Abstract
Anterior temporal lobectomy and amygdalohippocampectomy (ATL) is the gold standard surgical treatment for drug resistant mesial temporal lobe epilepsy (mTLE). Nevertheless, seizure recurrence after ATL is not uncommon. Insufficient resection of the mesial temporal structures remains one of the most common reasons for ATL failure. Extending the resection leads to improved seizure outcome in a majority of patients. However, repeat craniotomy can be higher risk for the patient and also can be technically challenging due to scarring and altered anatomy. Laser interstitial thermal therapy (LITT) is a novel minimally invasive alternative to ATL, and it has been shown to be safe and effective. However, it is unclear if LITT has a role in managing post-ATL mTLE patients with recurrent seizures and residual epileptogenic structures. LITT is an attractive option for post-ATL patients with residual mesial structures because the surgery is minimally invasive, and it allows precise targeting and real time confirmation of tissue ablation under MRI guidance. We present a case of an mTLE patient with recurrent seizures after ATL who achieved long-term seizure-freedom after successfully undergoing LITT to ablate the residual hippocampus. This approach, if demonstrated to be safe, effective and durable, can benefit select post-ATL mTLE patients.
Keywords: Ablation, Intraoperative MRI, Laser, Temporal Lobe Epilepsy, Residual, Seizure Outcome
Highlights
-
•
Persistent seizures at long-term follow-up after anterior temporal lobectomy (ATL) is not uncommon.
-
•
Residual mesial temporal lobe is one of the most common reasons for seizure recurrence after ATL.
-
•
Laser interstitial thermal therapy (LITT) is a minimally invasive surgery with advantages of intraoperative MRI guidance.
-
•
LITT can be used to selectively ablate residual mesial temporal structures in post-ATL patients with recurrent seizures.
1. Introduction
Anterior temporal lobectomy and amygdalohippocampectomy (ATL) is the gold standard surgical therapy for patients with drug resistant mesial temporal lobe epilepsy (mTLE) [1]. However, 20–30% of patients fail to achieve seizure-freedom after ATL [2,3]. Insufficient resection of the mesial structures is one of the most common reasons for seizure recurrence following ATL [2,[4], [5], [6], [7], [8]]. Further resection of the residual mesial structures can lead to seizure-freedom in approximately 50–60% of these patients [[5], [6], [7], [8]]. Therefore, those who failed initial ATL due to insufficient resection have traditionally undergone repeat open surgery.
Laser interstitial thermal therapy (LITT) is a minimally invasive surgery that is increasingly becoming a first-line alternative surgical option for drug-resistant lesional temporal lobe epilepsy (TLE), and particularly for mTLE [9,10]. LITT involves stereotactic insertion of an optical laser fiber into the mesial temporal structures to ablate them under real time MR feedback. LITT has been associated with seizure outcomes and complication rates that are comparable to those of ATL [9]. Although there is no widely accepted protocol, patients who respond inadequately to LITT have subsequently undergone a repeat LITT or ATL with improved seizure outcomes [[11], [12], [13]]. However, it remains unknown whether LITT is safe and effective in mTLE patients with recurrent seizures after initial ATL. This is an important consideration because patients are increasingly opting for minimally invasive surgical options due to potentially lower morbidity, better cosmesis, and faster recovery. Repeat craniotomy after a failed ATL can be technically challenging due to scarring and altered anatomy [4,6]. Furthermore, repeat craniotomy is associated with higher risks of post-operative cerebrospinal fluid leak, stroke, incisional complications, and neurocognitive decline [14]. LITT is an attractive option for post-ATL patients with residual mesial structures because the surgery is minimally invasive, and it allows precise targeting and real time confirmation of tissue ablation under MRI guidance [[9], [10], [11], [12], [13]].
Currently, there is no guideline or consensus on how to surgically manage mTLE patients who fail their first surgery. With LITT becoming an important part of an epilepsy team's armamentarium, it is important and timely to define its role in managing this challenging population. Here we report a case of an mTLE patient with recurrent intractable seizures after ATL who achieved long-term seizure-freedom after successfully undergoing LITT to ablate the residual hippocampus.
2. Case presentation
A 42-year-old woman presented with a history of recurrent focal aware and, focal impaired awareness-non-motor seizures, and focal to bilateral tonic–clonic seizures, despite ATL twelve years ago. She had febrile seizures at 10-months-old, followed by recurring focal onset seizures starting at age two, consisting of “heart racing” and “disorienting” episodes. Her seizures were managed well with medications. At the age of 26, she began having frequent auras and 10–20 seizures a month in clusters of two to three events per day. Her auras consisted of a 30 s period, during which she experienced tachycardia, depersonalization, and fear. In 30–40% of the time, her auras progressed to focal onset seizures that consisted of 1–2 min of partial awareness, tonic posturing of the right arm, and aphasia followed by postictal confusion. She also had focal to bilateral tonic–clonic seizures every 6 months. Her seizures were refractory to lamotrigine, phenytoin, valproic acid, carbamazepine, and levetiracetam. A 3 T brain MRI demonstrated increased signal in the left hippocampus without volume asymmetry. No other intracranial abnormalities were detected. Video EEG (vEEG) monitoring captured three typical auras and six focal impaired awareness seizures associated with rhythmic 3–4 Hz slowing, which evolved into ictal spiking at 6–8 Hz in the left temporal region. Interictal EEG showed left temporal slowing and sharp waves. Neuropsychological evaluation demonstrated no deficits and Wada testing suggested left language dominance and shared memory function between the two hemispheres. She was diagnosed with mTLE.
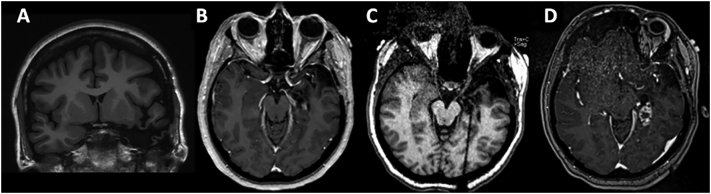
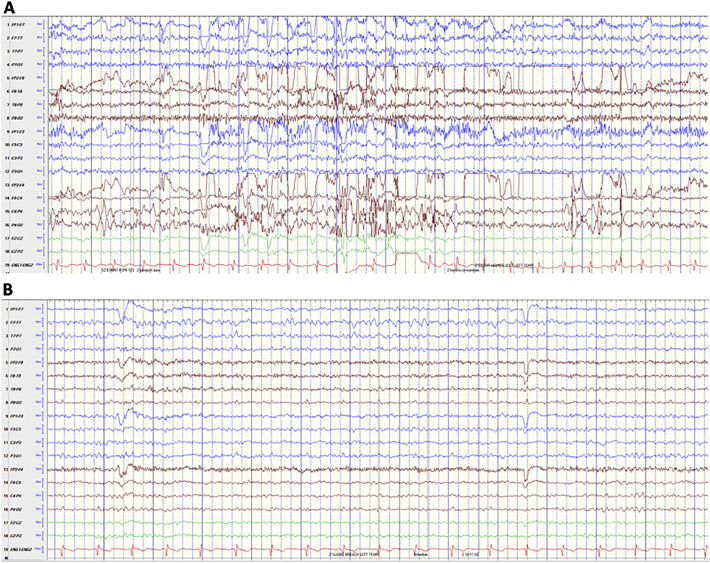
The patient was deemed a good surgical candidate, and subsequently underwent a left ATL. Post-operative MR was notable for complete resection of the amygdala and residual posterior hippocampus (Fig. 1A). Pathology showed severe loss of hippocampal pyramidal neurons in CA1 and the hilus and dispersion and focal duplication of the granule cell layer consistent with mesial temporal sclerosis (MTS). Although she developed new difficulty with naming and word retrieval, she otherwise recovered well and was seizure-free on levetiracetam and lamotrigine for 21 months. She then had recurrent seizures of similar semiology as her previous ones, which progressively worsened in frequency and severity with focal to bilateral tonic–clonic seizures despite up-titration of levetiracetam, lamotrigine, and lacosamide. Repeat MRI confirmed the presence of residual posterior hippocampus (Fig. 1B). On vEEG, the patient had four typical focal impaired awareness seizures associated with rhythmic theta (5–7 Hz) activity seen maximally over the left posterior temporal region (T7, Fig. 2A). There was also intermittent focal slowing (1–3 Hz) and breach pattern over the left temporal region seen maximally at T7 (Fig. 2B).
Fig. 1.
Post-ATL, pre-ablation coronal T1 MRI without contrast (A). Pre-ablation axial T1 MRI with contrast demonstrating residual hippocampus (B). Intra-operative post-laser catheter placement, pre-ablation MPRAGE axial MRI demonstrates the catheter placed within the residual mesial hippocampus (C). Post-ablation axial T1 MRI with contrast demonstrates enhancement of the ablated residual hippocampus (D). MPRAGE, magnetization-prepared rapid gradient echo.
Fig. 2.
Onset of typical focal impaired awareness seizure demonstrating evolution of 1–3 Hz focal slow activity into theta (6 Hz) activity (A). Inter-ictal focal slowing (1–3) over the left temporal region, maximum at T7 and breach pattern over the same region (B).
She was recommended a reoperation with LITT to target the residual hippocampus as an initial step with the plan to proceed with stereoencephalography (sEEG) if she were to fail the surgery. Extraoperative invasive monitoring was deemed unnecessary in her case because the residual posterior hippocampus could be readily identified on brain MRI and the prominent autonomic features of her seizures suggested involvement of the temporal lobe. The vEEG also indicated that the left posterior temporal region was still involved in the seizure onset, taking into account the possible bias localization due to breach seen interictally. The patient's long period of seizure freedom following her initial surgery was also thought to be a favorable sign for extending the original resection boundaries. Therefore, she underwent LITT 12 years after her ATL. A Visualase™ (Medtronic, Plc, Minneapolis, MN) laser cooling catheter was stereotactically inserted into the target area using the ClearPoint™ navigation system (ClearPoint Neuro, Inc., Irvine, CA) and 1.5 T Siemens intraoperative MRI (iMRI) with radial placement error < 1.0 mm (Fig. 1C). The details of LITT workflow using the Visualase™ and ClearPoint™ systems have been described elsewhere [14]. Two successive ablations were performed at approximately 10 W for a total of 4 min. An intra-operative T1 post-gadolinium MRI confirmed the ablation of the residual hippocampus (Fig. 1D). She tolerated the procedure well without any complication and was discharged home the next day. She was followed closely for the next 39 months. She initially reported three auras at one-month, one focal impaired awareness seizure after missing two doses of antiseizure medications one year after LITT, and no auras or seizures since then on reduced doses of levetiracetam and lacosamide.
3. Discussion
We present an mTLE patient who developed recurrent drug-resistant seizures after ATL who subsequently achieved long-term seizure-freedom after undergoing LITT to ablate the residual hippocampus. Using the iMRI, residual hippocampus was readily identified, laser insertion into the target was achieved with high accuracy, and adequacy of the ablation was confirmed in real time. iMRI also enabled real time monitoring of complications, such as hemorrhage, throughout the case. The patient had a short hospital stay and recovered without complication. Notably, seizure-free duration after LITT was nearly twice as long as that after ATL. Because the patient was seizure-free for nearly 2 years after ATL, a longer follow-up is desirable to determine LITT's true therapeutic durability in this patient. This is particularly true because the patient underwent LITT under the assumption that the known residual hippocampus was the primary epileptogenic zone (EZ), a hypothesis that was not confirmed with presurgical invasive monitoring. This may represent a unique scenario and invasive monitoring may be necessary in other post-craniotomy cases for precise EZ characterization. Nevertheless, the case suggests that it is feasible to use LITT to discretely ablate the suspected EZ in the setting of insufficient surgical resection to achieve seizure control.
ATL is a safe and highly effective surgical treatment for drug-resistant mTLE, with seizure recurrence rate of approximately 20% at 1–2 years follow-up [1,3]. However, long-term outcomes have been associated with recurrence in up to 60% of patients at 10 years [3,16,17], with reoperation being performed most commonly 3–6 years after the index surgery [[6], [7], [8]]. Reoperation tends to be performed much sooner after LITT at 6–9 months potentially due to lower treatment efficacy [11,13]. Although there are many possibilities for ATL failure, insufficient resection of the mesial temporal structures remains one of the most common reasons [2,[5], [6], [7], [8]]. Intra-operative overestimation of resection, variable extent of resection due to unintended deviation from the surgical plan, and limitations of image-guided navigation have been cited as potential contributors [2,4]. Although many patients do well after an open reoperation, scarring and distorted anatomy can render accurate identification and complete resection of residual structures challenging [4,6]. Surgical risks can also increase with repeat craniotomy. The minimal invasiveness inherent to LITT along with the advantages of iMRI can be leveraged in carefully selected patients to overcome the limitations of a repeat open surgery. High placement accuracy (radial error < 1.0 mm) associated with iMRI compatible stereotactic neuro-navigation systems can be used to selectively ablate residual epileptogenic regions while accounting for the variable anatomy of post-surgical patients [9,15]. Repeat LITT can also be performed after the first LITT failure with potentially lower surgical morbidity than multiple repeat craniotomies [11,13].
Applications of LITT in epilepsy management are growing rapidly and potential indications include tuberous sclerosis, focal cortical dysplasia, cerebral cavernous malformation, periventricular nodular heterotopia, poststroke epilepsy, and hypothalamic hamartoma [18]. Presently, mTLE is the epilepsy disorder most commonly treated with LITT [18]. Furthermore, Selective laser amygdalohippocampectomy (SLAH) using LITT has become the first-line surgical therapy over resection for mTLE at some centers [18]. Under this ‘LITT-First’ paradigm, mTLE patients who fail LITT can either undergo another LITT or open surgery after additional work-up, which can include invasive monitoring [[10], [11], [12], [13]]. Those who fail their initial resection can proceed with either LITT or repeat craniotomy depending on patient characteristics, as well as suspected EZs (e.g. type, location, number, and size) and implicated networks. As in the presented case, it may be reasonable to use LITT to selectively target residual epileptogenic structures in the setting of biopsy-proven mTLE with no suspected extratemporal lesions. In cases of adequate initial resection, other hypotheses should be considered, and optimal surgical technique should be selected based on EZs delineated through additional work-up.
However, the role of LITT in epilepsy management is still being defined. Our case report demonstrates technical feasibility in a carefully selected patient and attempts to raises awareness of this relatively under-recognized application of LITT. Studies with large cohorts and long-term follow up are needed to determine whether LITT should be considered as one of the viable options in the management of mTLE patients who have failed ATL either due incomplete mesial temporal resection or other active EZs. Also, comparative therapeutic durability of LITT versus ATL after failed initial surgery with either of the surgical modalities needs to be explored. Importantly, patient selection criteria based on anatomic, electrophysiologic and clinical characteristics needs to be further defined so that a subset of post-ATL patients who benefit the most from the approach can be identified. In addition, technical considerations and challenges associated with performing LITT in post-surgical population is currently lacking in the literature and must be discussed further. These endeavors will help establish a standardized surgical management protocol that incorporates invasive monitoring, open surgery, and LITT to achieve the highest chance of long-term seizure freedom with the fewest number of procedures and the lowest total surgical risk.
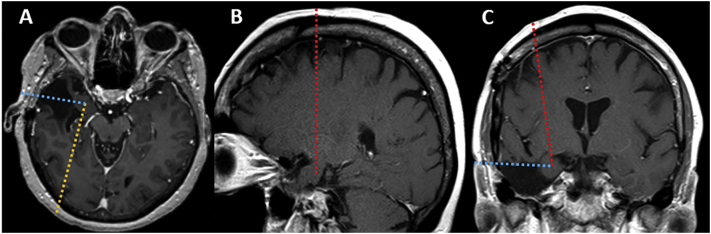
Hippocampus is the structure most frequently associated with reoperation for a failed epilepsy surgery in mTLE [2,5]. Nevertheless, extrahippocampal mesial temporal structures, such as the amygdala, entorhinal cortex, and parahippocampal gyrus can be implicated in recurrent seizures [2,4]. One key advantage of LITT is its ability to target virtually anywhere in the brain to cause a discrete thermal lesion with minimal disruption to the surrounding tissues. LITT has been shown to selectively and safely ablate the hippocampus and the extrahippocampal mesial temporal structures to achieve good seizure outcome [9,11]. Standard occipital approach can be considered when performing LITT in post-ATL patients, because it can reach nearly all the mesial temporal structures (Fig. 3A). Non-standard approaches, such as frontal and temporal trajectories, can be considered when the region of interest predominantly implicates the anterior mesial temporal structures. A frontal trajectory may allow maximal targeting of the residual amygdala, head of the hippocampus, and entorhinal cortex while entirely avoiding the previous surgical site (Fig. 3B, C). One must be mindful of the laser catheter's proximity to the basal ganglia during a frontal approach, and real time MR thermometry can help mitigate the risk of unintended heat spread. The advantage of the temporal trajectory is that it provides excellent approach to the residual structures while minimizing traversing of the normal brain (Fig. 3A, C). However, the temporal approach may not be feasible in patients with significant amount of missing temporal bone or healing craniotomy because iMRI stereotaxic navigation systems require rigid fixation to the skull. Also, traversing a resection cavity may affect targeting accuracy and treatment efficacy through catheter deflection and heat sink effect, respectively. Also, traversing of resection cavities with laser catheters can cause hemorrhage. Thus, risks and benefits of each must be weighed against individual anatomical characteristics (i.e. location and shape of the target structures, proximity to eloquent structures, degree of scarring, proximity to large blood vessels or cerebrospinal fluid filled spaces that can act as heat sink, etc.). Determining the safety and efficacy of different laser trajectories in post-surgical patients as well as improving our understanding of LITT thermodynamics remain critical in establishing a standardized practice protocol.
Fig. 3.
Standard occipitotemporal (A, yellow line), frontal (B, red line), and temporal (C, blue line) trajectories can be considered when ablating residual amygdala and hippocampal head after anterior temporal lobectomy. Axial (A) and coronal (C) views also demonstrate temporal (blue line) and frontal (red line) trajectories, respectively.
LITT has brought exciting advances to epilepsy management and techniques, strategies and procedures will continue to evolve in the foreseeable future. Ultimately, continued efforts to devise and standardize novel management strategies and surgical approaches will be important in improving long-term seizure outcomes and quality of lives of epilepsy patients.
CRediT author statement
Brian Y. Hwang: Conceptualization, Investigation, Writing – Original Draft, Writing – Review and Editing. David Mampre: Investigation, Writing – Original Draft, Writing – Review and Editing. Joon Y. Kang: Conceptualization, Investigation, Writing – Review and Editing. Gregory Krauss: Conceptualization, Investigation, Writing – Review and Editing. William S. Anderson: Conceptualization, Investigation, Writing – Review and Editing, Supervision.
Ethics statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Declaration of competing interest
WSA sits on Advisory Boards for Longeviti Neuro Solutions, and NeuroLogic. He is also a paid consultant for Globus Medical. The rest of the authors have no conflict of interest to disclose.
Acknowledgements
None.
References
- 1.Wiebe S., Blume W.T., Girvin J.P., Eliasziw M. Effectiveness, efficiency of surgery for temporal lobe epilepsy study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Harroud A., Bouthillier A., Weil A.G., Nguyen D.K. Temporal lobe epilepsy surgery failures: a review. Epilepsy Res Treat. 2012;2012:201651. doi: 10.1155/2012/201651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeha L.E., Najm I.M., Bingaman W.E., Khandwala F., Widdess-Walsh P., Morris H.H. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz T.H., Marks D., Pak J., Hill J., Mandelbaum D.E., Holodny A.I. Standardization of amygdalohippocampectomy with intraoperative magnetic resonance imaging: preliminary experience. Epilepsia. 2002;43:430–436. doi: 10.1046/j.1528-1157.2002.39101.x. [DOI] [PubMed] [Google Scholar]
- 5.Wyler A.R., Hermann B.P., Richey E.T. Results of reoperation for failed epilepsy surgery. J Neurosurg. 1989;71:815–819. doi: 10.3171/jns.1989.71.6.0815. [DOI] [PubMed] [Google Scholar]
- 6.Awad I.A., Nayel M.H., Luders H. Second operation after the failure of previous resection for epilepsy. Neurosurgery. 1991;28:510–518. doi: 10.1097/00006123-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Germano I.M., Poulin N., Olivier A. Reoperation for recurrent temporal lobe epilepsy. J Neurosurg. 1994;81:31–36. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 8.Salanova V., Markand O., Worth R. Temporal lobe epilepsy: analysis of failures and the role of reoperation. Acta Neurol Scand. 2005;111:126–133. doi: 10.1111/j.1600-0404.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Jermakowicz W.J., Chakravorti S., Cajigas I., Sharan A.D., Jagid J.R. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: a multicenter study of 234 patients. Epilepsia. 2019;60:1171–1183. doi: 10.1111/epi.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petito G.T., Wharen R.E., Feyissa A.M., Grewal S.S., Lucas J.A., Tatum W.O. The impact of stereotactic laser ablation at a typical epilepsy center. Epilepsy Behav. 2018;78:37–44. doi: 10.1016/j.yebeh.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Willie J.T., Laxpati N.G., Drane D.L., Gowda A., Appin C., Hao C. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–584. doi: 10.1227/NEU.0000000000000343. [discussion 584-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaRiviere M.J., Gross R.E. Stereotactic laser ablation for medically intractable epilepsy: the next generation of minimally invasive epilepsy surgery. Front Surg. 2016;3:64. doi: 10.3389/fsurg.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J.Y., Wu C., Tracy J., Lorenzo M., Evans J., Nei M. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57:325–334. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 14.Brotis A.G., Giannis T., Kapsalaki E., Dardiotis E., Fountas K.N. Complications after anterior temporal lobectomy for medically intractable epilepsy: a systematic review and meta-analysis. Stereotact Funct Neurosurg. 2019;97:69–82. doi: 10.1159/000500136. [DOI] [PubMed] [Google Scholar]
- 15.Ho A.L., Sussman E.S., Pendharkar A.V., Le S., Mantovani A., Keebaugh A.C. Improved operative efficiency using a real-time MRI-guided stereotactic platform for laser amygdalohippocampotomy. J Neurosurg. 2018;128:1165–1172. doi: 10.3171/2017.1.JNS162046. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh A.M., Kalnins R.M., Mitchell L.A., Fabinyi G.C., Briellmann R.S., Berkovic S.F. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 17.Dupont S., Tanguy M.L., Clemenceau S., Adam C., Hazemann P., Baulac M. Long-term prognosis and psychosocial outcomes after surgery for MTLE. Epilepsia. 2006;47:2115–2124. doi: 10.1111/j.1528-1167.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 18.Youngerman B.E., Save A.V., McKhann G.M. Magnetic resonance imaging-guided laser interstitial thermal therapy for epilepsy: systematic review of technique, indications, and outcomes. Neurosurgery. 2020;86:E366–E382. doi: 10.1093/neuros/nyz556. [DOI] [PubMed] [Google Scholar]