Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal human malignancies. Chemotherapeutic agents, such as sorafenib and lenvatinib, can improve the outcomes of HCC patients. Nevertheless, chemoresistance has become a major hurdle in the effective treatment of HCC. Noncoding RNAs (ncRNAs), including mircoRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs), have been demonstrated to participate in the onset and progression of HCC. Moreover, multiple lines of evidence have indicated that ncRNAs also play a pivotal role in HCC drug resistance. ncRNAs can regulate drug efflux and metabolism, glucose metabolism, cellular death pathways, and malignant characteristics in HCC. A deeper understanding of the molecular mechanisms responsible for ncRNA-mediated drug resistance in HCC will provide new opportunities for improving the treatment of HCC. In this review, we summarize recent findings on the molecular mechanisms by which ncRNAs regulate HCC chemoresistance, as well as their potential clinical implications in overcoming HCC chemoresistance.
Keywords: hepatocellular carcinoma, chemoresistance, microRNAs, long noncoding RNAs, circular RNAs
Graphical Abstract
Noncoding RNAs (ncRNAs) have emerged as important regulators of drug resistance in hepatocellular carcinoma (HCC). This article reviews the molecular mechanisms underlying ncRNA-mediated HCC chemoresistance. Understanding the functional mechanisms of ncRNAs in coordinating resistance pathways provides insight into potentially novel therapeutic interventions that can overcome chemoresistance in HCC patients.
Main Text
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers worldwide and the third-most lethal human malignancy among cancer patients.1 Surgical resection, liver transplantation, tumor ablation, transarterial therapies, and systemic chemotherapies are currently the mainstays of treatment for HCC.2 In addition, immune-based therapies for HCC have been proposed. Several clinical trials are being conducted to investigate the efficacy of immune-based therapies. Remarkably, radical surgery, ablation, and liver transplantation are only curative in cases of early HCC diagnosis. Transarterial therapies should be performed in patients with intermediate-stage tumors. Systemic chemotherapies are recommended for patients with an advanced stage of HCC. To date, several agents, including sorafenib and lenvatinib, have been approved for systemic therapy for advanced HCC.3 However, the therapeutic effectiveness of these chemotherapeutic agents is significantly restricted by the chemoresistance of HCC cells. For this reason, drug resistance is a difficult obstacle in HCC therapy. Generally, cancer resistance to chemotherapies is classified into intrinsic and acquired resistance.4 Intrinsic resistance is an inherent capability of cancer cells to survive and persist through their first exposure to therapies. Distinct mechanisms are involved in the intrinsic resistance of cancer cells. Drug efflux pumps function to decrease drug concentrations within cancer cells,5 and therapeutic drugs can be biochemically degraded by detoxifying enzymes (e.g., cytochrome p450 and glutathione transferases).6,7 In contrast, acquired resistance is the molecular evolution of cancer cells, following treatment, to a persistent state, whereby cells can expand in the presence of subsequent treatment though natural selection of changes contributing to a survival advantage. This can be mediated by regulation of the expression of genes that are involved in cell proliferation, survival and death signaling pathways, genetic damage tolerance, and DNA repair capacity. Acquired resistance renders cancer cells resistant to more than one type of chemotherapeutic agent. The future of HCC treatment is correlated with our capability to elucidate the molecular mechanisms associated with HCC chemoresistance and to discover novel therapeutic targets.
Noncoding RNAs (ncRNAs) refer to transcripts that have no protein-coding potential and constitute a vast majority of cellular RNAs.8 ncRNAs are functional regulatory molecules that are involved in various cellular processes, including transcription, translation, chromatin remodeling, and post-translational modification.9 More importantly, ncRNAs can affect multiple molecular targets associated with cell proliferation and apoptosis.9 Accordingly, ncRNAs serve as vital mediators of cellular functions in both physiological and pathological contexts. ncRNAs are generally grouped into small or short ncRNAs and long ncRNAs (lncRNAs) with a transcript size cutoff of 200 nucleotides (nt) in length.10 There are several kinds of small ncRNAs, including microRNAs (miRNAs), endogenous small interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), extracellular RNAs (exRNAs), and small Cajal body-specific RNAs (scaRNAs).11 The most well-known class of small ncRNAs, miRNAs, can negatively regulate gene expression by inducing mRNA degradation or translational repression. lncRNAs are a heterogeneous group of ncRNAs that are larger than 200 nt.12 lncRNAs control gene expression at epigenetic, transcriptional, or translational levels. Among lncRNAs, circular RNAs (circRNAs) are an emerging type of endogenous RNA molecule that are generated from the back-splicing of precursor mRNAs.13 circRNAs form a covalently closed continuous loop and are generally stable relative to linear RNAs. circRNAs can work as miRNA sponges and also combine with proteins.13 circRNAs can be spatially or temporally regulated in a disease-specific manner, implying that they may possess significant functions in disease pathology. miRNAs, lncRNAs, and circRNAs have been identified to take part in key steps during HCC carcinogenesis and progression.14 These ncRNAs also play an important role in the drug resistance of HCC. Although there are several reviews on the roles of ncRNAs in HCC chemoresistance, some recent advances in this field have not yet been reviewed. In a recent review, Wei et al.15 concluded that miRNAs and lncRNAs were involved in HCC chemoresistance, mainly by governing cell proliferation, death, cell cycle, and epithelial-mesenchymal transition (EMT). Additional mechanisms associated with ncRNA-mediated HCC drug resistance have been elucidated, including upregulation of drug-metabolizing enzymes, alteration in the availability of drug targets. and enhanced glucose metabolism. Here, we review the molecular mechanisms identified, to date, that contribute to miRNA/lncRNA-mediated HCC chemoresistance. In addition to miRNAs and lncRNAs, other types of ncRNAs (siRNAs and circRNAs) have been linked to drug resistance in HCC. In this review, we also summarize the underlying mechanisms by which siRNAs and circRNAs affect HCC chemoresistance. In another review by Ding et al.,16 only 9 lncRNAs and their implications in HCC chemoresistance were discussed. So far, more than 20 lncRNAs have been confirmed to play a critical role in the drug resistance of HCC. By contrast, we present a comprehensive overview of lncRNA-mediated regulatory networks involved in HCC chemoresistance. Lai et al.17 previously summarized the relationship between miRNAs/lncRNAs and sorafenib resistance in HCC. Here, we review the regulatory mechanisms of ncRNAs in HCC resistance to various chemotherapeutic agents, such as sorafenib, cisplatin, oxaliplatin, and paclitaxel. Furthermore, we discuss the possibility of the clinical application of ncRNAs in HCC management. A better understanding of the functional mechanisms of ncRNAs in coordinating s resistance pathways may offer an opportunity to develop novel therapeutic interventions that can overcome drug resistance in HCC patients.
Underlying Mechanisms of miRNA-Mediated Chemoresistance in HCC
miRNAs are a class of endogenous small ncRNA molecules with a length of approximately 19–25 nt.18 miRNAs function in the post-transcriptional modulation of gene expression and thus, manipulate pivotal cellular processes, including cell metabolism, proliferation, differentiation, and death.19 In general, miRNAs interact with target mRNAs through complementary base paring to govern their stability or translation. Emerging evidence indicates that miRNAs can regulate the sensitivity of HCC cells to chemotherapeutic drugs by modifying diverse molecular processes (Table 1). The contribution of miRNAs to HCC chemoresistance is outlined below.
Table 1.
Overview of Dysregulated ncRNAs Related to Drug Resistance in HCC
| ncRNA | Gene Type | Alteration | Target/Pathway | Effect on Drug Resistance | References |
|---|---|---|---|---|---|
| miR-325-3p | tumor suppressor | downregulated | DPAGT1 | sensitivity to DOX | 21 |
| miR-590-5p | tumor suppressor | downregulated | YAP1 | sensitivity to DOX | 22 |
| miR-375 | tumor suppressor | downregulated | AEG1 | sensitivity to DOX | 24, 25, 26 |
| miR-125b | tumor suppressor | downregulated | ABCC1, ABCG2, P-gp; HK II | sensitivity to DOX, sorafenib, 5-FU | 27,51 |
| miR-141 | oncogene | upregulated | Keap1 | resistance to 5-FU | 29 |
| miR-340 | tumor suppressor | downregulated | Nrf2-dependent antioxidant pathway | sensitivity to cisplatin | 30 |
| miR-128-3p | oncogene | upregulated | CYP2C9 | – | 31 |
| miR-215 | oncogene | upregulated | DHFR, TS | resistance to DOX | 32 |
| miR-21-5p | oncogene | upregulated | FASLG | resistance to cisplatin | 33 |
| miR-301a-3p | oncogene | upregulated | VGLL4 | resistance to oxaliplatin | 34 |
| miR-16 | tumor suppressor | downregulated | IKBKB | sensitivity to paclitaxel | 35 |
| miR-26b | tumor suppressor | downregulated | NF-κB signaling pathway | sensitivity to DOX | 36 |
| miR-19a-3p | oncogene | upregulated | PTEN/Akt signaling pathway | resistance to sorafenib | 37 |
| miR-760 | tumor suppressor | downregulated | Notch1, PTEN | sensitivity to DOX | 40 |
| miR-205-5p | oncogene | upregulated | PTEN/JNK/ANXA3 pathway | resistance to 5-FU | 41 |
| miR-122 | tumor suppressor | downregulated | IGF-1R; PKM2; serpinB3 | sensitivity to sorafenib, DOX | 42,52,56 |
| miR-379 | tumor suppressor | downregulated | IGF-1R | sensitivity to 5-FU, paclitaxel, DOX | 44 |
| miR-182 | oncogene | upregulated | TP53INP1 | resistance to cisplatin | 46 |
| miR-34a | tumor suppressor | downregulated | Bcl-2 | sensitivity to sorafenib | 47 |
| miR-34a-5p | tumor suppressor | downregulated | AXL | sensitivity to cisplatin | 48 |
| miR-539 | tumor suppressor | downregulated | STAT3 signaling pathway | sensitivity to arsenic trioxide | 49 |
| miR-142-3p | tumor suppressor | downregulated | ATG5, ATG16L1 | sensitivity to sorafenib | 53 |
| miR-26 | tumor suppressor | downregulated | ULK1 | sensitivity to DOX | 54 |
| miR-101 | tumor suppressor | downregulated | RAB5A, STMN1, ATG4D | sensitivity to cisplatin | 55,120 |
| miR-589-5p | oncogene | upregulated | STAT3 signaling cascade, Nanog, BMI-1, Oct4, Sox2 | resistance to DOX | 57 |
| miR-383 | oncogene | upregulated | EIF5A2 | sensitivity to DOX | 59 |
| miR-145 | tumor suppressor | downregulated | SMAD3 | sensitivity to DOX | 61 |
| miR-144 | tumor suppressor | downregulated | SMAD4 | sensitivity to 5-FU | 62 |
| miR-106a | tumor suppressor | downregulated | Twist1 | sensitivity to gemcitabine | 63 |
| KCNQ1OT1 | oncogene | upregulated | MRP5, MDR1, LRP1 | resistance to oxaliplatin | 68 |
| NR2F1-AS1 | oncogene | upregulated | miR-363 | resistance to oxaliplatin | 69 |
| HOTAIR | oncogene | upregulated | STAT3, ABCB1; miR-145 | resistance to cisplatin, imatinib | 70,72 |
| linc-VLDLR | oncogene | upregulated | ABCG2, ABCC1 | resistance to sorafenib, DOX | 73 |
| H19 | oncogene | upregulated | MDR1, P-gp | resistance to DOX | 74 |
| H19 | tumor suppressor | downregulated | miR-193a-3p/PSEN1 | sensitivity to DOX, sorafenib, docetaxel, paclitaxel, vinorelbine and 5-FU | 75,77 |
| KRAL | tumor suppressor | downregulated | miR-141 | sensitivity to 5-FU | 79 |
| NRAL | oncogene | upregulated | miR-340-5p | resistance to cisplatin | 80 |
| PDIA3P1 | oncogene | upregulated | miR-125a/b, miR-124 | resistance to DOX | 81 |
| SNHG1 | oncogene | upregulated | the Akt pathway | resistance to sorafenib | 82 |
| NEAT1 | oncogene | upregulated | miR-335 | resistance to sorafenib | 83 |
| lncARSR | oncogene | upregulated | PTEN/PI3K/Akt pathway; STAT3 signaling pathway | resistance to DOX, cisplatin | 84,103 |
| linc-ROR | oncogene | upregulated | p53; TICs | resistance to arsenic trioxide, sorafenib | 85,95 |
| CASC2 | tumor suppressor | downregulated | miR-24, miR-221 | sensitivity to TRAIL | 86 |
| SNHG16 | tumor suppressor | downregulated | miR-93 | sensitivity to 5-FU | 88 |
| SNHG16 | oncogene | upregulated | – | resistance to sorafenib | 89 |
| GAS5 | tumor suppressor | downregulated | miR-21 | sensitivity to DOX | 90 |
| TUC338 | oncogene | upregulated | RASAL1 | resistance to sorafenib | 91 |
| SNHG6-003 | oncogene | upregulated | miR-26a/b | resistance to 5-FU | 92 |
| HULC | oncogene | upregulated | USP22, Sirt1 | resistance to oxaliplatin, 5-FU, pirarubicin | 93 |
| MALAT1 | oncogene | upregulated | miR-216b | resistance to 5-FU | 94 |
| lnc-PDZD7 | oncogene | upregulated | miR-101 | resistance to 5-FU, sorafenib | 101 |
| HANR | oncogene | upregulated | GSKIP | resistance to DOX | 106 |
| HOTTIP | oncogene | upregulated | HOXA13 | resistance to sorafenib | 107 |
| circRNA_101505 | tumor suppressor | downregulated | miR-103 | sensitivity to cisplatin | 108 |
| circ_0003418 | tumor suppressor | downregulated | Wnt/β-catenin pathway | sensitivity to cisplatin | 109 |
miRNAs Alter the Expression of Drug Efflux Pumps and Metabolizing Enzymes
One major obstacle to successful cancer treatment is the existence of tumor cells that demonstrate the multidrug resistance (MDR) genotype. MDR is commonly a result of the upregulation of drug efflux pumps, including ATP-binding cassette (ABC) transporters. ABC transporters, including ABC transporter-subfamily B member 1 (ABCB1), -subfamily C member 1 (ABCC1), and -subfamily G member 2 (ABCG2), are responsible for the efflux of chemotherapeutic drugs from tumor cells, hence leading to MDR.20 Multiple miRNAs have been reported to affect ABC transporter-mediated drug efflux (Figure 1). MicroRNA (miR)-325-3p was found to target the hexosamine pathway molecule dolichyl-phosphate N-acetylglucosamine phosphotransferase 1 (DPAGT1), thus repressing the growth of doxorubicin (DOX)-resistant HCC cells in vitro and in vivo.21 Mechanistically, DPAGT1 expression was positively correlated with upregulation of stemness-related markers and ABC drug efflux transporters in DOX-treated HCC cells. Yes-associated protein 1 (YAP1) elevated the expression of stemness markers (Oct4, Sox2, Notch1, Nanog, and Nestin) and ABC transporters (ABCB1 and ABCC1) in DOX-resistant HCC cells.22 miR-590-5p markedly enhanced the sensitivity of HCC cells to DOX by downregulating YAP1 in vitro and in vivo.
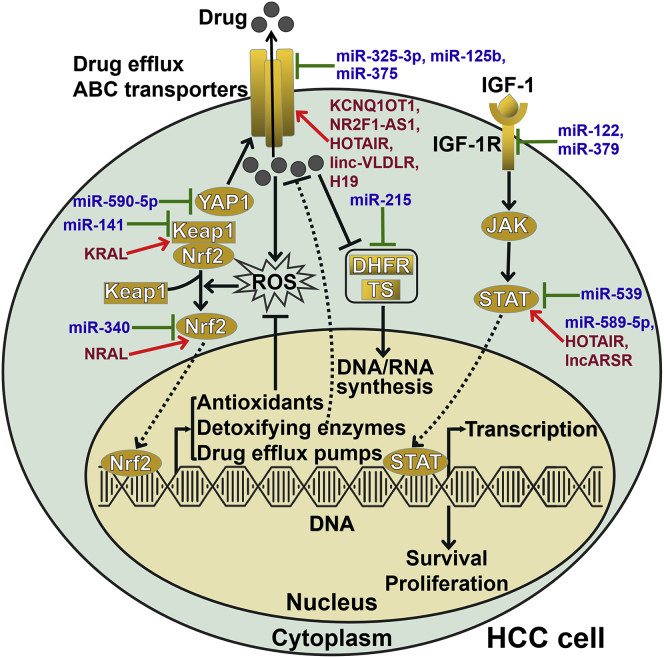
Figure 1.
ncRNAs Affect Drug Efflux and Nrf2-Dependent Drug Metabolism in HCC Cells
Various miRNAs and lncRNAs can regulate intracellular drug concentrations in HCC cells by targeting drug efflux pumps. Several miRNAs and lncRNAs dominate the expression of drug efflux pumps and metabolizing enzymes in HCC cells through the Nrf2 signaling pathway. miR-215 inhibits the expression of DHFR and TS, which are important targets of chemotherapeutic agents. miRNAs and lncRNAs also orchestrate the survival and proliferation of HCC cells by regulating the JAK/STAT signaling pathway. ABC transporter, ATP-binding cassette transporter; YAP1, Yes-associated protein 1; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor-erythroid 2-related factor 2; ROS, reactive oxygen species; DHFR, dihydrofolate reductase; TS, thymidylate synthase; IGF-1, insulin-like growth factor-1; IGF-1R, type 1 insulin-like growth factor receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
Astrocyte-elevated gene-1 (AEG1) prompts the translation of MDR1, which contributes to drug resistance in cancer.23 miR-375 reduced efflux and increased accumulation of DOX in HCC tissues by descending MDR1 expression through downregulation of AEG1.24 Codelivery of miR-375 and DOX by liposomes dramatically reversed DOX resistance by downregulating MDR1. The combination of miR-375 and DOX displayed enhanced antitumor efficiency and overcame DOX resistance in a xenograft mouse model of HCC. Likewise, codelivery of miR-375 and DOX by nanoparticles produced enhanced antitumor effects and reversed DOX resistance in HCC mouse models.25,26 These studies suggested that the combination of DOX and miR-375 might be useful in treating HCC. miR-125b attenuated DOX/sorafenib resistance in HCC cells by negatively modulating MDR genes, including ABCC1, ABCG2, and P-glycoprotein (P-gp).27 Nuclear factor-erythroid 2-related factor 2 (Nrf2) dominated the expression of drug transporters and drug-metabolizing enzymes.28 Activation of the Nrf2 signaling pathway is crucial for the acquisition of drug resistance in cancer cells. miR-141 significantly repressed fluorouracil (5-FU)-induced apoptosis in HCC cells.29 In terms of the mechanism, miR-141 enhanced 5-FU resistance in HCC cells by directly targeting Kelch-like ECH-associated protein 1 (Keap1) and activating the Nrf2-dependent antioxidant pathway. In contrast, miR-340 sensitized HCC cells to cisplatin by blocking the Nrf2-dependent antioxidant pathway.30 Further exploration of the detailed mechanisms underlying miRNA-mediated regulation of drug efflux may offer new insight into HCC chemoresistance. In addition, miRNAs can regulate the expression of drug-metabolizing enzymes. For instance, miR-128-3p was reported to suppress the expression of cytochrome p450 2C9 (CYP2C9) in HCC cells.31 The expression of miR-215 was increased in DOX-resistant HCC cells compared to nonresistant cells.32 The upregulation of miR-215 contributed to the development of DOX chemoresistance in HCC cells. Mechanistic investigation indicated that miR-215 targeted dihydrofolate reductase (DHFR) and thymidylate synthase (TS), both of which were important enzymes in DNA synthesis. These two enzymes are also critical targets of chemotherapeutic drugs.
miRNAs Affect the Cellular Apoptotic Pathway
Inhibition of drug-induced apoptosis is a vital mechanism contributing to drug resistance in HCC. miRNAs are capable of influencing HCC chemoresistance by manipulating the apoptotic pathway (Figure 2). miR-21-5p restrained HCC cell apoptosis by directly targeting FAS ligand (FASLG).33 As a result, miR-21-5p attenuated the sensitivity of HCC cells to cisplatin treatment. miR-301a-3p promoted HCC cell proliferation, invasion, and resistance to oxaliplatin by targeting proapoptotic vestigial-like protein 4 (VGLL4).34 Moreover, miR-301a-3p overexpression was strongly correlated with poor prognosis in HCC patients. miR-16 was expressed at low levels in HCC tissues and cell lines.35 miR-16 enhanced paclitaxel sensitivity of HCC cells by targeting inhibitor of nuclear factor κB (NF-κB) kinase β (IKBKB), a critical protein of the NF-κB signaling pathway. miR-16 also decreased tumor sensitivity to paclitaxel in nude mice. miR-26b improved the chemosensitivity of HCC cells to DOX by negatively regulating the NF-κB signaling pathway.36 miR-19a-3p was obviously upregulated in HCC cells compared to normal hepatic cells.37 miR-19a-3p conferred sorafenib resistance to HCC cells by regulating the phosphatase and tensin homolog (PTEN)/protein kinase B (Akt) signaling pathway. Both the NF-κB and PTEN/Akt pathways are associated with cell apoptosis in cancer.38 Thus, miR-16, miR-26b, and miR-19a-3p mediated HCC chemoresistance by controlling apoptosis-related signaling cascades. Notch1, an important component in Notch signaling, mediated the chemoresistant phenotype in cancer cells.39 miR-760 was downregulated in HCC cells compared to normal liver cells, whereas DOX treatment evidently lowered miR-760 expression.40 miR-760 sensitized HCC cells to DOX-mediated apoptosis by downregulating Notch1 and upregulating PTEN. miR-205-5p conferred resistance to 5-FU in HCC cells through regulation of the PTEN/c-Jun N-terminal kinase (JNK)/annexin A3 (ANXA3) pathway.41 miR-122 conferred drug-tolerant HCC cells sensitive to sorafenib treatment by supporting cell apoptosis.42 miR-122 could target type 1 insulin-like growth factor receptor (IGF-1R), which possessed an antiapoptotic effect.43 Likewise, miR-379 sensitized HCC cells to 5-FU, paclitaxel, and DOX by reducing IGF-1R expression.44 Tumor protein 53-induced nuclear protein 1 (TP53INP1), a tumor suppressor, induced p53-mediated apoptosis.45 miR-182 decreased the expression of TP53INP1 and thus induced resistance to cisplatin in HCC cells.46 miR-34a promoted sorafenib-mediated cytotoxicity and apoptosis in HCC cells by lowering the expression of B cell lymphoma-2 (Bcl-2).47 Another study also demonstrated that miR-34a-5p promoted apoptosis and reversed cisplatin resistance in HCC cells by targeting the receptor tyrosine kinase AXL though the phosphorylated (p)-JNK/Bcl-2 signaling pathway.48 The expression of miR-539 was low in HCC tissues compared to adjacent normal liver tissues.49 miR-539 obviously promoted cell apoptosis and overcame arsenic trioxide resistance in HCC cells by inactivating the signal transducer and activator of transcription 3 (STAT3) signaling pathway. In addition, intratumoral delivery of miR-539 mimics remarkably repressed the growth of xenograft tumors from arsenic trioxide-resistant HCC cells. It has been reported that cancer cells have a high rate of glucose metabolism, and repression of glucose metabolism can foster cancer cell apoptosis.50 The expression of miR-125b was decreased in 5-FU-resistant HCC cells compared to 5-FU-sensitive cells.51 miR-125b increased the sensitivity of HCC cells to 5-FU by targeting hexokinase II (HK II) to suppress glucose metabolism. The expression level of miR-122 was decreased in DOX-resistant HCC cells compared to nonresistant cells.52 miR-122 downregulated pyruvate kinase M2 (PKM2) to limit glucose metabolism, thereby promoting apoptosis and reversing DOX resistance in HCC cells. Targeting glucose metabolism is conducive to improving the sensitivity of cancer cells to chemotherapy. Additional work is required to fully disclose the regulatory role of miRNAs in cellular apoptotic pathways.
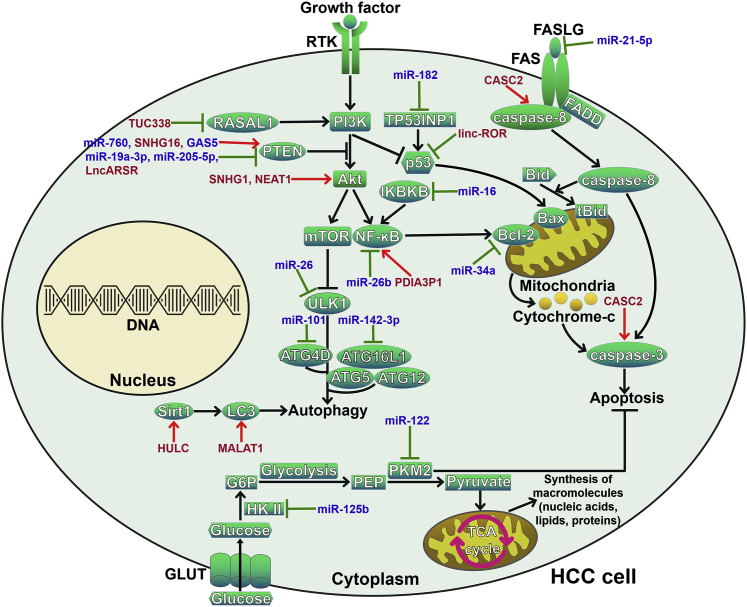
Figure 2.
ncRNAs Regulate Cell Death Pathways to Influence HCC Chemoresistance
Multiple miRNAs and lncRNAs act as mediators of the autophagic process in HCC cells by regulating the PI3K/Akt signaling pathway. They also control the expression of key autophagy-related proteins, including Sirt1, LC3, and ATGs. Several miRNAs and lncRNAs participate in FAS-mediated extrinsic apoptosis in HCC cells by targeting FASLG, caspase-8, and caspase-3. miRNAs and lncRNAs are also involved in mitochondria-mediated intrinsic apoptosis by modulating the NF-κB and p53 signaling pathways. In addition, miR-125b and miR-122 can suppress glucose metabolism and thus regulate HCC cell growth and apoptosis. RTK, receptor tyrosine kinase; RASAL1, RAS protein activator like-1; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin; ULK1, unc-51-like kinase 1; ATG, autophagy-related gene; ATG16L1, ATG16-like 1; Sirt1, silent information regulator 1; LC3, microtubule-associated protein 1 light chain 3; TP53INP1, tumor protein 53-induced nuclear protein 1; NF-κB, nuclear factor-B; IKBKB, inhibitor of NF-κB kinase β; FASLG, FAS ligand; FADD, FAS-associating protein with death domain; Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein; Bid, Bcl-2-interacting domain death agonist; tBid, truncated Bid; GLUT, glucose transporter; HK II, hexokinase II; G6P, glucose-6-phosphate; PEP, phosphoenolpyruvate; PKM2, pyruvate kinase M2; TCA, tricarboxylic acid.
miRNAs Regulate the Cellular Autophagic Pathway
Protective autophagy serves as a critical mechanism contributing to cancer chemoresistance. Targeting the autophagic pathway may be a useful therapeutic strategy to improve clinical outcomes in cancer patients. Previously, Zhang et al.53 revealed that miR-142-3p inhibited sorafenib-induced autophagy and improved the responsiveness of HCC cells to sorafenib by targeting autophagy-related gene 5 (ATG5) and ATG16-like 1 (ATG16L1). In addition, miR-142-3p promoted apoptosis and repressed the growth of sorafenib-treated HCC cells. Restoration of miR-142-3p expression boosted the in vivo sensitivity of HCC cells to sorafenib. Upregulation of miR-142-3p may be a promising therapeutic measure for overcoming sorafenib resistance in HCC cells. The expression of miR-26 was reduced in HCC cells after DOX treatment.54 miR-26 suppressed DOX-induced autophagy by targeting the autophagy initiator unc-51-like kinase 1 (ULK1). Accordingly, miR-26 sensitized HCC cells to DOX treatment and induced apoptosis through repression of autophagy. miR-26 sensitized hepatomas to DOX treatment in a tumor xenograft mouse model. The miRNA-26/ULK1/autophagy axis might be a potential target for developing a sensitizing strategy to treat HCC. Similarly, miR-101 potentiated cisplatin-induced apoptosis in HCC through inhibition of autophagy.55 Specifically, miR-101 blocked the autophagic pathway by targeting RAB GTPase 5A (RAB5A), Stathmin 1 (STMN1), and ATG4D. The miR-101/autophagy axis played an important role in cisplatin resistance in HCC and was proposed as a promising therapeutic strategy for HCC. miRNAs may simultaneously regulate apoptotic and autophagic pathways. It is essential to comprehensively identify miRNAs associated with these death pathways in HCC.
miRNAs Modulate the Stemness Feature and EMT Program in HCC
The acquisition of chemoresistance also involves a minority of tumor cells with stem cell-like features that show intrinsic resistance to anticancer agents. miR-122 negatively regulated the stemness features of HCC cells by targeting oncogenic serpinB3.56 Moreover, miR-122 increased the chemosensitivity of HCC cells to sorafenib treatment. The exploitation of miR-122 mimics might contribute to the improvement of HCC treatment. The expression of miR-589-5p was increased in HCC tissues compared to the matched adjacent normal tissues.57 Mechanistically, miR-589-5p mediated the resistance of HCC cells to DOX by activating the STAT3 signaling cascade. In addition, miR-589-5p maintained the cancer stem cell (CSC)-like characteristics of HCC cells by upregulating the pluripotency-associated markers Nanog, BMI-1, Oct4, and Sox2. Therefore, miR-589-5p antagonists might be used in combination with traditional chemotherapeutic strategies for better HCC treatment. Cells undergoing EMT exhibit properties similar to CSCs, including antiapoptotic capabilities and enhanced drug efflux.58 EMT plays a pivotal role in cancer metastasis and chemoresistance. miRNAs have been proven to regulate the EMT process in HCC (Figure 3). miR-383 sensitized HCC cells to DOX in vitro and in vivo by reducing the expression of eukaryotic translation initiation factor 5A2 (EIF5A2).59 Previously, EIF5A2 was demonstrated to favor the EMT program in HCC.60 It was likely that miR-383 governed HCC chemoresistance by impeding EIF5A2-mediated EMT. miR-145 was markedly decreased in DOX-resistant HCC cells compared to the chemosensitive parental cells.61 miR-145 inhibited the EMT program in HCC cells by directly targeting small mothers against decapentaplegic homolog 3 (SMAD3). As a result, miR-145 enhanced the chemosensitivity of HCC cells to DOX. Upregulation of miR-145 might be an effective therapeutic strategy for HCC treatment. Likewise, miR-144 inhibited the EMT process in HCC and increased cell sensitivity to 5-FU by targeting SMAD4.62 miR-106a increased sensitivity of HCC cells to gemcitabine treatment by targeting Twist1 to restrict the EMT process.63
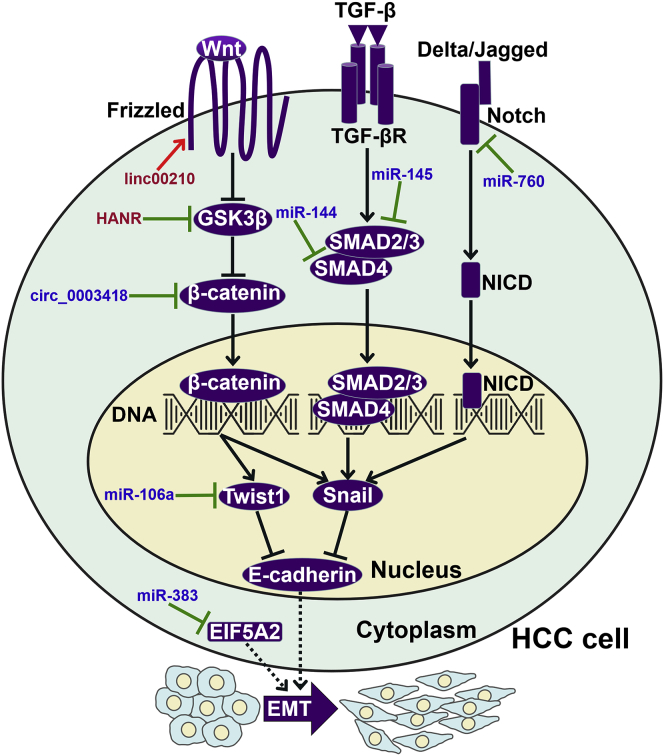
Figure 3.
ncRNAs Modulate the EMT Program in HCC Cells
ncRNAs can interfere with the Wnt/β-catenin, TGF-β/SMAD, and Notch signaling cascades to regulate the expression of EMT-inducing transcription factors (Twist1 and Snail) in HCC cells. Specifically, miR-106a directly targets Twist1 to restrict the EMT process in HCC cells. miR-383 can restrain the EIF5A2-mediated EMT program in HCC cells. GSK3β, glycogen synthase kinase 3β; EIF5A2, eukaryotic translation initiation factor 5A2; EMT, epithelial-mesenchymal transition; TGF-β, transforming growth factor-β; TGF-βR, TGF-β receptor; SMAD, small mothers against decapentaplegic homolog; NICD, the intracellular domain of Notch.
miRNAs can regulate the expression of multiple targets; thus, they are implicated in a variety of biological processes, such as drug efflux and metabolism, apoptosis, autophagy, EMT, glucose metabolism, carcinogenesis, and malignant transformation. Owing to their broad impact on cancer cell behaviors, miRNAs exert profound effects on drug resistance in HCC. It is proposed that miRNA antagonists/mimics, in combination with chemotherapy, will be a valuable strategy for HCC management. However, further investigation is required to elucidate the exact functions and underlying mechanisms of miRNAs in HCC chemoresistance. To date, a large number of miRNAs have been reported to be involved in HCC chemoresistance. These miRNAs may act synergistically or antagonistically. Accordingly, the complex interplay among chemoresistance-related miRNAs deserves further exploration. In addition, a particular miRNA may exert opposite roles in HCC chemoresistance by targeting different mRNAs. Therefore, it is necessary to determine the genuine function of specific miRNAs in the drug resistance of HCC. Notably, some miRNAs, such as miR-325-3p, miR-590-5p, miR-375, miR-16, miR-539, miR-142-3p, miR-26, and miR-383, have been shown to be involved in HCC cell resistance to chemotherapeutic agents, both in vitro and in vivo. However, additional work is needed to validate their regulatory roles in HCC drug resistance. Furthermore, it is also important to elucidate the effect of these miRNAs on drug resistance in HCC patients. More importantly, several miRNAs, including miR-375, miR-125b, and miR-122, have been verified to regulate HCC drug resistance in different studies. These miRNAs may be key therapeutic targets for overcoming chemoresistance in HCC. Accordingly, their utility in HCC treatment should be assessed in further studies.
Involvement of siRNAs in Regulating HCC Chemoresistance
Apart from miRNAs, siRNAs also have the ability to modulate HCC drug resistance. α7 nicotinic acetylcholine receptor (α7nAChR) could confer drug resistance to various cancer cells.64 α7nAChR-specific siRNAs dramatically sensitized HCC cells to sorafenib treatment. Thus, α7nAChR-specific siRNAs might represent a promising therapeutic strategy for HCC. Downregulation of MRP1–4 by siRNAs restored the sensitivity of HCC cells to DOX cytotoxicity.65 Thus, MRP1–4 siRNAs might serve as a novel therapeutic option for HCC. In contrast, siRNAs targeting certain factors could confer HCC resistance to chemotherapeutic agents. For example, depletion of dual-specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2) by siRNAs was reported to favor HCC cell proliferation and enhance resistance to oxaliplatin.66 These studies demonstrated that specific siRNAs had considerable effects on HCC drug resistance. Collectively, siRNAs have tremendous potential as a novel class of therapeutic agents that can silence the genes of interest.
Underlying Mechanisms of HCC Chemoresistance Regulated by lncRNAs
lncRNAs are a class of RNA transcripts longer than 200 nt that can affect gene expression at the transcriptional, post-transcriptional, or epigenetic levels.67 Therefore, lncRNAs serve important functions in diverse physiological and pathological states. More importantly, aberrant expression of lncRNAs is closely linked to HCC progression and chemoresistance.16 lncRNAs play dual roles in HCC chemoresistance mediation by altering the intracellular accumulation of drugs, regulating cell death signaling pathways, controlling the EMT process, and monitoring the function of liver CSCs (Table 1).
lncRNAs Control ABC Transporter-Mediated Drug Efflux
MDR is primarily caused by upregulation of ABC transporters in cancer cell membranes. The lncRNA KCNQ1 overlapping transcript 1 (KCNQ1OT1) elevated the expression of drug-resistant genes (MRP5, MDR1, and LRP1) and enhanced oxaliplatin resistance in HCC cells.68 Moreover, KCNQ1OT1 increased ABCC1 expression by directly targeting miR-7-5p. Likewise, the lncRNA NR2F1 antisense RNA 1 (NR2F1-AS1) enhanced oxaliplatin resistance in HCC cells by sponging miR-363 to upregulate ABCC1 expression.69 As a result, NR2F1-AS1 promoted the growth, migration, and invasion of oxaliplatin-resistant HCC cells. Conversely, silencing NR2F1-AS1 slowed the growth of oxaliplatin-resistant HCC cells in vivo. The lncRNA homeobox (HOX) transcript antisense RNA (HOTAIR) reduced chemosensitivity to cisplatin in HCC cells by enhancing STAT3 activity and ABCB1 expression.70 Transforming growth factor-β1 (TGF-β1) is associated with cancer drug resistance.71 A recent study indicated that TGF-β1 elevated HOTAIR expression in HCC cells and induced resistance of HCC cells to imatinib.72 HOTAIR inhibited the expression of miR-145 and thus upregulated its downstream targets, P-gp and breast cancer resistance protein (BCRP). These studies revealed a novel mechanism contributing to HOTAIR-mediated MDR in HCC and provided new opportunities for improved therapeutic strategies against HCC.
Long intergenic noncoding (linc)-very-low-density-lipoprotein receptor (VLDLR) mediated acquired chemoresistance to sorafenib and DOX in HCC cells by upregulating ABCG2 and ABCC1.73 The lncRNA H19 increased MDR1/P-gp expression, reduced the level of intracellular DOX, and enhanced DOX resistance in HCC cells.74 In contrast, another study indicated that H19 inhibited HCC chemoresistance to DOX and sorafenib by affecting HCC cell proliferation and death.75 In vivo tumorigenesis assays indicated that silencing H19 enhanced HCC tumor growth. Presenilin 1 (PSEN1) activated the DNA damage response pathway and was negatively associated with drug resistance.76 H19 sensitized HCC cells to chemotherapeutic agents (docetaxel, paclitaxel, vinorelbine, and 5-FU) by regulating the miR-193a-3p/PSEN1 axis.77 The above results suggested that H19 played dual roles in orchestrating HCC responsiveness to anticancer drugs. It seems that diverse pathways participate in H19-mediated regulation of HCC drug resistance. However, the crosstalk between H19-regulated signaling cascades during HCC chemoresistance needs to be further characterized. Nrf2 was confirmed to increase cancer resistance to chemotherapy by manipulating drug efflux.78 Keap1 regulation-associated lncRNA (KRAL) functioned as a miR-141 sponge to elevate Keap1 expression, restraining the Nrf2-dependent antioxidant pathway and hence, reversing 5-FU resistance in HCC cells.79 Nrf2 regulation-associated lncRNA (NRAL) was highly expressed in HCC cells.80 NRAL increased Nrf2 expression by acting as a competing endogenous RNA (ceRNA) against miR-340-5p. Thus, the miR-340-5p/Nrf2 pathway mediated NRAL regulation of cisplatin resistance in HCC cells. Collectively, lncRNAs control ABC transporter-mediated drug efflux mainly through the regulation of miRNAs. The biological function of the lncRNA/miRNA axes in the drug efflux pathway awaits further investigation.
lncRNAs Regulate Cellular Death Pathways
lncRNAs modulate drug resistance in HCC through interference with cellular apoptosis or proliferation pathways. The lncRNA protein disulfide isomerase family A member 3 pseudogene 1 (PDIA3P1) protected HCC cells from DOX-induced apoptosis and promoted tumor growth.81 In terms of the mechanism, PDIA3P1 enhanced the expression of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) by acting as a sponge of miR-125a/b and miR-124. The upregulation of TRAF6 caused the activation of the NF-κB signaling pathway. DOX increased PDIA3P1 expression by inhibiting its degradation. PDIA3P1 knockdown sensitized tumor xenografts to DOX treatment. Collectively, the PDIA3P1-miR-125/124 regulatory axis played a vital role in supporting the drug resistance of HCC cells. The lncRNA small nucleolar RNA host gene 1 (SNHG1) decreased the chemosensitivity of HCC cells to sorafenib by activating the Akt pathway.82 Moreover, antiapoptotic miR-21 induced the nuclear expression of SNHG1. SNHG1 might be a valuable target for overcoming HCC chemoresistance. The lncRNA nuclear-enriched abundant transcript 1 (NEAT1) increased sorafenib resistance in HCC cells by inhibiting drug-induced apoptosis.83 Further study indicated that NEAT1 negatively regulated miR-335, thereby relieving its inhibition of the c-Met-Akt pathway. In vivo tumorigenesis assays indicated that silencing NEAT1 could inhibit HCC tumor growth. Long noncoding activated in renal cell carcinoma with sunitinib resistance (lncARSR) reduced the sensitivity of HCC cells to DOX through regulation of the PTEN/phosphatidylinositol 3-kinase (PI3K)/Akt pathway.84 Overexpression of lncARSR conferred HCC cell resistance to DOX treatment in a mouse subcutaneous xenograft model. Thus, lncARSR might be a promising therapeutic target to overcome HCC chemoresistance. linc-regulator of reprogramming (ROR) was found to be highly expressed in arsenic trioxide-treated HCC cells.85 linc-ROR alleviated arsenic trioxide-induced cell apoptosis by lowering p53 expression. miR-24 and miR-221 were verified to downregulate caspase-8 and capsase-3, respectively.86 The tumor-suppressive lncRNA CASC2 sensitized HCC cells to TNF-related apoptosis-inducing ligand (TRAIL) and enhanced TRAIL-induced apoptosis in HCC cells by directly targeting miR-24 and miR-221. miR-93 suppressed HCC cell apoptosis by restricting the expression of the tumor suppressor PTEN and cyclin-dependent kinase inhibitor 1A (CDKN1A).87 The lncRNA SNHG16 functioned as a miR-93 sponge to enhance the sensitivity of HCC cells to 5-FU, hence reducing HCC cell proliferation.88 On the other hand, SNHG16 induced sorafenib resistance in HCC cells.89 These results suggested that SNHG16 played a crucial role in HCC chemoresistance. The exact impact of SNHG16 on HCC drug resistance should be further validated. A recent report indicated that the lncRNA growth arrest-specific 5 (GAS5) was downregulated in HCC tissues and cell lines.90 It was able to sponge miR-21 to increase PTEN expression. As a consequence, overexpression of GAS5 restored the sensitivity of DOX-resistant HCC cells to DOX both in vitro and in vivo. The lncRNA TUC338 increased the expression of tumor growth genes and contributed to sorafenib resistance in HCC cells by inactivating the tumor suppressor RAS protein activator like-1 (RASAL1).91 Knockdown of TUC338 increased HCC cell sensitivity to sorafenib in vivo. The lncRNA SNHG6-003 supported cell proliferation and enhanced 5-FU resistance in HCC cells.92 Importantly, SNHG6-003 increased the expression of TGF-β-activated kinase 1 (TAK1) by sponging miR-26a/b. Modulation of the SNHG6-003/miR-26a/b axis might be a therapeutic strategy against HCC.
Chemotherapy-mediated activation of autophagy functions to protect cancer cells from drug-induced apoptosis. Anticancer drugs (oxaliplatin, 5-FU, and pirarubicin) significantly upregulated the expression of the lncRNA highly upregulated in liver cancer (HULC).93 HULC increased the expression of ubiquitin-specific peptidase 22 (USP22) and stabilized silent information regulator 1 (Sirt1), thus inducing protective autophagy in HCC cells. Accordingly, HULC enhanced the resistance of HCC cells to chemotherapeutic agents. The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) inhibited 5-FU-induced apoptosis and promoted autophagy in HCC cells.94 MALAT1 was shown to reduce the expression of miR-216b. The MALAT1/miR-216b axis promoted microtubule-associated protein 1 light chain 3 (LC3)-II upregulation and p62 downregulation, thereby inducing autophagy in HCC. These results uncovered the role of the MALAT1/miR-216 axis in modifying drug resistance in HCC cells.
lncRNAs Orchestrate Malignant Characteristics of HCC Cells
Tumor-initiating cells (TICs) play a critical role in cancer drug resistance. linc-ROR inhibited sorafenib-induced apoptosis and contributed to sorafenib resistance in HCC cells by affecting the growth of TICs.95 In addition, some lncRNAs have also been found to regulate liver TIC expansion. The lncRNA HOX A10 (lncHOXA10) activated the transcription of HOXA10 and drove the self-renewal of liver TICs.96 The lncRNA G protein-coupled receptor 107 (lncGPR107) facilitated the self-renewal of liver TICs by promoting the transcriptional activation of GPR107 in HCC.97 The lncRNA frizzled 6 (lncFZD6) promoted the self-renewal of liver TICs by activating the transcription of BRM/SWI2-related gene 1 (BRG1)-dependent FZD6.98 The lncRNA Zic family member 2 (lncZic2) was found to be required for the self-renewal of liver TICs.99 Specifically, lncZic2 activated the expression of myristoylated alanine-rich protein kinase C substrate (MARCKS) and MARCKS-like 1 (MARCKSL1) by combining with the transcription factor BRG1. The lncZic2/BRG1/MARCKS/MARCKSL1 pathway can be targeted to eliminate liver TICs. linc00210 promoted the self-renewal and tumor-initiating activity of liver TICs by triggering the Wnt/β-catenin signaling pathway.100 Mechanistically, linc00210 bound β-catenin-interacting protein 1 (CTNNBIP1) and alleviated its suppressive effect on Wnt/β-catenin activation. It could be envisioned that these lncRNAs may contribute to drug resistance in HCC. Their association with HCC chemoresistance should be investigated in the future.
Meanwhile, lncRNAs are able to govern the stemness and malignant features of HCC cells, hence exerting regulatory effects on drug resistance in HCC. The histone methyltransferase enhancer of zeste homolog 2 (EZH2) strengthened the stemness features of HCC by lowering the expression of the stemness regulator atonal homolog 8 (ATOH8).101 lnc-PDZD7 sponged miR-101 to elevate EZH2 expression, thereby suppressing the sensitivity of HCC cells to 5-FU and sorafenib in vitro and in vivo. The high expression of lnc-PDZD7 correlated with tumor stage, size, differentiation, and vascular invasion of HCC patients. lnc-PDZD7 might represent a potential drug target to benefit HCC treatment. Liver CSCs are characterized by the stemness-related marker CD133 and are responsible for cancer drug resistance.102 lncARSR promoted the expansion of liver CSCs by activating the STAT3 pathway.103 Consistently, lncARSR reduced the responsiveness of HCC cells to cisplatin treatment. H19 inhibited the chemosensitivity of CD133+ CSCs by activating the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway in HCC.104 It is well established that glycogen synthase kinase 3β (GSK3β) plays a vital role in cancer growth and metastasis by manipulating the Wnt/β-catenin signaling cascade.105 HCC-associated long noncoding RNA (HANR) mediated DOX resistance in HCC cells partially by binding to GSK3β-interacting protein (GSKIP) to inhibit GSK3β activation.106 Knockdown of HANR significantly inhibited HCC xenograft/orthotopic tumor growth and restored the sensitivity of in vivo tumors to DOX treatment. HOXA13 was capable of increasing HCC cell migration and aggressiveness.107 More importantly, HOXA13 also reduced the response to sorafenib in HCC cells. The lncRNA HOXA transcript at the distal tip (HOTTIP) could regulate HOXA13 expression through an epigenetic mechanism. HOTTIP might function in enhancing resistance to sorafenib in HCC.
Altogether, a list of lncRNAs has been validated to be associated with chemotherapy resistance in HCC. The mechanisms underlying the effects of lncRNAs on HCC resistance to chemotherapy involve their regulation of MDR gene expression, cellular apoptotic and autophagic pathways, liver TIC self-renewal, CSC expansion, and cancer cell metastasis. Remarkably, lncRNAs can serve as miRNA sponges. Additionally, lncRNAs can affect gene expression via epigenetic patterns. Therefore, it can be speculated that chemoresistance-related lncRNAs influence various target genes and cellular signaling pathways through different mechanisms. The mechanisms behind lncRNA-mediated HCC chemoresistance should be extensively explored in future studies. Moreover, it is essential to delve into the lncRNA/miRNA axes involved in HCC drug resistance.
Remarkably, both in vitro and in vivo experiments have proven the contribution of several lncRNAs, such as NR2F1-AS1, H19, PDIA3P1, NEAT1, lncARSR, GAS5, TUC338, lnc-PDZD7, and HANR, to HCC drug resistance. These lncRNAs may have great potential to be utilized as therapeutic targets for HCC management. Further studies are required to investigate the effectiveness and feasibility of lncRNA-based therapies in the clinical setting. HOTAIR, H19, lncARSR, and linc-ROR are well-characterized lncRNAs that are involved in HCC chemoresistance. Therefore, the regulation of their expression in tumors may represent an effective therapeutic treatment for HCC patients.
Emerging Roles of circRNAs in HCC Chemoresistance
Although the role of circRNAs in HCC development has recently become a research hotspot, the study of the contribution of circRNAs to HCC chemoresistance is still at the initial stage. circRNA_101505 was downregulated in cisplatin-resistant HCC cells compared to cisplatin-sensitive cells.108 circRNA_101505 improved the chemotherapeutic response to cisplatin in HCC cells by functioning as a miR-103 sponge. This resulted in the upregulation of oxidored-nitro domain-containing protein 1 (NOR1), a tumor suppressor. Consistently, circRNA_101505 also inhibited HCC cell proliferation. Likewise, circ_0003418 was identified to be expressed at low levels in HCC tissues and cell lines.109 Its expression was associated with tumor size and tumor-node-metastasis (TNM) stage in HCC patients. circ_0003418 inhibited HCC cell proliferation, migration, and invasion. It also increased the sensitivity of HCC cells to cisplatin by blocking the Wnt/β-catenin pathway. Knockdown of circ_0003418 facilitated HCC growth and cisplatin resistance in a mouse xenograft model. circRNAs have been shown to be differentially expressed in HCC and exert significant effects on HCC progression.110 It has been well documented that circRNAs usually act as miRNA sponges.13 There is no doubt that circRNAs possess multifaceted roles in HCC drug resistance, given the broad involvement of miRNAs in cancer chemoresistance. circRNAs can also combine with other molecules and then repress their functions. For instance, circZKSCAN1 sequestered fragile X mental retardation protein (FMRP) and prevented its interaction with the cell cycle and apoptosis regulator 1 (CCAR1), thus blocking the Wnt/β-catenin signaling cascade.111 Particular circRNAs may be involved in HCC drug resistance by regulating the key steps during HCC pathogenesis and metastasis. Further studies are necessary to fully disclose the functional roles of circRNAs in HCC chemoresistance. Additionally, the circRNA/miRNA regulatory networks associated with chemotherapeutic responsiveness in HCC should be delineated.
Potential ncRNA Targets in HCC
Poor therapy responses to conventional chemotherapeutics and the emergence of chemoresistance are still the major challenges in HCC therapy.112 Increasing evidence has confirmed the involvement of ncRNAs in HCC drug resistance. An ncRNA may function as an oncogene or a tumor suppressor, depending on its targets during cancer pathogenesis.113 The rapid progress in ncRNA research points to the great potential of ncRNAs as novel therapeutic targets in cancer. Therapeutic strategies that make use of ncRNAs or directly target ncRNAs may be helpful to improve HCC intervention. One of the potential ways to exploit ncRNAs in HCC therapy would be the direct delivery of tumor-suppressive ncRNAs to target cells. It has been proposed that several tumor-suppressive ncRNAs are attractive therapeutic targets for HCC. In preclinical studies, the therapeutic efficacy of ncRNAs can be evaluated by detecting cancer cell viability or by measuring tumor volume and survival rate in tumor-bearing mice. For instance, miR-26 family members were downregulated in HCC and were correlated with poor prognosis in HCC patients.36 miR-26b enhanced DOX-induced apoptosis in HCC cells by inhibiting NF-κB signaling. Another report indicated that miR-26a inhibited HCC growth by inducing cell-cycle arrest.114 Systematic administration of miR-26a dramatically inhibited tumor progression without toxicity in a mouse model of HCC. miR-26a/b-sensitized HCC cells to DOX treatment and promoted cell apoptosis through suppression of autophagy in vitro and in vivo.54 Upregulation of tumor-suppressive miR-26a/b may represent an effective strategy to suppress HCC progression. miR-122, a hepatocyte-specific miRNA, constitutes 70% of the total adult liver miRNA contents.115 miR-122 is generally downregulated in HCC and correlates with poor prognosis in HCC patients. This miRNA suppressed HCC cell proliferation, invasion, and drug resistance by targeting various genes involved in the Wnt/β-catenin signaling pathway.116 Restoration of tumor-suppressive miR-122 expression is a potential therapeutic option in HCC. Animal models that target miR-122 have been developed. In an orthotopic miR-122-deficient HCC mouse model, intratumoral administration of adenovirus vectors expressing miR-122-regulated thymidine kinase (TK) achieved effective antitumor effects and enhanced the safety of intratumoral delivery of adenovirus-mediated TK/ganciclovir gene therapy.117 A novel MS2 bacteriophage virus-like particle (VLP)-based miR-122 delivery system, crosslinked with the HIV transactivator of transcription (TAT) peptide, penetrated the cytomembrane and significantly inhibited HCC cell proliferation in tumor-bearing mice.118 In addition, the delivery of miR-122 by multifunctional graphene-P-gp loaded with miR-122-InP@ZnS quantum dots (QDs) nanocomposites (GPMQNs) selectively targeted HCC xenograft tumors and alleviated DOX resistance by inducing apoptosis in HCC.119 These studies regarding experimental miR-122-based therapies are encouraging, but their therapeutic efficacy warrants further evaluation in clinical trials. Similarly, miR-101 has been affirmed to be a critical tumor-suppressive miRNA in HCC. miR-101 repressed the proliferation and malignant properties of HCC cells, as well as increased HCC sensitivity to chemotherapeutic agents.120 The intratumoral delivery of miR-101, in combination with DOX by liposome nanoparticles, resulted in enhanced antitumor effects in a HCC xenograft model.121 Taken together, these findings might provide evidence for further development of ncRNA/drug-based combination therapies in HCC.
On the other hand, the silencing of oncogenic ncRNAs could be an alternative strategy to restrain HCC progression. The lncRNA HOTAIR was highly expressed and acted as an oncogene in HCC.122 Its overexpression was strongly correlated with lymph node metastasis, larger tumor size, poor prognosis, and tumor recurrence in HCC patients. HOTAIR regulated the invasive and aggressive features of HCC cells by various mechanisms, including induction of the EMT process, interaction between tumor-suppressive miRNAs, and promotion of autophagy.123 It also played an important role in the intrinsic chemoresistance of HCC cells.70 HOTAIR may be a promising therapeutic target for HCC and could be silenced in several ways.123 First, potential agents that mask the binding sites may prevent the interaction between HOTAIR and its molecular targets, thus blocking the pro-carcinogenic function of HOTAIR. Second, miRNA-141-mediated degradation of HOTAIR may be an effective approach to silence HOTAIR in HCC.124 Finally, key molecules that mediate the oncogenic ability of HOTAIR probably serve as novel targets for HCC therapy. High expression of miR-221 correlated with poorly differentiated HCC and poor outcome in HCC patients.125 miR-221 facilitated HCC growth and chemoresistance by acceleration of cell-cycle progression or by suppression of cell apoptosis.86,126,127 Knockdown of miR-221 suppressed the proliferation, invasion, and migration of HCC cells by negatively regulating the Janus-activated kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signaling pathway.128 Downregulation of highly expressed miR-221 using a 2′-O-methyl phosphorothioate-modified anti-miR-221 oligonucleotide prevented tumor growth in an orthotopic HCC mouse model.129 Thus, oncogenic miR-221 might be used as an ideal candidate for therapeutic approaches aimed at miRNA silencing. Upregulation of the lncRNA TUC338 was significantly associated with disease progression in HCC patients.130 It was found to promote HCC cell growth by regulating cell-cycle progression.131 Therefore, targeting oncogenic TUC338 may be an effective strategy to counteract HCC growth. Hepatic uptake occurs rapidly following systemic administration of oligonucleotides. Thus, ncRNA-based therapeutic approaches are particularly suitable for the treatment of hepatic tumors. The activation of tumor-suppressive ncRNAs or inactivation of oncogenic ncRNAs may be critical mechanisms that restore drug sensitivity in HCC. Despite the considerable promise of ncRNAs as therapeutic targets, ncRNA-based therapies have not yet reached clinical practice. The currently known repertoire of ncRNAs may only represent a small fraction of functionally relevant ncRNAs in HCC. A better understanding of the molecular mechanism of ncRNA-mediated HCC carcinogenesis will make a significant contribution to the treatment of HCC. Before ncRNA-based therapeutics can become an effective approach of cancer care, there are still many challenges that remain to be addressed, including the lack of specific targeting of the drug, poor cellular uptake, low bioavailability, off-target effects, and long-term safety in humans. Therefore, more efforts are warranted to facilitate the clinical translation of ncRNA-based therapeutics in cancer intervention.
Conclusions and Future Perspectives
HCC is one of the most common and lethal cancers globally, due to its high rates of metastasis and relapse. Acquired drug resistance contributes to the unsatisfactory effects of chemotherapy in HCC. Drug resistance has become an immense obstacle in the treatment of HCC patients. Therefore, a better understanding of the mechanisms responsible for HCC chemoresistance will provide opportunities for improved treatments for HCC. ncRNA research has already added a new layer of complexity to the comprehension of HCC pathogenesis. It is well established that the dysregulation of ncRNAs is tightly correlated with HCC progression and chemoresistance. The expression level of ncRNAs in drug-resistant HCC cells could be 2–10 times higher than that in control cells. Nevertheless, it is unclear how the expression pattern of ncRNAs can affect their biological function. The effects of ncRNA abundance on their function should be defined in future studies. It has been reported that some ncRNAs display tissue-specific expression patterns.132,133 Hepatocyte-specific ncRNAs may be involved in HCC drug resistance. Further research is required to identify hepatocyte-specific ncRNAs and determine their biological function. The mechanisms underlying the functional role of ncRNAs in HCC chemoresistance involve sophisticated networks. ncRNAs serve as critical players in HCC chemoresistance by affecting cell death, malignant behaviors, and drug efflux and metabolism. With the development of microarray and high-throughput sequencing techniques, numerous ncRNAs have been identified in HCC. So far, only a small proportion of ncRNAs has been functionally characterized. Most of the studies regarding the connection between specific ncRNAs and HCC drug resistance were only reported once. Substantial efforts should be undertaken to further reveal the actual function of these ncRNAs in HCC chemoresistance. Notably, various in vitro and in vivo preclinical studies have highlighted the great potential of several ncRNAs as therapeutic targets for HCC treatment. Further clinical studies are warranted to evaluate the feasibility, safety, and effectiveness of ncRNA-based therapeutic approaches in HCC treatment. So far, it is still not clear how many ncRNAs are implicated in HCC drug resistance. Undoubtedly, comprehensive elucidation of ncRNA function in HCC will advance our knowledge of the exact mechanisms associated with HCC chemoresistance. Both lncRNAs and circRNAs can act as miRNA sponges. The lncRNA/miRNA and circRNA/miRNA regulatory axes in HCC drug resistance are worthy of further investigation. Some lncRNAs play a role in HCC chemoresistance through interaction with miRNAs. However, the detailed mechanisms of how the interactions affect HCC chemoresistance have not yet been fully revealed. Further integrative analyses of lncRNAs and miRNAs with potential crosstalk will contribute to elucidating the complex mechanisms behind HCC chemoresistance. At present, there are a very limited number of studies exploring the biological function of circRNAs in HCC chemoresistance. It can be hypothesized that certain circRNAs affect HCC chemoresistance by targeting miRNAs. Therefore, further studies are needed to figure out whether circRNAs serve as upstream regulators of miRNA-mediated HCC drug resistance. In addition, lncRNAs and circRNAs may interfere with the function of their associated miRNAs in HCC drug resistance. It is likely that different types of ncRNA form intricate regulatory axes in HCC during chemotherapy. More intensive studies are necessary to disclose the complicated interplay among diverse classes of chemoresistance-associated ncRNAs. ncRNAs can modulate various targets and signaling pathways. The effects of ncRNAs on HCC carcinogenesis and development are broad and diverse. Therefore, it is necessary to adequately understand the molecular processes underlying HCC progression and chemoresistance for the identification of appropriate ncRNAs as therapeutic targets.
Therapies targeting ncRNAs might overcome drug resistance in cancer. At present, several strategies have been adopted to develop ncRNA-based therapies, which include controlling ncRNA expression with mimics or sponges, antisense oligonucleotide-mediated repression of ncRNA function, and small molecular inhibitors of specific ncRNAs (Figure 4). More importantly, ncRNA-directed therapeutics for different cancers have entered into clinical trials.134 Nevertheless, some issues remain to be addressed. The in vivo stability, bioactivity, and targeted delivery efficiency of ncRNA mimics or antagonists are pivotal components in the successful development of ncRNA-based therapies. Chemical modifications can improve the stability of therapeutic ncRNAs and prolong their half-lives in vivo. Previously, N-acetylgalactosamine (GalNAc)-conjugated siRNAs were developed, and these siRNAs could be accurately transferred to HCC cells and specifically downregulate target mRNAs.135 Moreover, the development of novel delivery systems may foster efficient ncRNA transport in vivo and enhance the bioavailability of delivered ncRNAs. For example, incorporation of therapeutic oligonucleotides into nanoparticles was reported to improve their delivery efficiency.136 The combination of delivery systems with cell-specific receptors may facilitate targeted ncRNA delivery. In addition, approaches aimed at evading nuclease degradation or the host immune system should be established to enhance the bioavailability of ncRNAs in vivo. More clinical trials must be further launched to advance the development of ncRNA-based therapies to benefit HCC patients. Notably, accumulating evidence highlights the great prospect of employing ncRNAs coupled with traditional therapeutic measures to maximize the efficacy of HCC intervention. The safety and efficacy of the combined therapy must be validated before its clinical application. It is hoped that these efforts could ultimately open up potential approaches for overcoming HCC drug resistance.
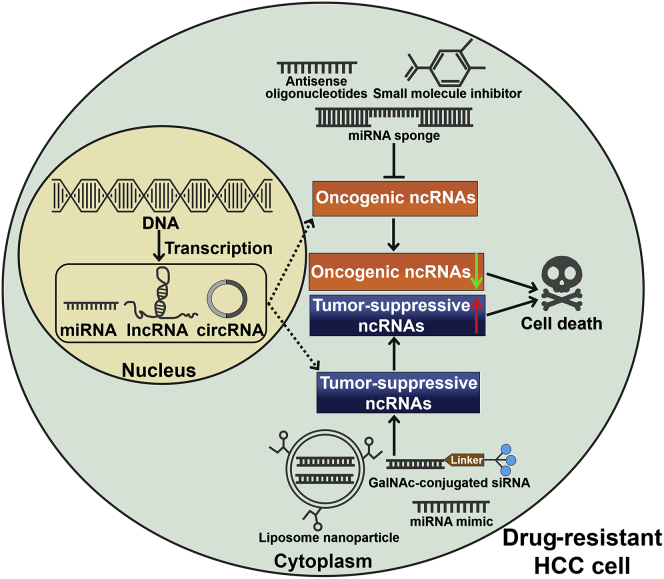
Figure 4.
Schematic Illustration of ncRNA-Based Therapeutic Strategies in Drug-Resistant HCC Cells
Antisense oligonucleotides, small molecule inhibitors, and miRNA sponges can be employed to suppress the expression of oncogenic ncRNAs, thus reversing HCC chemoresistance. Nanoparticle-encapsulated ncRNAs, chemically modified ncRNAs, or exogenous ncRNA mimics can be delivered to inhibit the expression of their target genes that mediate drug resistance in HCC cells. GalNAc, N-acetylgalactosamine; siRNA, small interfering RNA.
Author Contributions
M.W. and K.W. conceived this article. F.Y. and X.C. collected the related papers. M.W. drew the figures and wrote the manuscript. P.L. and K.W. revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81701991) and Applied Basic Research Programs of Qingdao, China (no. 17-1-1-59-jch).
Contributor Information
Man Wang, Email: wangman@qdu.edu.cn.
Kun Wang, Email: wangk696@163.com.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Llovet J.M., Montal R., Sia D., Finn R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelison R., Llaneza D.C., Landen C.N. Emerging therapeutics to overcome chemoresistance in epithelial ovarian cancer: a mini-review. Int. J. Mol. Sci. 2017;18:2171. doi: 10.3390/ijms18102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman M.M., Pastan I.H. The role of multidrug resistance efflux pumps in cancer: revisiting a JNCI publication exploring expression of the MDR1 (P-glycoprotein) gene. J. Natl. Cancer Inst. 2015;107:djv222. doi: 10.1093/jnci/djv222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Antona C., Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 7.Pathania S., Bhatia R., Baldi A., Singh R., Rawal R.K. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed. Pharmacother. 2018;105:53–65. doi: 10.1016/j.biopha.2018.05.117. [DOI] [PubMed] [Google Scholar]
- 8.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 10.Patil V.S., Zhou R., Rana T.M. Gene regulation by non-coding RNAs. Crit. Rev. Biochem. Mol. Biol. 2014;49:16–32. doi: 10.3109/10409238.2013.844092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agirre E., Eyras E. Databases and resources for human small non-coding RNAs. Hum. Genomics. 2011;5:192–199. doi: 10.1186/1479-7364-5-3-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao R.W., Wang Y., Chen L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Y., Du Y., Yang X., Mo Y., Fan C., Xiong F., Ren D., Ye X., Li C., Wang Y. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol. Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.M., Tsang F.H., Ng I.O. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat. Rev. Gastroenterol. Hepatol. 2018;15:137–151. doi: 10.1038/nrgastro.2017.169. [DOI] [PubMed] [Google Scholar]
- 15.Wei L., Wang X., Lv L., Liu J., Xing H., Song Y., Xie M., Lei T., Zhang N., Yang M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol. Cancer. 2019;18:147. doi: 10.1186/s12943-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding B., Lou W., Xu L., Fan W. Non-coding RNA in drug resistance of hepatocellular carcinoma. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180915. BSR20180915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai Y., Feng B., Abudoureyimu M., Zhi Y., Zhou H., Wang T., Chu X., Chen P., Wang R. Non-coding RNAs: emerging regulators of sorafenib resistance in hepatocellular carcinoma. Front. Oncol. 2019;9:1156. doi: 10.3389/fonc.2019.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garofalo M., Croce C.M. microRNAs: Master regulators as potential therapeutics in cancer. Annu. Rev. Pharmacol. Toxicol. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 19.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z., Shi T., Zhang L., Zhu P., Deng M., Huang C., Hu T., Jiang L., Li J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016;370:153–164. doi: 10.1016/j.canlet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Li R., Xu T., Wang H., Wu N., Liu F., Jia X., Mi J., Lv J., Gao H. Dysregulation of the miR-325-3p/DPAGT1 axis supports HBV-positive HCC chemoresistance. Biochem. Biophys. Res. Commun. 2019;519:358–365. doi: 10.1016/j.bbrc.2019.08.116. [DOI] [PubMed] [Google Scholar]
- 22.Chen M., Wu L., Tu J., Zhao Z., Fan X., Mao J., Weng Q., Wu X., Huang L., Xu M., Ji J. miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. EBioMedicine. 2018;35:142–154. doi: 10.1016/j.ebiom.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo B.K., Chen D., Su Z.Z., Gredler R., Yoo J., Shah K., Fisher P.B., Sarkar D. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Y.P., Liao J.Z., Lu Y.Q., Tian D.A., Ye F., Zhao P.X., Xiang G.Y., Tang W.X., He X.X. MiR-375 and doxorubicin co-delivered by liposomes for combination therapy of hepatocellular carcinoma. Mol. Ther. Nucleic Acids. 2017;7:181–189. doi: 10.1016/j.omtn.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao P., Wu S., Cheng Y., You J., Chen Y., Li M., He C., Zhang X., Yang T., Lu Y. MiR-375 delivered by lipid-coated doxorubicin-calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomedicine (Lond.) 2017;13:2507–2516. doi: 10.1016/j.nano.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Xue H., Yu Z., Liu Y., Yuan W., Yang T., You J., He X., Lee R.J., Li L., Xu C. Delivery of miR-375 and doxorubicin hydrochloride by lipid-coated hollow mesoporous silica nanoparticles to overcome multiple drug resistance in hepatocellular carcinoma. Int. J. Nanomedicine. 2017;12:5271–5287. doi: 10.2147/IJN.S135306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J.N., Zeng Q., Wang H.Y., Zhang B., Li S.T., Nan X., Cao N., Fu C.J., Yan X.L., Jia Y.L. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62:801–815. doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 28.Bai X., Chen Y., Hou X., Huang M., Jin J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab. Rev. 2016;48:541–567. doi: 10.1080/03602532.2016.1197239. [DOI] [PubMed] [Google Scholar]
- 29.Shi L., Wu L., Chen Z., Yang J., Chen X., Yu F., Zheng F., Lin X. MiR-141 activates Nrf2-dependent antioxidant pathway via down-regulating the expression of Keap1 conferring the resistance of hepatocellular carcinoma cells to 5-Fluorouracil. Cell. Physiol. Biochem. 2015;35:2333–2348. doi: 10.1159/000374036. [DOI] [PubMed] [Google Scholar]
- 30.Shi L., Chen Z.G., Wu L.L., Zheng J.J., Yang J.R., Chen X.F., Chen Z.Q., Liu C.L., Chi S.Y., Zheng J.Y. miR-340 reverses cisplatin resistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Asian Pac. J. Cancer Prev. 2014;15:10439–10444. doi: 10.7314/apjcp.2014.15.23.10439. [DOI] [PubMed] [Google Scholar]
- 31.Yu D., Green B., Marrone A., Guo Y., Kadlubar S., Lin D., Fuscoe J., Pogribny I., Ning B. Suppression of CYP2C9 by microRNA hsa-miR-128-3p in human liver cells and association with hepatocellular carcinoma. Sci. Rep. 2015;5:8534. doi: 10.1038/srep08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Wang Y.M., Xu S., Wang W.G., Chen Y., Mao J.Y., Tian B.L. MicroRNA-215 is upregulated by treatment with Adriamycin and leads to the chemoresistance of hepatocellular carcinoma cells and tissues. Mol. Med. Rep. 2015;12:5274–5280. doi: 10.3892/mmr.2015.4012. [DOI] [PubMed] [Google Scholar]
- 33.Chen S., Yang C., Sun C., Sun Y., Yang Z., Cheng S., Zhuge B. miR-21-5p suppressed the sensitivity of hepatocellular carcinoma cells to cisplatin by targeting FASLG. DNA Cell Biol. 2019;38:865–873. doi: 10.1089/dna.2018.4529. [DOI] [PubMed] [Google Scholar]
- 34.Hu J., Ruan J., Liu X., Xiao C., Xiong J. MicroRNA-301a-3p suppressed the progression of hepatocellular carcinoma via targeting VGLL4. Pathol. Res. Pract. 2018;214:2039–2045. doi: 10.1016/j.prp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y., Chen G., Wang Y., He R., Du J., Jiao X., Tai Q. Inhibition of microRNA-16 facilitates the paclitaxel resistance by targeting IKBKB via NF-κB signaling pathway in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018;503:1035–1041. doi: 10.1016/j.bbrc.2018.06.113. [DOI] [PubMed] [Google Scholar]
- 36.Zhao N., Wang R., Zhou L., Zhu Y., Gong J., Zhuang S.M. MicroRNA-26b suppresses the NF-κB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol. Cancer. 2014;13:35. doi: 10.1186/1476-4598-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang X.M., Yu X.N., Liu T.T., Zhu H.R., Shi X., Bilegsaikhan E., Guo H.Y., Song G.Q., Weng S.Q., Huang X.X. microRNA-19a-3p promotes tumor metastasis and chemoresistance through the PTEN/Akt pathway in hepatocellular carcinoma. Biomed. Pharmacother. 2018;105:1147–1154. doi: 10.1016/j.biopha.2018.06.097. [DOI] [PubMed] [Google Scholar]
- 38.Wang G., Reed E., Li Q.Q. Apoptosis in prostate cancer: progressive and therapeutic implications (Review) Int. J. Mol. Med. 2004;14:23–34. [PubMed] [Google Scholar]
- 39.Yuan X., Wu H., Xu H., Xiong H., Chu Q., Yu S., Wu G.S., Wu K. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369:20–27. doi: 10.1016/j.canlet.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Tian T., Fu X., Lu J., Ruan Z., Nan K., Yao Y., Yang Y. MicroRNA-760 inhibits doxorubicin resistance in hepatocellular carcinoma through regulating Notch1/Hes1-PTEN/Akt signaling pathway. J. Biochem. Mol. Toxicol. 2018;32:e22167. doi: 10.1002/jbt.22167. [DOI] [PubMed] [Google Scholar]
- 41.Shao P., Qu W.K., Wang C.Y., Tian Y., Ye M.L., Sun D.G., Sui J.D., Wang L.M., Fan R., Gao Z.M. MicroRNA-205-5p regulates the chemotherapeutic resistance of hepatocellular carcinoma cells by targeting PTEN/JNK/ANXA3 pathway. Am. J. Transl. Res. 2017;9:4300–4307. [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y., Huang J., Ma L., Shan J., Shen J., Yang Z., Liu L., Luo Y., Yao C., Qian C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371:171–181. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 43.Christopoulos P.F., Msaouel P., Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang D.J., Huang J.Z., Yang J., Li Y.H., Luo Y.C., He H.Y., Huang H.J. Bioinformatic identification of IGF1 as a hub gene in hepatocellular carcinoma (HCC) and in-vitro analysis of the chemosensitizing effect of miR-379 via suppressing the IGF1/IGF1R signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2016;20:5098–5106. [PubMed] [Google Scholar]
- 45.Tomasini R., Seux M., Nowak J., Bontemps C., Carrier A., Dagorn J.C., Pébusque M.J., Iovanna J.L., Dusetti N.J. TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene. 2005;24:8093–8104. doi: 10.1038/sj.onc.1208951. [DOI] [PubMed] [Google Scholar]
- 46.Qin J., Luo M., Qian H., Chen W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene. 2014;538:342–347. doi: 10.1016/j.gene.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 47.Yang F., Li Q.J., Gong Z.B., Zhou L., You N., Wang S., Li X.L., Li J.J., An J.Z., Wang D.S. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol. Cancer Res. Treat. 2014;13:77–86. doi: 10.7785/tcrt.2012.500364. [DOI] [PubMed] [Google Scholar]
- 48.Li X.Y., Wen J.Y., Jia C.C., Wang T.T., Li X., Dong M., Lin Q.U., Chen Z.H., Ma X.K., Wei L.I. MicroRNA-34a-5p enhances sensitivity to chemotherapy by targeting AXL in hepatocellular carcinoma MHCC-97L cells. Oncol. Lett. 2015;10:2691–2698. doi: 10.3892/ol.2015.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu C., Zhou R., Zhou Q., Chang Y., Jiang M. microRNA-539 suppresses tumor growth and tumorigenesis and overcomes arsenic trioxide resistance in hepatocellular carcinoma. Life Sci. 2016;166:34–40. doi: 10.1016/j.lfs.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Vaughn A.E., Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat. Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang J.X., Gao S., Pan Y.Z., Yu C., Sun C.Y. Overexpression of microRNA-125b sensitizes human hepatocellular carcinoma cells to 5-fluorouracil through inhibition of glycolysis by targeting hexokinase II. Mol. Med. Rep. 2014;10:995–1002. doi: 10.3892/mmr.2014.2271. [DOI] [PubMed] [Google Scholar]
- 52.Pan C., Wang X., Shi K., Zheng Y., Li J., Chen Y., Jin L., Pan Z. MiR-122 reverses the doxorubicin-resistance in hepatocellular carcinoma cells through regulating the tumor metabolism. PLoS ONE. 2016;11:e0152090. doi: 10.1371/journal.pone.0152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang K., Chen J., Zhou H., Chen Y., Zhi Y., Zhang B., Chen L., Chu X., Wang R., Zhang C. PU.1/microRNA-142-3p targets ATG5/ATG16L1 to inactivate autophagy and sensitize hepatocellular carcinoma cells to sorafenib. Cell Death Dis. 2018;9:312. doi: 10.1038/s41419-018-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin F., Wang Y., Li M., Zhu Y., Liang H., Wang C., Wang F., Zhang C.Y., Zen K., Li L. MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis. 2017;8:e2540. doi: 10.1038/cddis.2016.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y., An Y., Wang Y., Zhang C., Zhang H., Huang C., Jiang H., Wang X., Li X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013;29:2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- 56.Turato C., Fornari F., Pollutri D., Fassan M., Quarta S., Villano G., Ruvoletto M., Bolondi L., Gramantieri L., Pontisso P. MiR-122 targets SerpinB3 and is involved in sorafenib resistance in hepatocellular carcinoma. J. Clin. Med. 2019;8:171. doi: 10.3390/jcm8020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long J., Jiang C., Liu B., Dai Q., Hua R., Chen C., Zhang B., Li H. Maintenance of stemness by miR-589-5p in hepatocellular carcinoma cells promotes chemoresistance via STAT3 signaling. Cancer Lett. 2018;423:113–126. doi: 10.1016/j.canlet.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 58.Du B., Shim J.S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:965. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tu C., Chen W., Wang S., Tan W., Guo J., Shao C., Wang W. MicroRNA-383 inhibits doxorubicin resistance in hepatocellular carcinoma by targeting eukaryotic translation initiation factor 5A2. J. Cell. Mol. Med. 2019;23:7190–7199. doi: 10.1111/jcmm.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang D.J., Dong S.S., Ma N.F., Xie D., Chen L., Fu L., Lau S.H., Li Y., Li Y., Guan X.Y. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- 61.Ju B.L., Chen Y.B., Zhang W.Y., Yu C.H., Zhu D.Q., Jin J. miR-145 regulates chemoresistance in hepatocellular carcinoma via epithelial mesenchymal transition. Cell. Mol. Biol. 2015;61:12–16. [PubMed] [Google Scholar]
- 62.Yu M., Lin Y., Zhou Y., Jin H., Hou B., Wu Z., Li Z., Jian Z., Sun J. MiR-144 suppresses cell proliferation, migration, and invasion in hepatocellular carcinoma by targeting SMAD4. OncoTargets Ther. 2016;9:4705–4714. doi: 10.2147/OTT.S88233. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Wang R., Li Y., Hou Y., Yang Q., Chen S., Wang X., Wang Z., Yang Y., Chen C., Wang Z., Wu Q. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget. 2015;6:7000–7010. doi: 10.18632/oncotarget.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hajiasgharzadeh K., Somi M.H., Mansoori B., Khaze Shahgoli V., Derakhshani A., Mokhtarzadeh A., Shanehbandi D., Baradaran B. Small interfering RNA targeting alpha7 nicotinic acetylcholine receptor sensitizes hepatocellular carcinoma cells to sorafenib. Life Sci. 2020;244:117332. doi: 10.1016/j.lfs.2020.117332. [DOI] [PubMed] [Google Scholar]
- 65.Su Z., Liu G., Fang T., Wang Y., Zhang H., Yang S., Wei J., Lv Z., Tan L., Liu J. Silencing MRP1-4 genes by RNA interference enhances sensitivity of human hepatoma cells to chemotherapy. Am. J. Transl. Res. 2016;8:2790–2802. [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X., Xu P., Ni W., Fan H., Xu J., Chen Y., Huang W., Lu S., Liang L., Liu J. Downregulated DYRK2 expression is associated with poor prognosis and Oxaliplatin resistance in hepatocellular carcinoma. Pathol. Res. Pract. 2016;212:162–170. doi: 10.1016/j.prp.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu H., Yang L., Li L., Zeng C. Long non-coding RNA KCNQ1OT1 modulates oxaliplatin resistance in hepatocellular carcinoma through miR-7-5p/ ABCC1 axis. Biochem. Biophys. Res. Commun. 2018;503:2400–2406. doi: 10.1016/j.bbrc.2018.06.168. [DOI] [PubMed] [Google Scholar]
- 69.Huang H., Chen J., Ding C.M., Jin X., Jia Z.M., Peng J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J. Cell. Mol. Med. 2018;22:3238–3245. doi: 10.1111/jcmm.13605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Zhou J.J., Cheng D., He X.Y., Meng Z., Ye H.L., Chen R.F. Knockdown of long non-coding RNA HOTAIR sensitizes hepatocellular carcinoma cell to cisplatin by suppressing the STAT3/ABCB1 signaling pathway. Oncol. Lett. 2017;14:7986–7992. doi: 10.3892/ol.2017.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oshimori N., Oristian D., Fuchs E. TGF-β promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963–976. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong J., Qiu Y., Li Y., Zhang H., Wang W. TGF-β1 elevates P-gp and BCRP in hepatocellular carcinoma through HOTAIR/miR-145 axis. Biopharm. Drug Dispos. 2019;40:70–80. doi: 10.1002/bdd.2172. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsang W.P., Kwok T.T. Riboregulator H19 induction of MDR1-associated drug resistance in human hepatocellular carcinoma cells. Oncogene. 2007;26:4877–4881. doi: 10.1038/sj.onc.1210266. [DOI] [PubMed] [Google Scholar]
- 75.Schultheiss C.S., Laggai S., Czepukojc B., Hussein U.K., List M., Barghash A., Tierling S., Hosseini K., Golob-Schwarzl N., Pokorny J. The long non-coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma. Cell Stress. 2017;1:37–54. doi: 10.15698/cst2017.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng H., Lv L., Li Y., Zhang C., Meng F., Pu Y., Xiao J., Qian L., Zhao W., Liu Q. The miR-193a-3p regulated PSEN1 gene suppresses the multi-chemoresistance of bladder cancer. Biochim. Biophys. Acta. 2015;1852:520–528. doi: 10.1016/j.bbadis.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Ma H., Yuan L., Li W., Xu K., Yang L. The LncRNA H19/miR-193a-3p axis modifies the radio-resistance and chemotherapeutic tolerance of hepatocellular carcinoma cells by targeting PSEN1. J. Cell. Biochem. 2018;119:8325–8335. doi: 10.1002/jcb.26883. [DOI] [PubMed] [Google Scholar]
- 78.Tsuchida K., Tsujita T., Hayashi M., Ojima A., Keleku-Lukwete N., Katsuoka F., Otsuki A., Kikuchi H., Oshima Y., Suzuki M., Yamamoto M. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic. Biol. Med. 2017;103:236–247. doi: 10.1016/j.freeradbiomed.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 79.Wu L., Pan C., Wei X., Shi Y., Zheng J., Lin X., Shi L. lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun. Signal. 2018;16:47. doi: 10.1186/s12964-018-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu L.L., Cai W.P., Lei X., Shi K.Q., Lin X.Y., Shi L. NRAL mediates cisplatin resistance in hepatocellular carcinoma via miR-340-5p/Nrf2 axis. J. Cell Commun. Signal. 2019;13:99–112. doi: 10.1007/s12079-018-0479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie C., Zhang L.Z., Chen Z.L., Zhong W.J., Fang J.H., Zhu Y., Xiao M.H., Guo Z.W., Zhao N., He X., Zhuang S.M. A hMTR4-PDIA3P1-miR-125/124-TRAF6 Regulatory Axis and Its Function in NF kappa B Signaling and Chemoresistance. Hepatology. 2020;71:1660–1677. doi: 10.1002/hep.30931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W., Dong X., He C., Tan G., Li Z., Zhai B., Feng J., Jiang X., Liu C., Jiang H., Sun X. LncRNA SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by miR-21 in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2019;38:183. doi: 10.1186/s13046-019-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S., Xia X. Long noncoding RNA NEAT1 suppresses sorafenib sensitivity of hepatocellular carcinoma cells via regulating miR-335-c-Met. J. Cell. Physiol. 2019 doi: 10.1002/jcp.27567. Published online April 1, 2019. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Ye Y., Feng B., Qi Y. Long noncoding RNA lncARSR promotes doxorubicin resistance in hepatocellular carcinoma via modulating PTEN-PI3K/Akt pathway. J. Cell. Biochem. 2017;118:4498–4507. doi: 10.1002/jcb.26107. [DOI] [PubMed] [Google Scholar]
- 85.Li X., Sun D., Zhao T., Zhang Z. Long non-coding RNA ROR confers arsenic trioxide resistance to HepG2 cells by inhibiting p53 expression. Eur. J. Pharmacol. 2020;872:172982. doi: 10.1016/j.ejphar.2020.172982. [DOI] [PubMed] [Google Scholar]
- 86.Jin X., Cai L., Wang C., Deng X., Yi S., Lei Z., Xiao Q., Xu H., Luo H., Sun J. CASC2/miR-24/miR-221 modulates the TRAIL resistance of hepatocellular carcinoma cell through caspase-8/caspase-3. Cell Death Dis. 2018;9:318. doi: 10.1038/s41419-018-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohta K., Hoshino H., Wang J., Ono S., Iida Y., Hata K., Huang S.K., Colquhoun S., Hoon D.S. MicroRNA-93 activates c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by directly inhibiting PTEN and CDKN1A. Oncotarget. 2015;6:3211–3224. doi: 10.18632/oncotarget.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu F., Zha G., Wu Y., Cai W., Ao J. Overexpressing lncRNA SNHG16 inhibited HCC proliferation and chemoresistance by functionally sponging hsa-miR-93. OncoTargets Ther. 2018;11:8855–8863. doi: 10.2147/OTT.S182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo Z., Zhang J., Fan L., Liu J., Yu H., Li X., Sun G. Long noncoding RNA (lncRNA) small nucleolar RNA host gene 16 (SNHG16) predicts poor prognosis and sorafenib resistance in hepatocellular carcinoma. Med. Sci. Monit. 2019;25:2079–2086. doi: 10.12659/MSM.915541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Ke S., Li M., Lin C., Liu X., Pan Q. Downregulation of LncRNA GAS5 promotes liver cancer proliferation and drug resistance by decreasing PTEN expression. Mol. Genet. Genomics. 2020;295:251–260. doi: 10.1007/s00438-019-01620-5. [DOI] [PubMed] [Google Scholar]
- 91.Jin W., Chen L., Cai X., Zhang Y., Zhang J., Ma D., Cai X., Fu T., Yu Z., Yu F., Chen G. Long non-coding RNA TUC338 is functionally involved in sorafenib-sensitized hepatocarcinoma cells by targeting RASAL1. Oncol. Rep. 2017;37:273–280. doi: 10.3892/or.2016.5248. [DOI] [PubMed] [Google Scholar]
- 92.Cao C., Zhang T., Zhang D., Xie L., Zou X., Lei L., Wu D., Liu L. The long non-coding RNA, SNHG6-003, functions as a competing endogenous RNA to promote the progression of hepatocellular carcinoma. Oncogene. 2017;36:1112–1122. doi: 10.1038/onc.2016.278. [DOI] [PubMed] [Google Scholar]
- 93.Xiong H., Ni Z., He J., Jiang S., Li X., He J., Gong W., Zheng L., Chen S., Li B. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36:3528–3540. doi: 10.1038/onc.2016.521. [DOI] [PubMed] [Google Scholar]
- 94.Yuan P., Cao W., Zang Q., Li G., Guo X., Fan J. The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance of hepatocellular carcinoma cells via modulating autophagy. Biochem. Biophys. Res. Commun. 2016;478:1067–1073. doi: 10.1016/j.bbrc.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi K., Yan I.K., Kogure T., Haga H., Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shao M., Yang Q., Zhu W., Jin H., Wang J., Song J., Kong Y., Lv X. LncHOXA10 drives liver TICs self-renewal and tumorigenesis via HOXA10 transcription activation. Mol. Cancer. 2018;17:173. doi: 10.1186/s12943-018-0921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang G., Jiang H., Lin Y., Xia W., Luo Y., Wu Y., Cai W., Zhou X., Jiang X. LncGPR107 drives the self-renewal of liver tumor initiating cells and liver tumorigenesis through GPR107-dependent manner. J. Exp. Clin. Cancer Res. 2018;37:121. doi: 10.1186/s13046-018-0794-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Chen Z., Gao Y., Yao L., Liu Y., Huang L., Yan Z., Zhao W., Zhu P., Weng H. LncFZD6 initiates Wnt/β-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene. 2018;37:3098–3112. doi: 10.1038/s41388-018-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen Z., Liu Y., Yao L., Guo S., Gao Y., Zhu P. The long noncoding RNA lncZic2 drives the self-renewal of liver tumor-initiating cells via the protein kinase C substrates MARCKS and MARCKSL1. J. Biol. Chem. 2018;293:7982–7992. doi: 10.1074/jbc.RA117.001321. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Fu X., Zhu X., Qin F., Zhang Y., Lin J., Ding Y., Yang Z., Shang Y., Wang L., Zhang Q., Gao Q. Linc00210 drives Wnt/β-catenin signaling activation and liver tumor progression through CTNNBIP1-dependent manner. Mol. Cancer. 2018;17:73. doi: 10.1186/s12943-018-0783-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Zhang Y., Tang B., Song J., Yu S., Li Y., Su H., He S. Lnc-PDZD7 contributes to stemness properties and chemosensitivity in hepatocellular carcinoma through EZH2-mediated ATOH8 transcriptional repression. J. Exp. Clin. Cancer Res. 2019;38:92. doi: 10.1186/s13046-019-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Yamashita T., Ji J., Budhu A., Forgues M., Yang W., Wang H.Y., Jia H., Ye Q., Qin L.X., Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang C., Cai W.C., Dong Z.T., Guo J.W., Zhao Y.J., Sui C.J., Yang J.M. lncARSR promotes liver cancer stem cells expansion via STAT3 pathway. Gene. 2019;687:73–81. doi: 10.1016/j.gene.2018.10.087. [DOI] [PubMed] [Google Scholar]
- 104.Ding K., Liao Y., Gong D., Zhao X., Ji W. Effect of long non-coding RNA H19 on oxidative stress and chemotherapy resistance of CD133+ cancer stem cells via the MAPK/ERK signaling pathway in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018;502:194–201. doi: 10.1016/j.bbrc.2018.05.143. [DOI] [PubMed] [Google Scholar]
- 105.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao J., Lv Y., Jin F., Liu Y., Ma Y., Xiong Y., Liu L., Zhang S., Sun Y., Tipoe G.L. LncRNA HANR promotes tumorigenesis and increase of chemoresistance in hepatocellular carcinoma. Cell. Physiol. Biochem. 2017;43:1926–1938. doi: 10.1159/000484116. [DOI] [PubMed] [Google Scholar]
- 107.Quagliata L., Quintavalle C., Lanzafame M., Matter M.S., Novello C., di Tommaso L., Pressiani T., Rimassa L., Tornillo L., Roncalli M. High expression of HOXA13 correlates with poorly differentiated hepatocellular carcinomas and modulates sorafenib response in in vitro models. Lab. Invest. 2018;98:95–105. doi: 10.1038/labinvest.2017.107. [DOI] [PubMed] [Google Scholar]
- 108.Luo Y., Fu Y., Huang R., Gao M., Liu F., Gui R., Nie X. CircRNA_101505 sensitizes hepatocellular carcinoma cells to cisplatin by sponging miR-103 and promotes oxidored-nitro domain-containing protein 1 expression. Cell Death Discov. 2019;5:121. doi: 10.1038/s41420-019-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen H., Liu S., Li M., Huang P., Li X. circ_0003418 inhibits tumorigenesis and cisplatin chemoresistance through Wnt/β-catenin pathway in hepatocellular carcinoma. OncoTargets Ther. 2019;12:9539–9549. doi: 10.2147/OTT.S229507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qiu L.P., Wu Y.H., Yu X.F., Tang Q., Chen L., Chen K.P. The emerging role of circular RNAs in hepatocellular carcinoma. J. Cancer. 2018;9:1548–1559. doi: 10.7150/jca.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu Y.J., Zheng B., Luo G.J., Ma X.K., Lu X.Y., Lin X.M., Yang S., Zhao Q., Wu T., Li Z.X. Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 2019;9:3526–3540. doi: 10.7150/thno.32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klingenberg M., Matsuda A., Diederichs S., Patel T. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J. Hepatol. 2017;67:603–618. doi: 10.1016/j.jhep.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Feng W., Su Z., Yin Q., Zong W., Shen X., Ju S. ncRNAs associated with drug resistance and the therapy of digestive system neoplasms. J. Cell. Physiol. 2019;234:19143–19157. doi: 10.1002/jcp.28551. [DOI] [PubMed] [Google Scholar]
- 114.Kota J., Chivukula R.R., O’Donnell K.A., Wentzel E.A., Montgomery C.L., Hwang H.-W., Chang T.C., Vivekanandan P., Torbenson M., Clark K.R. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coulouarn C., Factor V.M., Andersen J.B., Durkin M.E., Thorgeirsson S.S. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fu X., Calin G.A. miR-122 and hepatocellular carcinoma: from molecular biology to therapeutics. EBioMedicine. 2018;37:17–18. doi: 10.1016/j.ebiom.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang G., Dong X., Tian W., Lu Y., Hu J., Liu Y., Yuchi J., Wu X. Evaluation of miR-122-regulated suicide gene therapy for hepatocellular carcinoma in an orthotopic mouse model. Chin. J. Cancer Res. 2013;25:646–655. doi: 10.3978/j.issn.1000-9604.2013.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang G., Jia T., Xu X., Chang L., Zhang R., Fu Y., Li Y., Yang X., Zhang K., Lin G. Novel miR-122 delivery system based on MS2 virus like particle surface displaying cell-penetrating peptide TAT for hepatocellular carcinoma. Oncotarget. 2016;7:59402–59416. doi: 10.18632/oncotarget.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zeng X., Yuan Y., Wang T., Wang H., Hu X., Fu Z., Zhang G., Liu B., Lu G. Targeted imaging and induction of apoptosis of drug-resistant hepatoma cells by miR-122-loaded graphene-InP nanocompounds. J. Nanobiotechnology. 2017;15:9. doi: 10.1186/s12951-016-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu L., Beckebaum S., Iacob S., Wu G., Kaiser G.M., Radtke A., Liu C., Kabar I., Schmidt H.H., Zhang X. MicroRNA-101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity. J. Hepatol. 2014;60:590–598. doi: 10.1016/j.jhep.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 121.Xu F., Liao J.Z., Xiang G.Y., Zhao P.X., Ye F., Zhao Q., He X.X. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017;6:651–661. doi: 10.1002/cam4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Z., Zhou L., Wu L.M., Lai M.C., Xie H.Y., Zhang F., Zheng S.S. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann. Surg. Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 123.Wu L., Zhang L., Zheng S. Role of the long non-coding RNA HOTAIR in hepatocellular carcinoma. Oncol. Lett. 2017;14:1233–1239. doi: 10.3892/ol.2017.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chiyomaru T., Fukuhara S., Saini S., Majid S., Deng G., Shahryari V., Chang I., Tanaka Y., Enokida H., Nakagawa M. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J. Biol. Chem. 2014;289:12550–12565. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gramantieri L., Fornari F., Ferracin M., Veronese A., Sabbioni S., Calin G.A., Grazi G.L., Croce C.M., Bolondi L., Negrini M. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin. Cancer Res. 2009;15:5073–5081. doi: 10.1158/1078-0432.CCR-09-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fornari F., Gramantieri L., Ferracin M., Veronese A., Sabbioni S., Calin G.A., Grazi G.L., Giovannini C., Croce C.M., Bolondi L., Negrini M. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 127.Rong M., Chen G., Dang Y. Increased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitro. BMC Cancer. 2013;13:21. doi: 10.1186/1471-2407-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang S., Zhou D., Li Y.X., Ming Z.Y., Li K.Z., Wu G.B., Chen C., Zhao Y.N. In vivo and in vitro effects of microRNA-221 on hepatocellular carcinoma development and progression through the JAK-STAT3 signaling pathway by targeting SOCS3. J. Cell. Physiol. 2019;234:3500–3514. doi: 10.1002/jcp.26863. [DOI] [PubMed] [Google Scholar]
- 129.Park J.K., Kogure T., Nuovo G.J., Jiang J., He L., Kim J.H., Phelps M.A., Papenfuss T.L., Croce C.M., Patel T., Schmittgen T.D. miR-221 silencing blocks hepatocellular carcinoma and promotes survival. Cancer Res. 2011;71:7608–7616. doi: 10.1158/0008-5472.CAN-11-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Braconi C., Valeri N., Kogure T., Gasparini P., Huang N., Nuovo G.J., Terracciano L., Croce C.M., Patel T. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2011;108:786–791. doi: 10.1073/pnas.1011098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen H.J., Walsh M.P., Yan I.K., Takahashi K., Fields A., Patel T. Functional modulation of gene expression by ultraconserved long non-coding RNA TUC338 during growth of human hepatocellular carcinoma. iScience. 2018;2:210–220. doi: 10.1016/j.isci.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ha S.Y., Yu J.I., Choi C., Kang S.Y., Joh J.W., Paik S.W., Kim S., Kim M., Park H.C., Park C.K. Prognostic significance of miR-122 expression after curative resection in patients with hepatocellular carcinoma. Sci. Rep. 2019;9:14738. doi: 10.1038/s41598-019-50594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xia Q., Li Z., Zheng J., Zhang X., Di Y., Ding J., Yu D., Yan L., Shen L., Yan D. Identification of novel biomarkers for hepatocellular carcinoma using transcriptome analysis. J. Cell. Physiol. 2019;234:4851–4863. doi: 10.1002/jcp.27283. [DOI] [PubMed] [Google Scholar]
- 134.Wang W.T., Han C., Sun Y.M., Chen T.Q., Chen Y.Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019;12:55. doi: 10.1186/s13045-019-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 136.Yang T., Zhao P., Rong Z., Li B., Xue H., You J., He C., Li W., He X., Lee R.J. Anti-tumor efficiency of lipid-coated cisplatin nanoparticles co-loaded with microRNA-375. Theranostics. 2016;6:142–154. doi: 10.7150/thno.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]