar]
- 41.Hemmatinezhad B, Khamesipour F, Mohammadi M, Safarpoor Dehkordi F, Mashak Z. Microbiological investigation of O-serogroups, virulence factors and antimicrobial resistance properties of shiga toxin-producing Escherichia coli isolated from ostrich, turkey and quail meats. J Food Safety. 2015;35(4):491–500. [Google Scholar]
- 42.Safarpoor Dehkordi F, Barati S, Momtaz H, Hosseini Ahari SN, Nejat DS. Comparison of shedding, and antibiotic resistance properties of Listeria monocytogenes isolated from milk, feces, urine, and vaginal secretion of bovine, ovine, caprine, buffalo, and camel species in Iran. Jundishapur J Microbiol. 2013;6(3):284–294. [Google Scholar]
- 43.Ghorbani F, Gheisari E, Dehkordi FS. Genotyping of vacA alleles of Helicobacter pylori strains recovered from some Iranian food items. Trop J Pharm Res. 2016;15(8):1631–1636. [Google Scholar]
- 44.Dehkordi FS, Khamesipour F, Momeni M. Brucella abortus and Brucella melitensis in Iranian bovine and buffalo semen samples: the first clinical trial on seasonal, senile and geographical distribution using culture, conventional and real-time polymerase chain reaction assays. Kafkas Univ Vet Fak Derg. 2014;20(6):821–828. [Google Scholar]
- 45.Dehkordi FS, Haghighi N, Momtaz H, Rafsanjani MS, Momeni M. Conventional vs real-time PCR for detection of bovine herpes virus type 1 in aborted bovine, buffalo and camel foetuses. Bulgar J Vet Med. 2013;16(2):102–111. [Google Scholar]
- 46.Nejat S, Momtaz H, Yadegari M, Nejat S, Safarpour Dehkordi F, Khamesipour F. Seasonal, geographical, age and breed distributions of equine viral arteritis in Iran. Kafkas Univ Vet Fak Derg. 2015;21(1):111–116. [Google Scholar]
- 47.Dehkordi FS, Valizadeh Y, Birgani TA, Dehkordi KG. Prevalence study of Brucella melitensis and Brucella abortus in cow's milk using dot enzyme linked immuno sorbent assay and duplex polymerase chain reaction. J Pure Appl Microbiol. 2014;8(2):1065–1069. [Google Scholar]
- 48.Rahimi E, Yazdanpour S, Dehkordi FS. Detection of Toxoplasma gondii antibodies in various poultry meat samples using enzyme linked immuno sorbent assay and its confirmation by polymerase chain reaction. J Pure Appl Microbiol. 2014;8(1):421–427. [Google Scholar]
- 49.Dehkordi AH, Khaji L, Shahreza MS, Mashak Z, Safarpoor Dehkordi F, Safaee Y, et al. One-year prevalence of antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus recovered from raw meat. Trop Biomed. 2017;34(2):396–404. [PubMed] [Google Scholar]
- 50.Madah H, Rostami F, Rahimi E, Dehkord FS. Prevalence of enterotoxigenic Staphylococcus aureus isolated from chicken nugget in Iran. Jundishapur J Microbiol. 2014;7(8):e10237. doi: 10.5812/jjm.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Momtaz H, Dehkordi FS, Rahimi E, Asgarifar A, Momeni M. Virulence genes and antimicrobial resistance profiles of Staphylococcus aureus isolated from chicken meat in Isfahan province. Iran. J Appl Poult Res. 2013;22(4):913–921. [Google Scholar]
- 52.Momtaz H, Safarpoor Dehkordi F, Taktaz T, Rezvani A, Yarali S. Shiga toxin-producing Escherichia coli isolated from bovine mastitic milk: serogroups, virulence factors, and antibiotic resistance properties. Sci World J. 2012;2012:618709. doi: 10.1100/2012/618709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Momtaz H, Rahimian MD, Safarpoor DF. Identification and characterization of Yersinia enterocolitica isolated from raw chicken meat based on molecular and biological techniques. J Appl Poult Res. 2013;22(1):137–45.