Since December 2019, coronavirus disease 2019 (COVID-19) outbreak caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has produced a worldwide panic. Beyond the principal human-to-human transmission method by droplet and contact, there is still limited knowledge about possible alternate transmission methods to guide clinical care. Recent clinical studies have observed digestive symptoms in patients with COVID-19,1 possibly because of the enrichment and infection of SARS-CoV-2 in the gastrointestinal tract, mediated by virus receptor of angiotensin converting enzyme 2 (ACE2),2 which suggests the potential for a fecal-oral route of SARS-CoV-2 transmission.3 , 4 However, there is still a large gap in the biological knowledge of COVID-19. In this study, via a bulk-to-cell strategy focusing on ACE2, we performed an integrated omics analysis at the genome, transcriptome, and proteome levels in bulk tissues and single cells across species to decipher the potential routes for SARS-CoV-2 infection in depth.
Methods
Clinical and epidemiologic data of patients with COVID-19 were collected from a continually updated resource.5 The transcriptome and proteome derived from bulk tissues and cells were accessed from multiple databases. A phenome-wide association study data set was supplied for genetic analysis on the ACE2 pathway. P values were calculated from t test and gene set analysis. More details are shown in Supplementary Methods.
Results
Clinical Symptoms of Coronavirus Disease 2019 at Diagnosis
We constructed a user-friendly interface for the visualization of clinical symptoms of COVID-19 (https://mulongdu.shinyapps.io/map_covid/; Supplementary Figure 1). Fever was the most common symptom at onset of illness (>70%). Notably, 5.13% and 3.34% of patients had recorded digestive symptoms from Hubei and the outside (Supplementary Table 1), respectively; 1.67% of asymptomatic carriers were recorded with positive SARS-CoV-2.
ACE2 Expression Pattern in Bulk Tissues
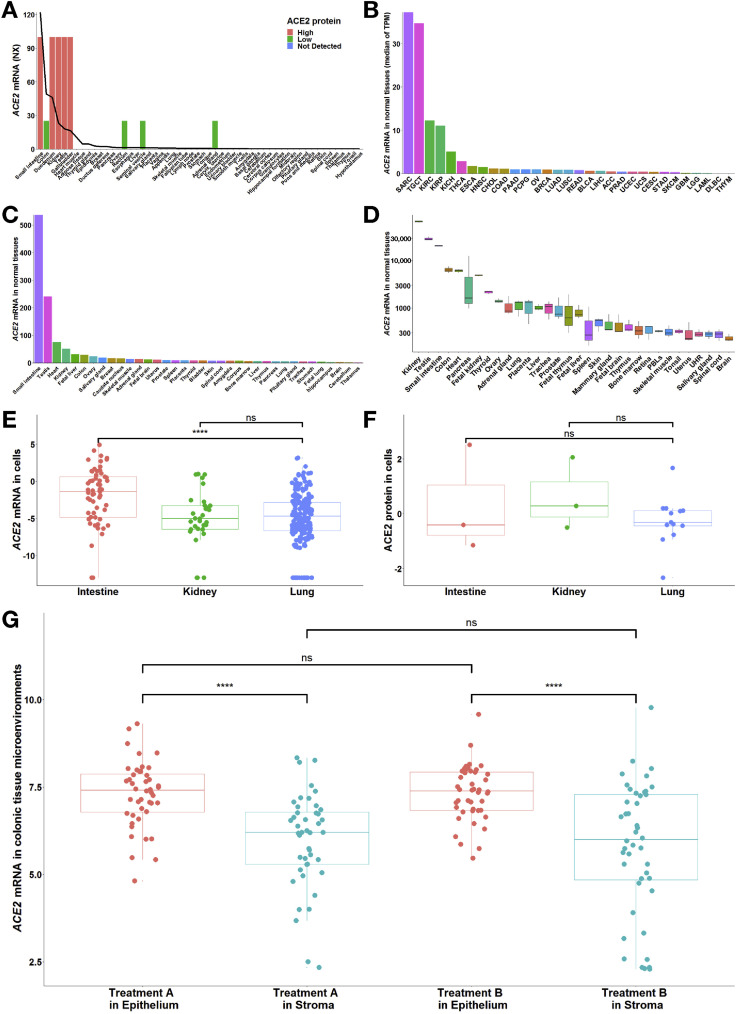
As shown in Figure 1 , ACE2 was widely expressed across tissues. ACE2 was considered intestine specific because its expression was enriched more than 4-fold in the intestinal tract (Figure 1 A) compared with other tissues. The protein detection supported the activity of ACE2 in digestive, excretory, and reproductive organs (Figure 1 A). A similar expression pattern could be found in extra data sets (Figure 1 B–D). However, ACE2 expression was mild in the lung.
Figure 1.
Expression pattern of ACE2 across tissues and cells in the human body. (A) At the mRNA level, in combined data from Human Protein Atlas, Genotype Tissue Expression, and Functional Annotation of The Mammalian Genome, the top 5 tissues included those from 3 digestive organs (intestinal tract), along with the kidney and testis, indicating the intestine specificity of ACE2 (with normalized expression values of 122, 49.1 and 46 in small intestine, colon, and duodenum, respectively). The line represents the normalized expression value of ACE2 across all tissues. At the protein level, in samples from Human Protein Atlas, 9 tissues were stained with ACE2, among which 5 tissues were highly stained, including tissues from 2 digestive organs (duodenum and small intestine), and 4 tissues were minimally stained, including tissues from digestive organs (colon and rectum). Furthermore, similar to ACE2 mRNA, ACE2 protein was enriched in the kidney and testis. The bars indicate the immunohistochemical results for ACE2 protein. Antibody staining was reported as not detected, low, medium, or high, and the score was based on the staining intensity and fraction of stained cells. (B–D) Among the normal tissues with ACE2 mRNA expression from (B) The Cancer Genome Atlas (TCGA) and (C, D) Gene Expression Omnibus, the top tissues also included those from the intestine, kidney, and testis. We excluded SARC in TCGA because it contained only 2 samples of normal tissues. (C) GSE2361-supplied gene expression profiles in 36 normal human tissues with only 1 sample per tissue. (D) GSE7905-collected gene expression profiles from 31 normal tissues and a Universal Human Reference RNA, which used 3 replicates per tissue for a total of 96 samples. (E) At the mRNA level, ACE2 expression in the intestinal cell lines (n = 60; –2.08 ± 4.02) was higher than that in the lung cell lines (n = 194; –5.00 ± 3.51; ∗∗∗∗P = 2.88 × 10–6), but there was no significant difference between the kidney (n = 32; –4.92 ± 3.59) and lung cell lines. (F) At the protein level, the differences in ACE2 expression among the intestine (n = 3; 0.31 ± 1.94), kidney (n = 3; 0.61 ± 1.31) and lung (n = 13; –0.26 ± 0.89) cell lines were not statistically significant. Values are reported as mean ± standard deviation. Both mRNA and protein expression levels were adjusted by the global normalization. (G) GSE71571-provided gene expression profiles in the epithelial and stromal cells of normal colon in 44 healthy individuals recruited for an aspirin intervention trial. Briefly, participants were randomly assigned to the order in which they received treatment A or treatment B, respectively. (The type of treatment included active [aspirin] and placebo but was masked. The study authors must be contacted directly for unblinding). A paired t test was used for the comparison of ACE2 mRNA between epithelial and stromal cells (∗∗∗∗P = 4.60 × 10–8 in treatment A; ∗∗∗∗P = 8.81 × 10–7 in treatment B). An unpaired t test was applied for the comparison of ACE2 mRNA between treatment A and B. More details are provided in the Supplementary Methods. ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, Lymphoid neoplasm diffuse large B-cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and Neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML, acute myeloid leukemia; LGG, brain lower-grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; ns, no significance; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THY, thymoma; TPM, transcripts per million; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UHR, Universal Human Reference RNA; UVM, uveal melanoma.
ACE2 Expression Pattern in Cells
We further performed cell-specific analysis to decipher the expression pattern of ACE2 in target organs. ACE2 messenger RNA (mRNA) in the intestinal cell lines was significantly higher than in the lung cell lines (P = 2.88 × 10–6) (Figure 1 E) but did not differ significantly between the kidney and lung cell lines. Moreover, the mean values of ACE2 protein in both the intestinal (0.31) and kidney (0.61) cell lines were higher than those in the lung (–0.26), although there were no significant differences among the cells (Figure 1 F).
In colonic tissue microenvironments, ACE2 exhibited significantly higher expression in epithelia than in stroma (P = 4.60 × 10–8 in treatment A; P = 8.81 × 10–7 in treatment B) (Figure 1 G); however, intriguingly, there was no significant difference in ACE2 mRNA between aspirin intervention and placebo (Figure 1 G).
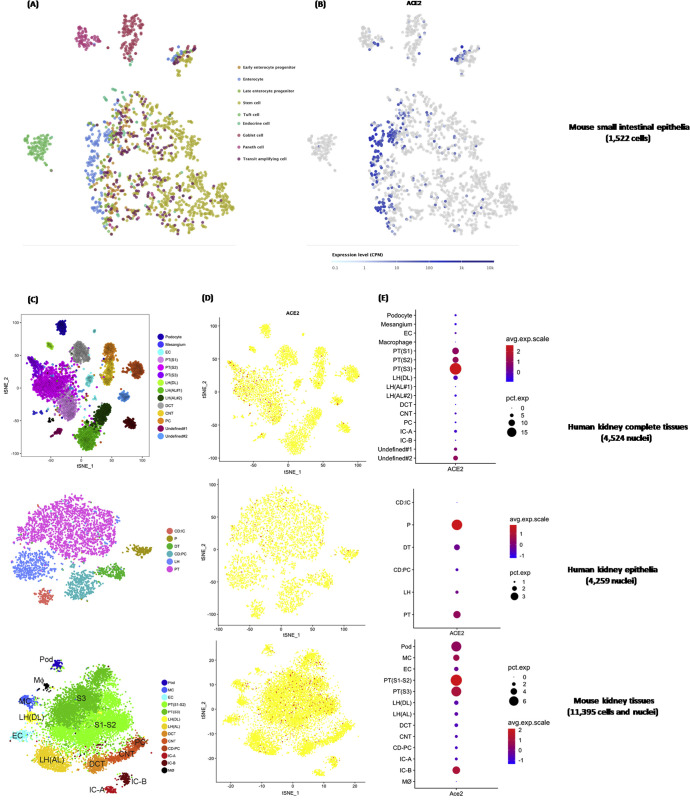
Subsequently, we carried out single-cell analysis to dissect the ACE2 expression pattern. ACE2 was enriched specifically in the enterocytes of mice small intestine epithelia (Supplementary Figure 2 A and B), which was consistent with the findings in humans.6 ACE2 was highly concentrated in epithelia at the renal proximal tubule in both humans and mice (Supplementary Figure 2 C–E).
Supplementary Figure 2.
Single-cell RNA sequencing data showing the ACE2 expression pattern in the intestinal tract and kidney. (A) tSNE plot of small intestinal epithelial cell subgroups. Cells were partitioned into 9 groups: early enterocyte progenitor, enterocyte, late enterocyte progenitor, stem cell, tuft cell, endocrine cell, goblet cell, Paneth cell, and transit amplifying cell. (B) Cells expressing ACE2 expression are colored blue, indicating enrichment of ACE2 in intestinal enterocytes. (C) tSNE plot of cell subgroups for each kidney data set. Cells from human and mouse kidney tissues were grouped by kidney anatomy: ascending limb (AL), collecting duct–principal cell (CD-PC), connecting tubule (CNT), distal convoluted tubule (DCT/DT), descending limb (DL), endothelial cell (EC), intercalated cell (IC), loop of Henle (LH), mesangial cell (MC), macrophage (MΦ), podocyte (Pod/P), and proximal tubule (PT). (D) Cells expressing ACE2 are colored red, indicating enrichment of ACE2 in the proximal tubule of the kidney. (E) The abundance of ACE2 expression across each cell subgroup in human and mouse kidneys. avg, average; exp, expression; CPM, counts per million; pct, percent; tSNE, t-distributed stochastic neighbor embedding.
Genetic Effect of the Angiotensin Converting Enzyme 2 Pathway on Clinical Symptoms
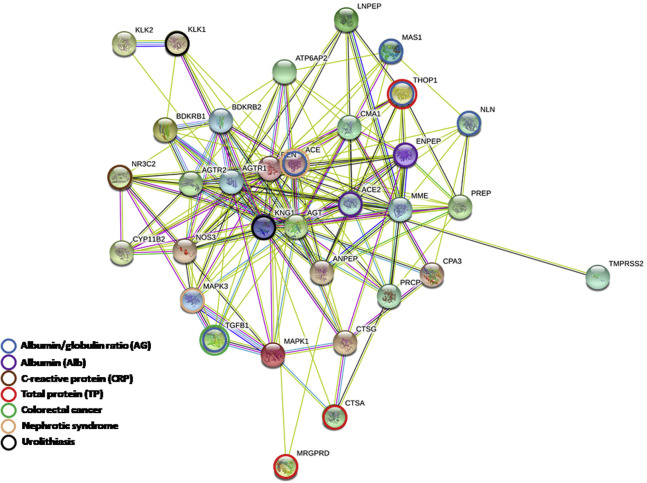
We obtained 7 phenotypes related to the intestinal tract and kidney deposited in the phenome-wide association study data set and extracted genome-wide association study summary statistics with more than 3000 single-nucleotide polymorphisms assigned to 33 genes of the ACE2 pathway for the gene set analysis (Supplementary Table 2 and Supplementary Figure 3). In the pathway-based level, we found no significant genetic association of the ACE2 pathway with 7 phenotypes but a modest performance of the prediction model in nephrotic syndrome (area under the receiver operating characteristic curve, 0.607) (Supplementary Table 3). Partitioned to the gene-based level, the significant joint effect of each gene extended across different phenotypes (Supplementary Table 3). In the disorders of the digestive and excretory systems, TGFB1 was associated with colorectal cancer, ACE and MAPK3 with nephrotic syndrome, and KLK1 and KNG1 with urolithiasis. Similarly, in the blood test parameters, ACE2, along with ENPEP, exhibited a significant association only with Alb.
Supplementary Figure 3.
Network of genes in the ACE2 pathway. The network was constructed by STRING (https://string-db.org/) with the default parameters.
Discussion
On the basis of prior evidence suggesting that ACE2 mediated the entry of SARS-CoV-2 into cells,2 we previously found that ACE2 expression was significantly higher in smokers than in nonsmokers, especially in distinct lung cell types.7 This was corroborated by the clinical observation that the patients with severe cases of COVID-19 were more likely to have a smoking history (22.1%) than those with nonsevere COVID-19 (13.1%).1 In this study, ACE2 was confirmed to be enriched in the epithelia of the intestinal tract; therefore, a mutual interaction potentially occurred such that SARS-CoV-2 disrupted ACE2 activity, infected the intestinal epithelium by its cytotoxicity, and shed into feces, resulting in gastrointestinal manifestations and/or positive SARS-CoV-2 in stool.4 , 8 Considering the physiologic renewal of intestinal epithelia every 4–5 days, our results warn that more attention must be given to the possibility of fecal-oral transmission of SARS-CoV-2, especially by asymptomatic carriers.
The renal proximal tubule enriched in ACE2 indicated that viral shedding in the urine was feasible; however, no evidence supported the actual detection of SARS-CoV-2 in the urine.4 Nevertheless, renal impairment was common in patients with severe COVID-19,1 which could be supported by the potential that SARS-CoV-2 damaged renal tubular cells and induced the disruption of the ACE2 pathway referring to ACE2, ACE, ENPEP, TGFB1, THOP1, MAS1, and NLN involved in kidney dysfunction.
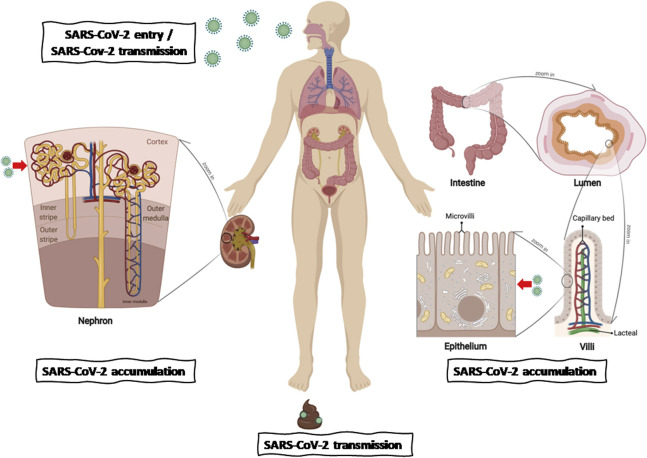
In summary, ACE2 enriched in the intestinal tract and kidney—more specifically, in the epithelium—could mediate the entry of SARS-CoV-2 into cells to accumulate and cause cytotoxicity but does not respond to nonsteroidal anti-inflammatory drugs. It is reasonable to emphasize the monitoring of digestive and excretory system complications in patients with COVID-19 and the possibility of SARS-CoV-2 transmission via the fecal-oral route by individuals with suspected infection and asymptomatic carriers (Supplementary Figure 4).
Supplementary Figure 4.
Schematic of the bulk-to-cell strategy for evaluating SARS-CoV-2 infection, accumulation, and transmission across hosts. The diagram was constructed with BioRender (https://biorender.com/).
Acknowledgments
The authors acknowledge Professor David C. Christiani (Departments of Environmental Health and Department of Epidemiology, Harvard T.H. Chan School of Public Health) as the senior author of this study. The authors would like to thank Dr Duo Peng (Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health) for his helpful advice and comments on the notion of this project and editing of the manuscript. The authors would like to thank Junyi Xin, Shuai Ben, and Silu Chen (Department of Environmental Genomics, Jiangsu Key Laboratory of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China), Peng Huang (Department of Epidemiology, Key Laboratory of Infectious Diseases, Nanjing Medical University, Nanjing, China), Qiang Cao (Department of Urology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China), and Lijuan Lin (Department of Biostatistics, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China) for their invaluable contributions to this article.
CRediT Authorship Contributions
Mulong Du, PhD (Conceptualization: Equal; Methodology: Lead; Writing – original draft: Lead); Guoshuai Cai, PhD (Formal analysis: Lead; Investigation: Equal; Writing – review & editing: Supporting); Feng Chen, PhD (Supervision: Supporting; Visualization: Supporting; Writing – review & editing: Supporting); David C. Christiani, MD (Supervision: Equal; Writing – review & editing: Supporting); Zhengdong Zhang, PhD (Conceptualization: Equal; Supervision: Lead); Meilin Wang, PhD (Conceptualization: Equal; Funding acquisition: Equal; Supervision: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine, China).
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.03.045.
Supplementary Methods
Clinical and Epidemiologic Information
The clinical and epidemiologic data of patients with COVID-19 were collected from a continually updated resource (https://tinyurl.com/s6gsq5y),1 including 26,357 cases worldwide. The analyzed data set was downloaded on Feb 23, 2020, which enrolled 14,729 confirmed cases from Hubei, Wuhan province, and 11,628 cases from the outside of Hubei around the world. The visualization of symptoms of COVID-19 cases worldwide was built using plotly and shiny packages in the R library (R Core Team, Vienna, Austria).
Bulk Tissue Resources
The consensus normalized expression value of ACE2 mRNA was extracted from the combined transcriptomics data sets by Human Protein Atlas,2 Genotype Tissue Expression,3 and Functional Annotation of The Mammalian Genome,4 which enrolled 55 tissue types and 6 blood cell types. Briefly, the Human Protein Atlas provided transcriptome and proteome profiles across approximately 40 kinds of cells, tissues, and organs in the human body and grouped genes into categories describing the tissue specificity. The Genotype Tissue Expression project collected samples from 54 nondiseased tissue sites from nearly 1000 individuals. The Functional Annotation of The Mammalian Genome project supplied transcriptome data for every major human organ and more than 200 cancer cell lines.
The Cancer Genome Atlas and Gene Expression Omnibus also supplied transcriptome profiles derived from bulk tissues. We extracted ACE2 mRNA in normal tissues from both The Cancer Genome Atlas, spanning 33 cancer types deposited in the Gene Expression Profiling Interactive Analysis platform,5 and the Gene Expression Omnibus, including major organs (GSE2361,6 GSE7905,7 and GSE715718). We further grouped cells into the subtypes intestine, kidney, and lung according to the cell progenitor using the Cancer Cell Line Encyclopedia data set and performed differential expression analysis for ACE2 mRNA and protein across each subgroup using the t test. The Cancer Cell Line Encyclopedia project provided omics data, including transcriptome9 and proteome,10 covering 1100 cell lines.
Single-Cell RNA Sequencing Analysis
We used the Single Cell Expression Atlas (https://www.ebi.ac.uk/gxa/sc/home) and Kidney Interactive Transcriptomics (http://humphreyslab.com/SingleCell/) to visualize the expression pattern of ACE2 mRNA in each cell cluster of intestinal tract and kidney. Briefly, single-cell RNA sequencing of intestinal and kidney tissues were carried out using the InDrop, DropSeq, or 10× Chromium platforms, and the distribution of ACE2 was shown in the cell subgroups derived from both humans (including 4524 nuclei in complete kidney tissues11 and 4259 nuclei in kidney epithelia12) and mice (including 1522 cells in small intestinal epithelia13 and 11,395 cells and nuclei in kidney epithelia14).
Selection of Angiotensin Converting Enzyme 2 Pathways and Genetic Variants
The key pathways containing ACE2 were accessed from the Kyoto Encyclopedia of Genes and Genomes (Renin-angiotensin system; hsa04614) and PathCards (ACE Inhibitor Pathway, Pharmacodynamics SuperPath). Considering that TMPRSS2 could cooperate with ACE2 to enable the virus to enter the host cells,15 we ultimately included 33 genes in the ACE2 network for further genetic analysis (Supplementary Table 2), among which ACE2, ATP6AP2, and AGTR2 were located on chromosome X. The ACE2 pathway network was constructed using STRING (https://string-db.org/) (Supplementary Figure 3). Genomic information for the candidate genes was extracted from the National Center for Biotechnology Information assembly by GRCh 37 (Supplementary Table 2). The 1000 Genomes Projects (phase 3) provided the genetic information for each gene, along with the following quality control criteria for selecting genetic variants underlying east Asian ancestry (CHB: Han Chinese in Beijing, China; JPT: Japanese in Tokyo, Japan): minor allele frequency >0.01, P value for Hardy-Weinberg equilibrium >1.0 × 10–6, and genotyping call rate >95%.
Analysis of Phenome-wide Association Studies
We initially used the largest Asian phenome-wide association study database, the Japanese Encyclopedia of Genetic Associations by Riken,16 , 17 to evaluate the genetic effect of the ACE2 pathway on the clinical symptoms of COVID-19 and the disease process that occurs in ACE2-enriched tissues. More than 200 traits or diseases with related genome-wide association studies were deposited in the Japanese Encyclopedia of Genetic Associations by Riken, of which the summary statistics were calculated by logistic or linear regression analysis for the association between each single nucleotide polymorphism and phenotypes. When mapping the phenotypes related to the intestinal tract and kidney deposited in the phenome-wide association study data set, we used colorectal cancer, nephrotic syndrome, and urolithiasis for candidate digestive and excretory system diseases. In addition, we considered the blood test parameters, including the albumin/globulin ratio and the Albumin, C-reactive protein, and total protein concentrations, as reflections of patients’ health conditions in terms of the clinical symptoms of COVID-19.18
To avoid unexplainable and weak effects of a single genetic variant on a phenotype, we thus performed gene set analysis using Multi-marker Analysis of genomic Annotation (MAGMA),19 which aggregated genetic variants into candidate gene or pathway sets, to evaluate and predict the phenotypes susceptibility. We also used SummaryAUC to evaluate the performance of polygenic risk prediction models in binary outcomes underlying summary statistics.20
Ethics Approval
All public databases involving human participants were approved by the ethics committees of original studies, and this study was approved by the institutional review board of Nanjing Medical University (NJMUIRB -2020-020).
Supplementary Figure 1.
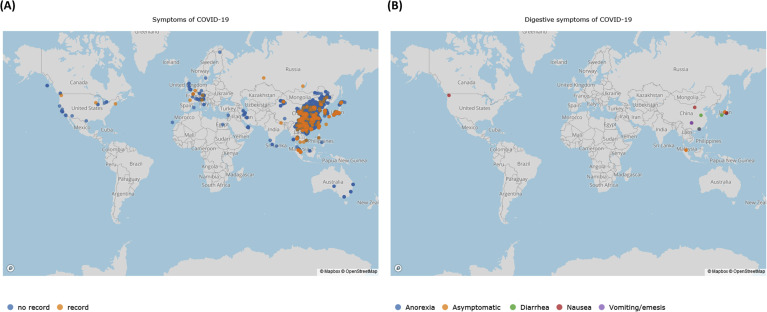
The global distribution of COVID-19 cases. The data set was downloaded on February 23, 2020 (https://tinyurl.com/s6gsq5y). (A) On that date, a total of 26,357 cases were distributed around the world, among which 398 cases (39 cases derived from Hubei and 359 from outside Hubei) recorded with complete clinical symptoms at the onset of illness are marked in orange, and the cases without recorded clinical symptoms are shown in blue. (B) Patients with symptoms in digestive system or asymptomatic carriers are marked in varying colors. A Web site location provided a user-friendly interface for visualization of clinical symptoms (https://mulongdu.shinyapps.io/map_covid/).
Supplementary Table 1.
Clinical Characteristics of Patients Infected With SARS-CoV-2
| Characteristics | Patients in Hubei |
Patients outside of Hubei |
||
|---|---|---|---|---|
| n = 39 | % | n = 359 | % | |
| Age, y | ||||
| <18 | 0 | 0.00 | 12 | 3.34 |
| 18–29 | 0 | 0.00 | 44 | 12.26 |
| 30–39 | 1 | 2.56 | 73 | 20.33 |
| 40–49 | 2 | 5.13 | 73 | 20.33 |
| 50–59 | 4 | 10.26 | 65 | 18.11 |
| 60–69 | 11 | 28.21 | 56 | 15.60 |
| 70–79 | 9 | 23.08 | 14 | 3.90 |
| ≥80 | 11 | 28.21 | 5 | 1.39 |
| Sex | ||||
| Male | 26 | 66.67 | 201 | 55.99 |
| Female | 13 | 33.33 | 148 | 41.23 |
| Countries | ||||
| Belgium | 1 | 0.28 | ||
| Cambodia | 1 | 0.28 | ||
| China | 256 | 71.31 | ||
| France | 3 | 0.84 | ||
| Germany | 1 | 0.28 | ||
| Italy | 1 | 0.28 | ||
| Japan | 52 | 14.48 | ||
| Malaysia | 7 | 1.95 | ||
| Nepal | 1 | 0.28 | ||
| Philippines | 1 | 0.28 | ||
| Russia | 2 | 0.56 | ||
| Singapore | 9 | 2.51 | ||
| South Korea | 9 | 2.51 | ||
| Thailand | 5 | 1.39 | ||
| United States | 3 | 0.84 | ||
| Vietnam | 7 | 1.95 | ||
| Symptoms | ||||
| Fever | 31 | 79.49 | 266 | 74.09 |
| Cough | 21 | 53.85 | 128 | 35.65 |
| Fatigue | 5 | 12.82 | 16 | 4.46 |
| Digestive (diarrhea, nausea, vomiting/emesis, anorexia) | 2 | 5.13 | 12 | 3.34 |
| Asymptomatic | 0 | 0.00 | 6a | 1.67 |
3 patients were from Japan, and 3 were from Malaysia.
Supplementary Table 2.
Information on 33 Genes in the ACE2 Pathway
| Gene name | Gene ID | Chromosome | Position | Strand |
|---|---|---|---|---|
| REN | 5972 | 1 | 204123944-204135465 | – |
| AGT | 183 | 1 | 230838269-230850336 | – |
| AGTR1 | 185 | 3 | 148415658-148460790 | + |
| CPA3 | 1359 | 3 | 148583043-148614874 | + |
| MME | 4311 | 3 | 154797436-154901518 | + |
| KNG1 | 3827 | 3 | 186435098-186462199 | + |
| ENPEP | 2028 | 4 | 111397229-111484493 | + |
| NR3C2 | 4306 | 4 | 148999915-149365850 | – |
| NLN | 57486 | 5 | 65018023-65125111 | + |
| LNPEP | 4012 | 5 | 96271346-96365115 | + |
| PREP | 5550 | 6 | 105725442-105850999 | – |
| MAS1 | 4142 | 6 | 160320218-160329339 | + |
| NOS3 | 4846 | 7 | 150688144-150711687 | + |
| CYP11B2 | 1585 | 8 | 143991975-143999259 | – |
| MRGPRD | 116512 | 11 | 68747490-68748455 | – |
| PRCP | 5547 | 11 | 82535409-82612733 | – |
| CMA1 | 1215 | 14 | 24974712-24977471 | – |
| CTSG | 1511 | 14 | 25042724-25045466 | – |
| BDKRB2 | 624 | 14 | 96671016-96710666 | + |
| BDKRB1 | 623 | 14 | 96721641-96735304 | + |
| ANPEP | 290 | 15 | 90328126-90358119 | – |
| MAPK3 | 5595 | 16 | 30125426-30134630 | – |
| ACE | 1636 | 17 | 61554422-61575741 | + |
| THOP1 | 7064 | 19 | 2785464-2813599 | + |
| TGFB1 | 7040 | 19 | 41836812-41859831 | – |
| KLK1 | 3816 | 19 | 51322402-51327043 | – |
| KLK2 | 3817 | 19 | 51376689-51383823 | + |
| CTSA | 5476 | 20 | 44519591-44527459 | + |
| TMPRSS2 | 7113 | 21 | 42836236-42880085 | – |
| MAPK1 | 5594 | 22 | 22113946-22221970 | – |
| ACE2 | 59272 | X | 15579156-15620192 | – |
| ATP6AP2 | 10159 | X | 40440141-40465889 | + |
| AGTR2 | 186 | X | 115301958-115306225 | + |
ID, identification.
Supplementary Table 3.
Gene Set Analysis to Evaluate the Genetic Effect of the ACE2 Pathway on the 7 Phenotypes
| Sources | Phenotypes | Pathway name | Number of genes/SNPs | Sample size, N | Ppathway based | AUC (variance) |
|---|---|---|---|---|---|---|
| Disease | Colorectal cancer | ACE2 pathway | 33 | 202,807 | .920 | 0.542 (1.21 × 10-5) |
| Nephrotic syndrome | ACE2 pathway | 33 | 212,453 | .933 | 0.607 (8.24 × 10-5) | |
| Urolithiasis | ACE2 pathway | 33 | 212,453 | .743 | 0.537 (1.29 × 10-5) | |
| Blood test |
Albumin | ACE2 pathway | 33 | 102,223 | .306 | NA |
| Albumin/globulin ratio | ACE2 pathway | 33 | 98,626 | .349 | ||
| C-reactive protein | ACE2 pathway | 33 | 75,391 | .365 | ||
| Total protein | ACE2 pathway | 33 | 113,509 | .541 | ||
| Gene name | Number of SNPs | |||||
| Disease | Colorectal cancer | TGFB1 | 23 | 202,807 | .028 | NA |
| Nephrotic syndrome | ACE | 37 | 212,453 | .012 | ||
| Nephrotic syndrome | MAPK3 | 3 | 212,453 | .012 | ||
| Urolithiasis | KLK1 | 13 | 212,453 | .001 | ||
| Urolithiasis | KNG1 | 146 | 212,453 | .002 | ||
| Blood test | Albumin | ENPEP | 130 | 102,223 | .020 | NA |
| Albumin | ACE2 | 18 | 102,223 | .029 | ||
| Albumin/globulin ratio | ACE | 33 | 98,626 | .012 | ||
| Albumin/globulin ratio | TGFB1 | 23 | 98,626 | .013 | ||
| Albumin/globulin ratio | THOP1 | 36 | 98,626 | .020 | ||
| Albumin/globulin ratio | MAS1 | 13 | 98,626 | .032 | ||
| Albumin/globulin ratio | NLN | 253 | 98,626 | .037 | ||
| C-reactive protein | NR3C2 | 624 | 75,391 | .021 | ||
| Total protein | MRGPRD | 1 | 113,509 | .008 | ||
| Total protein | CTSA | 16 | 113,509 | .012 | ||
| Total protein | THOP1 | 36 | 113,509 | .012 | ||
AUC, area under the receiver operating characteristic curve (calculated by SummaryAUC); NA, not available; SNP, single nucleotide polymorphism.
References
- 1.Guan W.J. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu J. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao F. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu B. Lancet Infect Dis. 2020;20:534. doi: 10.1016/S1473-3099(20)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H. bioRxiv. 2020: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1 2020.01.30.927806. Available at: [Google Scholar]
- 7.Cai G. Am J Respir Crit Care Med. 2020 [Google Scholar]
- 8.Xu Y. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 1.Xu B. Lancet Infect Dis. 2020;20:534. doi: 10.1016/S1473-3099(20)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhlen M. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 3.Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu N.Y. Nucleic Acids Res. 2015;43:6787–6798. doi: 10.1093/nar/gkv608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Z. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge X. Genomics. 2005;86:127–141. doi: 10.1016/j.ygeno.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Dezso Z. BMC Biol. 2008;6:49. doi: 10.1186/1741-7007-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas S.S. Genom Data. 2015;6:154–158. doi: 10.1016/j.gdata.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barretina J. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nusinow D.P. Cell. 2020;180:387–402. doi: 10.1016/j.cell.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H. Cell Stem Cell. 2018;23:869–881. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H. J Am Soc Nephrol. 2018;29:2069–2080. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haber A.L. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H. J Am Soc Nephrol. 2019;30:23–32. doi: 10.1681/ASN.2018090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai M. Nat Genet. 2018;50:390–400. doi: 10.1038/s41588-018-0047-6. [DOI] [PubMed] [Google Scholar]
- 17.Ishigaki K. bioRxiv. 2019:795948. [Google Scholar]
- 18.Chen N. Lancet. 2020 [Google Scholar]
- 19.de Leeuw C.A. PLoS Comput Biol. 2015;11 doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song L. Bioinformatics. 2019;35:4038–4044. doi: 10.1093/bioinformatics/btz176. [DOI] [PMC free article] [PubMed] [Google Scholar]