Abstract
Fungi of the genus Trichoderma are important microorganisms used in biocontrol processes and the promotion of plant development. However, they remain poorly studied in the context of forestry programs, especially those related to native Amazonian species. Thus, it is the aim of this study to evaluate the effects of different Trichoderma isolates on the germination and development of Handroanthus serratifolius seedlings. During in vitro germination tests, seeds were immersed for 24 h in respective fungal suspensions each prepared using one of five Trichoderma isolates. The suspensions were held in plastic trays and kept at a temperature of 24 ± 2 °C. Metrics related to germination and development assessed under laboratory conditions include: germination speed index (GSI), germination percentage, length of the roots, and hypocotyls, as well as fungal perseverance. In the nursery, Trichoderma were used in two different applications: pre-planting treatment and as a monthly, post-planting treatment. Pre-planting treatments consisted of 10 g of colonized rice grains bearing each isolate being placed into experimental bags five days before seeding. The post-planting treatment involved the application of 10 mL of fungal suspension per experimental bag. Each month, the height, stem diameter, and leaf number were measured for each seedling. At the end of the experiment, the length and mass of roots as well as the total dry mass were recorded. In laboratory conditions, seeds treated with Trichoderma asperellum -TAM03 obtained the greatest fractional germination (76.5%) and GSI. In the nursery experiments, isolates TAM01 and TAM03, when applied as a post-planting treatment, increased the height, stem diameter, and number of leaves of treated plants with respect to the control group by 180 days post-treatment. After 365 days, plants which received TAM01 pre-planting treatments were observed to have increased root and aerial part length, as well as root mass and overall dry mass. These results suggest that T. asperellum -TAM01 positively affects H. serratifolius development.
Keywords: Biotechnology, Biological science, Microbiology, Physiology, Plant biology, Environmental science, Sustainable forestry, Seedling production, Yellow ipe, Growth promotion, Biological treatment
Biotechnology, Biological Science, Microbiology, Physiology, Plant biology, Environmental science, sustainable forestry, seedling production, yellow ipe, growth promotion, biological treatment
1. Introduction
Recently, native tree species have become endangered through processes related to the expansion of agricultural fields, like slash-and-burn deforestation, as well as rampant exploitation of the trees themselves. Many of these species stand out for their wood quality, and have important applications in manufacturing industries, ecological reforestation efforts, medicine, and landscaping [1, 2, 3].
The species Handroanthus serratifolius (Vahl) S. Grose, popularly known as yellow ipe, is of great importance throughout the Amazon. This tree stands out among other native tree species with regards to reforestation capabilities and timber potential. It is widely distributed in Brazil [4, 5] and Latin America [5]. This species, as with other members of the Bignoniaceae family, is also widely exploited for its medicinal properties. These trees are known to be a source of many pharmacologically active compounds [6, 7], with several such compounds being actively studied as candidates for the treatment of ailments, including cancer [8, 9]. The primary compound of medicinal interest, lapachol, occurs in various parts of the plant [7, 10, 11].
Handroanthus serratifolius is also considered important for its timber properties. This species grows into large trees with medium canopy sizes. Trees typically reach heights between 5 and 25 m, with straight cylindrical trunks that can measure between 20 and 90 cm in diameter [5, 12]. The timber derived from these trees is of good quality with BRL values ranging from 136.67 to 570.00 m3. It is widely used in construction, shipbuilding, and furniture fabrication [5, 13]. Furthermore, the tree is often used for ornamental purposes due to the exuberance of its flowers, which come in different shades. In addition, it also offers great potential for reforestation efforts [4, 5, 14, 15].
Despite its importance, H. serratifolius is rarely utilized in forest plantations due to the lack of information regarding its development in both nursery and field conditions [16], being propagated mainly by seeds but also demonstrating germination and preservation issues, such as reduced physiological potential during storage [17, 18, 19].
In addition, the H. serratifolius occurs at extremely low densities (<1 adult tree ha−1) and is constantly under threat of being harvested [20]. This demonstrates a high potential conflict of use in economic terms [21], while also raising concern about its maintenance in natural ecosystems. The production of native forest seedlings is an effective way of counteracting depopulation brought about by anthropic activities [22]. Therefore, the investigation of techniques aimed at improving the developmental quality of seedlings is highly desirable, as healthy seedlings lead to successful plantings [23, 24].
To this end, the treatment of seeds and seedlings with microorganisms or their metabolites has been shown to be effective in protecting seeds, promoting germination, and controlling pathogens [25]. In addition, the use of microbes for bioprotection or biocontrol purposes is seen as a promising and eco-friendly alternative to using chemicals [26, 27, 28].
Cavero et al. [27], i.e., showed that T. atroviride (2.047) was the first isolate of this genus to show potential in controlling banana black Sigatoka under field conditions. Furthermore, conidial germination of this isolate was not inhibited by the synthetic fungicides tested after 3 h of exposure.
Species of the genus Trichoderma are promising candidates for the biological treatment of seedlings. Members of this genus are naturally occurring soil fungi, present in all soil types, and usually associated with plant roots [30]. For this reason, they have aroused great scientific interest. This fungus promotes biocontrol [31] and plant growth through multiple mechanisms of action including: antibiosis, mycoparasitism, competition, resistance induction, predation, hypovirulence, and inactivation of phytopathogen enzymes. Trichoderma is also thought to increase nutrient availability for associated roots due to the high nutrient absorption efficiency of this fungal species [29, 32, 33, 34].
Trichoderma is most commonly used in association with agricultural species. However, its application is also viable in forest systems, especially in nurseries, where environmental conditions can be controlled [35, 36, 37] to increase germination speed, increase the mass of the aerial part and of the roots [22], improve robustness, and enhance the mass of dry plant matter [17]. The benefits of Trichoderma treatments may be important in optimizing seedling production for several application and, consequently, reduce pressure on natural forests [22].
The use of bioagents, such Trichoderma, in agricultural production has been shown to improve plant growth, and it is generally regarded as the best alternative to synthetic products that are harmful to human health and the environment. However, the use of biocontrol agents requires proper formulation in order to ensure their survival during storage, as well as their rapid multiplication and colonization after inoculation [38].
Hence, the objective of this study was to evaluate the effect of five Trichoderma isolates, with two types of application in substrata (pre and post-planting), on the germination and development of H. serratifolius.
2. Material and methods
The study was conducted in the municipality of Santarém (2º24′52″S, 54º42′36″W), within the western state of Pará, Brazil. Local climate is Am according to the Köppen classification scheme [39], with maximum average temperatures ranging between 26 °C to 31 °C, and minimum temperatures between 21 °C and 23 °C. Average relative humidity in the area is 86%, and average monthly precipitation ranges from 170 to 300 mm during the rainy season (December and May) to less than 60 mm in the dry season (June and November) [40].
For seed treatment and seedling applications, Trichoderma isolates were mass produced in parboiled rice grains. Laboratory experiments sought to address the effects of fungal treatment directly to the seed, while nursery experiments evaluated the effects of treatment of both pre-planting and post-planting substrate. The seeds were collected from matrices present at the Tapajós Unit of Federal University of Western Pará, Santarém Campus.
A total of five Trichoderma isolates were used to treat the seeds in the laboratory test. Three isolates belonged to the species Trichoderma asperellum (TAM01, TAM02, and TAM03), and two to Trichoderma sp. (Tc and Tce). The isolates TAM01, TAM02, and TAM03 came from forest soil in the Urucu Petroleum Province, municipality of Coari, Amazonas, Brazil. Isolates Tc and Tce originated from soil under an agroforestry system containing cumaru (Dipetrix odorata) and curauá (Ananas erectifolius) (Santarem, Pará, Brazil). In the nursery assay, the isolate Trichoderma sp. - Tc was removed due to low germination rates observed with seeds treated with this fungus.
TAM01, TAM02, and TAM03 were ceded by the Federal Rural University of Amazonia (UFRA), and they were deposited in the fungal culture repository at the Plant Protection Laboratory. Tc and Tce were identified, with genera-level resolution, by the Federal University of Western Pará, and were deposited in the fungal culture repository at the Phytopathology Laboratory.
2.1. Trichoderma in the germination of H. serratifolius seeds
Prior to microbialization, seeds were superficially disinfected with 2% hypochlorite. Afterwards, the seeds were immersed for 24 h in fungal suspensions prepared with one of the five Trichoderma isolates at 1.0 x 107 conidia mL−1 [36]. The control treatment consisted of seeds immersed only in water for the same period of time. Following this treatment, the seeds were deposited in trays containing sterile filter paper moistened with sterile water. The seed trays were stored at 25 ± 2 °C [36]. The experimental design was completely randomized with four replicates. Each replicate contained 50 seeds, amounting to a total of 1,200 seeds.
The variables evaluated were (a) germination speed index (GSI), (b) germination rate, and (c) initial seedling development. The GSI was calculated by counting the number of germinated seeds on a daily basis until stabilization as described by Maguire [41]. Germination rate (%) was estimated by counting the number of seeds germinated over a 15-day period during which stabilization occurred. Initial seedling development was obtained by measuring the lengths of the root and hypocotyl of germinated seeds with a scaled ruler.
2.2. Trichoderma in the development of H. serratifolius seedlings
A total of four isolates of Trichoderma spp. (TAM01, TAM02, TAM03, and Tce) were used under nursery conditions. Treatments were conducted with two approaches: application in pre-planting substrate and monthly application in post-planting substrate.
The standardized seedlings used in this stage of experimentation were derived from germinated seeds obtained in laboratory experiments. To obtain these seedlings, seeds were equidistantly placed in plastic trays containing filter paper moistened with sterile water. The trays were sealed with transparent plastic film and kept at room temperature. 13 days after assemblage, seedlings with similarly-sized roots were selected and transplanted to 2.25 kg polypropylene bags containing forest soil as a substrate.
The Trichoderma isolates were applied to the substrate before planting through soil irrigation and the incorporation of 10 g per bag of colonized rice grains with the respective Trichoderma species for each treatment, five days before seedling transplant.
Substrate treatment after planting consisted of a monthly addition of 10 mL of prepared fungal suspension containing one isolate at 1.0 x 107 conidia.mL−1. The first application was performed at 15 days after transplantation, with a total of 12 monthly treatments occurring during the course of the experiment. All plants were kept under 30% shading with daily irrigation, and they were evaluated monthly for 180 days. A final evaluation was conducted 365 days after transplantation.
The following variables were evaluated monthly. Seedling height (cm) was obtained with a scaled ruler by measuring the distance from the seedling base to the apical bud. Stem diameter (mm) was measured using digital calipers with 0.01 mm accuracy. This measurement was taken at the base of the seedling, near the substrate. The third variable monitored monthly was the number of leaves present on the seedling. This metric was obtained by counting the number of leaves per plant. During the experimental period, no dead plants were observed in any of the treatment conditions.
The following variables were obtained 365 days after planting. Seedling height, stem diameter, and number of leaves were obtained following the same procedures as in the monthly assessment. Root length (cm) was obtained by measuring the root with a scaled ruler from the base of the seedling to the end of the main root. The dry mass of the aerial part (g), the dry mass of the roots (g), and the total dry mass (g) were determined after both washing the root system to remove the substrate and cutting the seedlings at the base of the stem to separate the root system from the aerial portion of the plant. The plant material was then dried in an oven (55 °C) with forced air circulation for 48 h and weighed on an analytical scale with a precision of 0.001 g.
From these aforementioned variables, four ratios were calculated: plant height/stem diameter, plant height/aerial part dry mass, root length/root mass, and aerial part dry mass/root dry mass. Dickson quality index values were also calculated [42].
3. Experimental design and statistical analysis
The experimental design was completely randomized with a factorial design of 4 × 2 + 1 (i.e., 4 Trichoderma isolates x 2 application types +1 control treatment) with 10 replicates.
Only data obtained at 180 and 365 days were used to evaluate the effect of the biological treatment on seedling development. To do this, a univariate analysis with a significance threshold of 0.05 was employed. The data collected were analyzed with an analysis of variance (ANOVA). The means of the treatments were compared to that of the control using a Dunnett test (p ≤ 0.05). The means of treatment groups were compared to each other using a Tukey Test (p ≤ 0.05) with the aid of the ExpDes package of software R, version3.5.1 [43]. This program also was used for data visualization and figure generation.
4. Results and discussion
4.1. Trichoderma in the germination of H. serratifolius seeds
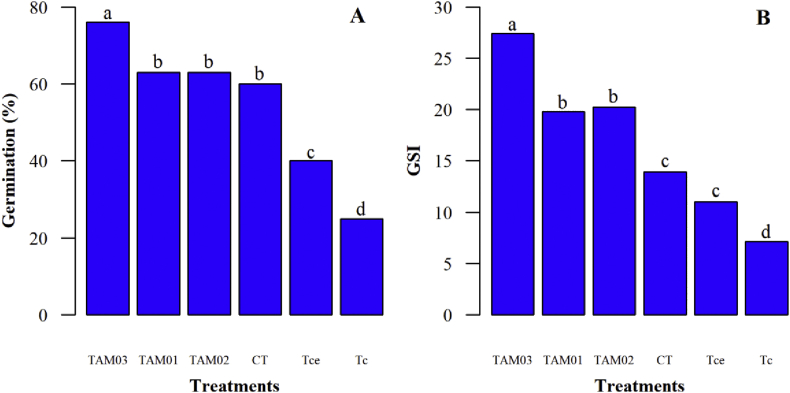
All the considered germination variables differed significantly among treatments (p ≤ 0.05). Yellow ipe seeds treated with isolate TAM03 exhibited a greater germination rate (76.5%) than the control treatment (60%). Seeds treated with the fungus Trichoderma sp. Tc exhibited the lowest germination rate (Figure 1A). The results of this study are interesting when compared with the results of Pereira et al. [44], who did not observe a decrease in germination rate when treating field lettuce seedlings with four evaluated strains of Trichoderma spp. In fact, all tested fungi from the study by Pereira et al. (2019) improved germination rate when compared to the control, noting both T. harzianum ESALQ 1306 and T. harzianum IBLF 006 SC isolates had a particularly positive effect.
Figure 1.
Means of germination rate (A) and germination speed index (GSI) (B) for Handroanthus serratifolius seeds treated and not treated with different Trichoderma isolates. TAM03 = T. asperellum - TAM03; TAM01 = T. asperellum – TAM01; TAM02 = T. asperellum – TAM02; CT = Control treatment; Tce = Trichoderma sp. - Tce; Tc = Trichoderma sp. - Tc. Means followed by the same letters in the columns do not differ from each other as determined by Tukey's test (p ≤ 0.05).
Seeds treated with the fungus T. asperellum TAM03 showed the highest germination speed, whereas seeds treated with Trichoderma sp. - Tc had the lowest GSI (Figure 1B). In addition to providing significant increases in germination rate, Trichoderma application can also increase germination precocity and speed [45]. The observed GSI results differ from those obtained by Santos et al. [46], who evaluated the effects of three T. asperellum isolates on GSI using seeds of Theobroma grandiflorum (cupuaçu), and they found no significant difference between control and experimental groups.
This study demonstrates that the application of T. asperellum TAM03 to seeds of H. serratifolius might provide a possible solution to the germination and preservation issues associated with yellow ipe seeds reported by Oliveira et al. [47]. Therefore, alternative seed treatments of a biological nature, such as the one addressed in this study, may have a significant effect on the successful propagation of this and other native species.
However, the effect on germination is often isolate-specific, and some isolates may inhibit germination [22]. Junges et al. [48] reported that Agrotrich (Trichoderma based commercial product) provided negative GSI results when used with soybean seeds. Junges et al. [3] evaluated the effects of Trichoderma spp. on seeds and seedling development for Parapiptadenia rigida (angico), Cedrela fissilis (ceder), and Peltophorum dubium (canafistula). The authors of this study observed that the application of Trichoderma spp. to angico seeds hindered seedling development, reducing seed emergence by 35.9% compared to untreated seeds.
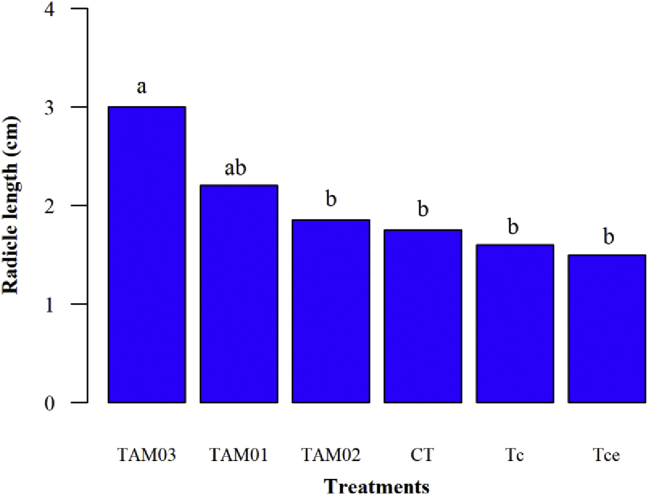
Only seeds treated with T. asperellum TAM03 exhibited root lengths which were significantly different from the control treatment (Figure 2). Santos et al. [46], when evaluating the effect of the Trichoderma isolates (TAM01, TAM02, and TAM03) on the treatment of T. grandiflorum seeds, observed that T. asperellum - TAM02 and T. asperellum - TAM03 isolates increased root length when compared to control, possibly due to improved phytosanitary seed conditions.
Figure 2.
Length of radicle of Handroanthus serratifolius treated and not treated with different Trichoderma isolates. TAM03 = Trichoderma asperellum - TAM03; TAM01 = Trichoderma asperellum - TAM01; TAM02 = Trichoderma asperellum - TAM02; CT = Control; Tc = Trichoderma sp. – Tc; Tce = Trichoderma sp. - Tce. Means followed by the same letters in the columns do not differ from each other as determined by Tukey's test (p ≤ 0.05).
During the laboratory study period, only seeds treated with T. asperellum TAM03 exhibited a hypocotyl. Patekoski and Pires-Zottarelli [49] evaluated the effect of Biotrich (Trichoderma based product) on the development of various hydroponic lettuce seedlings and reported a reduced root length along with stimulation of hypocotyl growth. Migliorini et al. [50] reported negative results when treating canola seeds with chemicals and Trichoderma spp., observing the frequency of hypocotyl on Trichoderma-treated seeds to be 24.4 % lower compared to chemically-treated seeds.
4.2. Trichoderma in the development of H. serratifolius seedlings
In the evaluation conducted 180 days after planting, the performed Dunnett test indicated differences between seedling heights, stem diameters, and leaf numbers, when comparing the experimental treatments to the control (Table 1). At least four treatments increased the height of the seedlings on average; two treatments resulted in larger stem diameters on average; and five treatments increased the number of leaves on average, all relative to the control. Treatments using T. asperellum isolates (TAM01 and TAM03) applied to the post-planting substrate yielded favorable results for all the variables analyzed (Table 1). Height increased by 0.7–1.1 cm, stem diameter increased by 0.3–0.4 cm, and the number of leaves increased by a factor of 1.3–1.5 with respect to the control.
Table 1.
Mean heights, stem diameters, and numbers of seedling leaves (followed by standard deviation) of Handroanthus serratifolius submitted to different treatments with Trichoderma spp. isolates at 180 days after transplantation.
| Treatments | Evaluations |
||
|---|---|---|---|
| Height (cm) | Stem diameter (cm) | Number of leaves | |
| TAM01 applied to the pre-planting substrate | 12.7 ± 0.70∗∗ | 2.0 ± 0.22ns | 18.7 ± 1.83∗∗ |
| TAM01 applied to the post-planting substrate | 13.4 ± 0.88∗∗ | 2.3 ± 0.30∗ | 20.1 ± 5.51∗∗ |
| TAM02 applied to the pre-planting substrate | 12.9 ± 1.03∗∗ | 2.1 ± 0.17ns | 17.6 ± 2.67ns |
| TAM02 applied to the post -planting substrate | 12.1 ± 1.07ns | 2.1 ± 0.32ns | 17.8 ± 2.70∗∗ |
| TAM03 applied to the pre-planting substrate | 11.9 ± 1.11ns | 1.9 ± 0.27ns | 16.6 ± 4.65ns |
| TAM03 applied to the post -planting substrate | 12.8 ± 0.65∗∗ | 2.2 ± 0.18∗ | 18.4 ± 3.66∗∗ |
| Tce applied to the pre-planting substrate | 12.5 ± 0.38ns | 1.9 ± 0.23ns | 17.5 ± 2.72ns |
| Tce applied to the post -planting substrate | 12.0 ± 0.77ns | 2.1 ± 0.25ns | 18.9 ± 4.01∗∗ |
| Control treatment | 11.6 ± 0.44 | 1.9 ± 0.24 | 13.2 ± 3.91 |
∗∗ significant at a 1% probability level (p < 0.01); ∗ significant at a 5% probability level (p < 0.05); ns non-significant by Dunnett's test.
Similar results were also observed using T. asperellum species as a growth promoter with Pinus taeda seedlings 150 days post-planting [51]. Amaral et al. [52] evaluated the effects of T. asperellum on caroba (Jacaranda micrantha) seedlings 90 days after planting. These results demonstrated an average dry mass of 129.8 mg for experimental cases, whereas the control group's average dry mass was 48.2 mg. Donoso et al. [53] reported that Trichoderma harzianum led to the highest average seedling height for Pinus radiata, 210 days after planting. Azevedo et al. [54] evaluated the effects of Trichoderma virens and T. harzianum on the growth of Eucalyptus camaldulensis clones, and they recorded satisfactory results. These authors noted that the use of these fungi provided a promising alternative to chemical treatments involved in the production processes of forest nurseries.
Significant differences were recorded for the primary effects of the factor Trichoderma, for the interaction between Trichoderma and application strategy with regards to plant height, and for application strategy with regards to stem diameter 180 days after planting, as determined using Tukey's test.
The fungi behaved differently depending on the application strategy. The isolates T. asperellum TAM01 and TAM03 applied post-planting substrate induced higher seedling growth as measured by seedling height. In turn, T. asperellum TAM02 yielded the best result of all isolates when applied to the pre-planting substrate. Plant height did not vary with application strategy for Trichoderma sp. Tce (Table 2).
Table 2.
Mean height (followed by standard deviation) of Handroanthus serratifolius plants subjected to two different strategies of application of Trichoderma sp. isolates 180 days after planting.
| Trichoderma isolates | Plant height (cm) |
|
|---|---|---|
| Application strategy | ||
| Pre-planting substrate | Post-planting substrate | |
| TAM01 | 12.7 ± 0.70 abB | 13.4 ± 0.88 aA |
| TAM02 | 12.9 ± 1.03 aA | 12.1 ± 1.07 bB |
| TAM03 | 11.9 ± 1.11 bB | 12.8 ± 0.65 abA |
| Tce | 12.5 ± 0.38 abA | 12.0 ± 0.77 bA |
| CV (%) | 6.6 | |
Means followed by the same lower case letters in the columns and the same upper case letters in the rows do not differ from each other by Tukey's test (p ≤ 0.05).
Soldan et al. [55] evaluated the effects of Trichoderma spp. isolate FS1 and Trichonat PM (a Trichoderma based product) on the development of Eugenia pyriformis (uvaieira) and Myrcianthes pungens (guabijuzeiro) seedlings, both of which are native species to southern Brazil. The authors reported positive effects on plant growth (height) for Eugenia pyriformis, with Trichonat PM treatment resulting in greater plant height when compared to the control group [55]. Machado et al. [22] also observed increases in plant height when using T. harzianum isolates to promote the growth of Gochnatia polymorpha (cambará) seedlings, a native tree species from southern Brazil.
The stem diameter, root length, dry weight of the aerial part of the plant, dry weight of the roots, and total dry mass showed significant differences in the evaluation conducted 365 days after planting as determined with Dunnet tests. However, this difference was observed only in seedlings treated with post-planting, monthly applications of T. asperellum TAM01, TAM02 and Tce. That said, TAM02 and Tce caused differences in relation to the control only for root length (Table 3).
Table 3.
Means followed by the standard deviation of variables analyzed in Handroanthus serratifolius seedlings submitted to different application methods with Trichoderma spp. isolates, 365 days after transplantation.
| Treatments | Evaluations |
||||||
|---|---|---|---|---|---|---|---|
| Height (cm) | Stem Diameter (cm) | Number of leaves | Root (cm) | APDM (g) | RDM (g) | TDM (g) | |
| TAM01 applied to the pre-planting substrate | 22.4 ± 4.3ns | 3.6 ± 0.5ns | 22.0 ± 6.1ns | 38.9 ± 14.7ns | 1.3 ± 0.6ns | 1.6 ± 0.8ns | 2.9 ± 1.4ns |
| TAM01 applied to the post-planting substrate | 24.6 ± 3.3ns | 3.9 ± 0.8∗ | 21.0 ± 8.2ns | 53.2 ± 13.1∗ | 1.8 ± 0.8∗ | 2.4 ± 1.5∗ | 4.1 ± 2.3∗ |
| TAM02 applied to the pre-planting substrate | 22.4 ± 3.9ns | 3.4 ± 0.6ns | 23.5 ± 6.1ns | 44.6 ± 14.8ns | 1.3 ± 0.5ns | 2.1 ± 1.0ns | 3.4 ± 1.4ns |
| TAM02 applied to the post-planting substrate | 20.9 ± 5.2ns | 3.6 ± 0.5ns | 20.4 ± 5.4ns | 46.8 ± 8.7∗ | 1.4 ± 0.6ns | 1.6 ± 0.9ns | 2.2 ± 1.5ns |
| TAM03 applied to the pre-planting substrate | 23.7 ± 3.9ns | 3.7 ± 0.3ns | 19.6 ± 5.8ns | 43.9 ± 14.2ns | 1.4 ± 0.4ns | 1.9 ± 0.7ns | 3.3 ± 0.9ns |
| TAM03 applied to the post-planting substrate | 26.3 ± 6.8ns | 3.7 ± 0.5ns | 21.0 ± 8.6ns | 44.3 ± 10.4ns | 1.4 ± 0.8ns | 1.7 ± 1.4ns | 3.1 ± 2.2ns |
| Tce applied to the pre-planting substrate | 20.4 ± 2.6ns | 3.4 ± 0.5ns | 20.5 ± 5.5ns | 33.2 ± 13.5ns | 1.1 ± 0.3ns | 1.4 ± 0.8ns | 2.5 ± 1.1ns |
| Tce applied to the post-planting substrate | 26.4 ± 3.2ns | 3.8 ± 0.4ns | 25.8 ± 5.7ns | 47.8 ± 7.6∗ | 1.5 ± 0.7ns | 2.0 ± 1.0ns | 3.5 ± 1.6ns |
| Control treatment | 21.6 ± 2.6 | 3.4 ± 0.4 | 19.6 ± 5.8 | 5.8 ± 8.6 | 1.2 ± 0.4 | 1.3 ± 0.7 | 2.5 ± 1.0 |
∗ significant at a 5% probability level; ns non-significant by Dunnet's test. APDM: aerial part dry matter; RDM: root dry matter; TDM: total dry matter.
Similar results, regarding improved biomass of plants treated with T. asperellum isolate TAM01, have been reported by Amaral et al. [52], who evaluated the effect of Trichoderma asperelloides on caroba (Jacaranda micrantha) seedlings 90 days after planting. The results of this study demonstrated a mean dry mass of 129.8 mg when the fungus was applied, compared to a mean dry mass of 48.2 mg obtained for the control treatment. Carvalho Filho et al. [56] tested the effects of T. asperellum CEN 162 and T. harzianum CEN 262 on seedling growth for a hybrid eucalyptus clone, and they recorded an improved average plant aerial portion and root dry weight in seedlings treated with these fungi. Soldan et al. [55] also reported increases in biomass when they tested an isolate of Trichoderma spp. and Trichonat PM in the development of Eugenia pyriformis and Myrcianthes pungens seedlings. Machado et al. [22] evaluated the effects of Trichoderma spp. isolates on the emergence and growth of cambará (G. polymorpha) seedlings. The authors reported that the isolate 2B22 (T. harzianum), applied at 4 g of biological powder per kg of substrate prior to planting, provided higher average aerial part dry mass and root dry mass when compared to the control treatment in both sterile and non-sterile substrates.
The production of metabolites by Trichoderma fungi is currently one of the major factors thought to promote seedling growth. These metabolites are thought to foster the multiplication of plant cells and, consequently, increase plant biomass [57]. Different isolates of Trichoderma spp. affect plants differently, and variable metabolite regulation and production might explain this observation. That said, many isolates are thought to increase nutrient absorption and thus biomass production, resulting in better seedling growth outcomes [32]. Although the effect of indole-3-acetic acid, a metabolite commonly produced by Trichoderma isolates, is not evaluated in this study, some authors attribute observed growth benefits in seedlings treated with Trichoderma species to improved nutrient solubility imparted by this compound [55, 56, 58].
The results recorded at 365 days showed that successive applications are necessary to maintain the observed positive growth effects of Trichoderma isolates on H. serratifolius. However, even with the monthly applications, only isolates of T. asperellum TAM01 and TAM02 increased at least one of the variables analyzed with respect to the control after 180 days of planting. It is important to emphasize that the fungi tested up to 180 days (seedling residence time in the nursery) provided an increase in one (TAM02 and Tce) or in all analyzed variables (TAM01 and TAM03) with monthly applications to the post-planting substrate. This demonstrates the importance of the fungus during the early period of plant development.
The presence of Trichoderma isolates in the substrate may be key for plant development after planting or transplanting, when seedlings are more exposed to pathogens [3]. This association between nutrient absorption in the roots and Trichoderma isolates is consistent with the current understanding of the nature of Trichoderma symbiosis, which is focused different levels of the root region [54]. Trichoderma lineages vary with respect to biocontrol activities, host action spectrum, physiological and biochemical properties, and ecological and environmental adaptability. Furthermore, varied plant development effects may also be due to culture type, inoculation dose, development conditions, and formulation type [25, 59]. All of these factors could also explain the variable performance of isolates in promoting growth [3].
Some strains of the fungus Trichoderma establish robust and lasting root surface colonization and penetrate the epidermis. Some fungal cells can even associate with deeper tissue levels for longer periods of time [33]. Strains that establish lasting interactions with the plant are considered the most effective in biological control and growth promoting applications because their effects remain throughout most of the plant's life cycle [60].
Root colonization by Trichoderma spp. induces root system growth and development, while also enhancing crop yield, resistance to abiotic stresses and nutrient uptake and use [33]. Moreover, the fungus’ role is decomposition makes absorbable nutrients readily available to the associated plant. In addition, as the fungus grows it competes with ecological phytopathogens thus acting as a biocontrol agent and promoting normal plant development [61]. Such characteristics are important for their use in biological treatment.
In this study, three of the four Trichoderma isolates demonstrated significant effects on the root growth of H. serratifolius seedlings when applied post-planting in monthly treatments, as determined after 365 days. This increased root development provides a strong indication that the roots and the fungus were directly interacting. Although still scarce in forest areas, other studies have also identified positive effects on root growth. For example, T. harzianum isolate treatments led to longer roots in studies with Pinus radiata and G. polymorpha seedlings [22, 53].
At 365 days after planting, significant differences were observed for the variable primary effects, as well as for their interactions (Trichoderma isolates and application strategies, as observed by Tukey test), with respect to plant height, number of leaves, root length, root dry mass, and total dry mass. Trichoderma sp. Tce was more efficient when applied to the substrate after planting with the exception of improved dry mass of the root (Table 4).
Table 4.
Height, number of leaves, root length, root dry mass and total dry mass of Handroanthus serratifolius individuals that received applications of Trichoderma sp. Isolates at 365 after planting, in two different strategies, pre and post planting.
| Trichoderma isolates | Application strategy |
|
|---|---|---|
| Pre-planting substrate |
Post planting substrate |
|
| Plant height (cm) | ||
| TAM01 | 22.4 aA | 24.6 abA |
| TAM02 | 22.4 aA | 20.9 bA |
| TAM03 | 23.7 aA | 26.3 aA |
| Tce | 20.4 aB | 26.4 aA |
| CV (%) | 13.3 | |
| Number of leaves | ||
| TAM01 | 22.0 aA | 21.0 aA |
| TAM02 | 23.5 aA | 20.4 aA |
| TAM03 | 19.6 aA | 21.0 aA |
| Tce | 20.5 aB | 25.9 aA |
| CV (%) | 19.7 | |
| Root length | ||
| TAM01 | 38.9 abA | 53.1 aA |
| TAM02 | 44.6 aA | 46.8 aA |
| TAM03 | 44.0 aA | 44.3 aA |
| Tce | 33.2 bB | 47.8 aA |
| CV (%) | 17.4 | |
| Dry root mass | ||
| TAM01 | 15.8 aB | 23.4 aA |
| TAM02 | 21.0 aA | 16.3 aA |
| TAM03 | 18.9 aA | 17.4 aA |
| Tce | 14.1 aA | 20.0 aA |
| CV (%) | 37.2 | |
| Total dry mass | ||
| TAM01 | 28.8 aB | 41.0 aA |
| TAM02 | 34.0 aA | 29.9 bA |
| TAM03 | 32.6 aA | 31.5 abA |
| Tce | 25.4 aB | 34.9 abA |
| CV (%) | 26.5 | |
Means followed by the same lower case letters in the columns and the same upper case letters in the rows do not differ from each other by the Tukey test (p ≤ 0.05).
The results of the evaluation performed at 180 and 365 days after planting with regards to previously discussed variables in addition to the Dickson Quality Index (DQI) are shown in Table 5. T. asperellum TAM01 isolates applied to the substrate after planting proved to be the treatment that provided the highest DQI after 365 days (Table 5). Azevedo et al. [54] also recorded maximum DQI value changes with regards to the control as a result of Trichoderma treatment. That particular study evaluated the effects of the fungus on seedlings of Eucalyptus camaldulensis clones.
Table 5.
Relationships between the variables obtained at 180 and 365 days after planting and Dickson quality index of Handroanthus serratifolius seedlings.
| Treatments | Evaluation period |
|||||
|---|---|---|---|---|---|---|
| 180 days |
365 days |
|||||
| H/SD | H/SD | H/DSM | H/DRM | DSM/DRM | DQI | |
| TAM01 applied in pre-planting substrate | 6.2ns | 6.4ns | 20.8ns | 22.8ns | 0.71ns | 0.46ns |
| TAM01 applied in post-planting substrate | 5.8ns | 6.4ns | 16.6ns | 20.2ns | 0.64ns | 0.70∗ |
| TAM02 applied in pre-planting substrate | 6.3ns | 6.4ns | 28.2ns | 32.0ns | 0.67ns | 0.44ns |
| TAM02 applied in post-planting substrate | 5.7ns | 6.1ns | 16.8ns | 27.8ns | 0.76ns | 0.51ns |
| TAM03 applied in pre-planting substrate | 6.3ns | 6.9ns | 21.2ns | 23.9ns | 0.62ns | 0.44ns |
| TAM03 applied in post-planting substrate | 5.7ns | 6.3ns | 19.2ns | 28.9ns | 0.69ns | 0.52ns |
| Tce applied in pre-planting substrate | 6.5ns | 5.6ns | 20.6ns | 24.6ns | 0.67ns | 0.44ns |
| Tce applied in post-planting substrate | 5.7ns | 6.5ns | 18.3ns | 24.5ns | 0.70ns | 0.57ns |
| Control | 6.3 | 6.7 | 25.1 | 32.2 | 0.78 | 0.33 |
∗ significant and ns non-significant, by Dunnett's test (p ≤ 0.05); H: height; SD: stem diameter; DSM: aerial part dry mass; DRM: root dry mass; DQI: Dickson Quality Score.
DQI is considered an important indicator in seedling quality evaluation. It is a composite metric taking into account the H/SD ratio, indicating robustness, and the DSM/DRM rate, indicates the balance of biomass distribution [62, 63]. Eloy et al. [64] evaluated the quality of Eucalyptus grandis seedlings and reported a high correlation between DQI and the other metrics. This confirms the importance of DQI as a tool to qualitatively evaluate the seedling development of forest species.
In agreement with the results of this study, Chandra Nayaka et al. [65] reported that Trichoderma spp. may increase plant growth, which is of great importance for the production and maintenance of forest seedlings. Given the economic and environmental importance of native forest species, it is critical to improve silvicultural techniques related to seedling production in nurseries. This would then allow for researchers to carry out other studies involving the promotion of forest seedling growth using bioagents such as Trichoderma [22].
The use of Trichoderma is a promising alternative strategy to chemical treatments for the improvement of seedling growth in forest nurseries, especially in the early stages of plant development. Beneficial effects can be provided in the stages of germination, emergence, seedling development and fruit production for many plant species [61]. In addition, Trichoderma may contribute to a reduction in fungicide use, as it could serve as an alternative biocontrol agent for phytopathogenic fungi [54]. Trichoderma could also improve soil fertility [66], increase nutrient content in forest nurseries, i.e. phosphorus [67] or nitrogen [68], and ultimately contribute to greater forestry sustainability [54].
5. Conclusion
Under laboratory conditions, H. serratifolius seeds treated with Trichoderma (TAM03) obtained the greatest germination rate and germination speed index.
In a nursery setting, isolates TAM01 and TAM03, applied to the substrate post-planting, increased the height, stem diameter, and number of leaves present on seedlings, as evaluated 180 days after planting. After 365 days, TAM01, applied to the substrate post-planting, provided an increase in root length and of the aerial portion of the plant, as well as an increase in root and total dry mass. The fungus T. asperellum TAM01 demonstrated the largest improvements for plant development.
Handroanthus serratifolius seedlings evaluated 180 days after planting showed greater sensitivity to biological treatment than after 365 days. This demonstrates the efficacy of Trichoderma treatment on the seedling production of this forest species, greatly reducing the need for chemical treatments. The results of this study indicate that a monthly, treatment with Trichoderma isolates after planting results in optimal conditions for the development of H. serratifolius seedlings. In conclusion, the research presented here indicates that, under laboratory and nursery conditions, Trichoderma isolate treatments can provide an effective, chemical-free strategy to promoting the growth of H. serratifolius seedlings. However, further research is recommended to investigate the behavior of individuals of this species under field conditions.
Declarations
Author contribution statement
M. Freitas dos Santos: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
L. Elizeário dos Santos: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
D. Lima da Costa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
T. Almeida Vieira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
D. Castro Lustosa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Institutional Scientific Initiation Scholarship Program ( PIBIC/UFOPA).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the anonymous reviewers for their suggestions and constructive comments of the manuscript, and to Michael James Stablein of the University of Illinois Urbana-Champaign for his translation services and review of this paper.
Contributor Information
Thiago Almeida Vieira, Email: thiago.vieira@ufopa.edu.br.
Denise Castro Lustosa, Email: denise.lustosa@ufopa.edu.br.
References
- 1.Angelo H., Pompermayer R.S., Almeida N.A., Magalhães J.M., Moreira A.P. O custo social do desmatamento da Amazônia Brasileira: o caso da Castanha-do-Brasil (Bertholletia excelsa) Ciênc. Florest. 2013;23:183–191. [Google Scholar]
- 2.Arraes R.A., Mariano F.Z., Simonassi A.G. Causas do Desmatamento no Brasil e seu ordenamento no contexto mundial. Rev. Econ. Sociol. Rural. 2012;50:119–140. [Google Scholar]
- 3.Junges E., Muniz M.F., Mezzomo R., Bastos B., Machado R.T. Trichoderma spp. na produção de mudas de espécies florestais. Floresta Amb. 2016;23:237–244. [Google Scholar]
- 4.Campos Filho E.M., Sartorelli P.A.R. first ed. INPUT; São Paulo: 2015. Guia de árvores com valor econômico. [Google Scholar]
- 5.Carrero G.C., Pereira R.S., Jacaúna M.A., Lima Júnior M.J.V. second ed. IDESAM; Manaus: 2014. Árvores do Sul do Amazonas: guia de espécies de interesse econômico e ecológico. [Google Scholar]
- 6.Gentry A.H. Bignoniaceae - part. II. (Tribe tecomae) Flora Neotrop. Monogr. 1992;25:1–370. http://www.jstor.org/stable/4393739 [Google Scholar]
- 7.Segoloni E., Di Maria F. UV–VIS spectral and GC–MS characterization of Handroanthus serratifolius (Vahl.) Grose (a.k.a. Tabebuia serratifolia (Vahl.) Nichols/Lapacho) heartwood main extractives: a comparison of protocols aimed at a practical evaluation of Lapachol and Dehydro-α-Lapachone content. Eur. J. Wood Wood Prod. 2018;76:1547–1561. [Google Scholar]
- 8.Oliveira M.F., Lemos T.L.G., Mattos M.C., Segundo T.A., Santiago G.M.P., Braz-Filho R. New enamine derivatives of lapachol and biological activity. An. Acad. Bras. Ciênc. 2002;74:211–221. doi: 10.1590/s0001-37652002000200004. [DOI] [PubMed] [Google Scholar]
- 9.Salustiano E.S.J., Netto C.D., Fernandes R.F., Silva A.J.M., Bacelar T.S., Castro C.P., Buarque C.D., Maia R.C., Rumjamanek V.M., Costs P.R.R. Comparison of the cytotoxic effect of lapachol, alphalapachone and pentacyclic 1,4-naphthoquinones on human leukemic cells. Invest. New Drug. 2010;28:139–144. doi: 10.1007/s10637-009-9231-y. [DOI] [PubMed] [Google Scholar]
- 10.Lemos T.L.G., Monte F.J.Q., Santos A.K.L., Fonseca A.M., Santos H.S., Oliveira M.F., Costa S.M.O., Pessoa O.D.L., Braz-Filho R. Quinones from plants of north-eastern Brazil: structural diversity, chemical transformation, NMR data and biological activities. Nat. Prod. Res. 2007;21:529–550. doi: 10.1080/14786410601130604. [DOI] [PubMed] [Google Scholar]
- 11.Romagnoli M., Segoloni E., Luna M., Margaritelli A., Gatti M., Santamaria U., Vinciguerra V. Wood color in Lapacho (Tabebuia serratifolia): chemical composition and industrial implications. Wood Sci. Technol. 2013;47:701–716. [Google Scholar]
- 12.Azevedo C., Sanquetta C. Efeito da exploração de madeira e dos tratamentos silviculturais no agrupamento ecológico de espécies. Floresta. 2008;2:53–69. [Google Scholar]
- 13.Lorenzi H. Instituto Plantarum; Nova Odessa: 2008. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. 5. [Google Scholar]
- 14.Ferreira L., Chalub D., Muxfeldt R. Vol. 5. Informativo Técnico Rede de Sementes da Amazônia; 2004. (Ipê-amarelo: Tabebuia Serratifolia (Vahl) Nichols. Manaus: Inpa). [Google Scholar]
- 15.Dousseau S., Alvarenga A.A., Castro E.M., Soares R.P., Emrich E.B., Melo L.A. Anatomia foliar de Tabebuia serratifolia (Vahl) Nich. (Bignoniaceae) propagadas in vitro, in vivo e durante a aclimatização. Ciênc. agrotec. 2008;32:1694–1700. [Google Scholar]
- 16.Vieira C., Weber O. Saturação por bases no crescimento e na nutrição de mudas de ipê-amarelo. Floresta e Ambiente. 2017;24:e20160019. [Google Scholar]
- 17.Oliveira A.R., Boechat C.L., Amorim S.P.N., Souza M.E.L., Duarte L.S.L., Silva H.F. Growth and quality of Handroanthus serratifolius seedlings in soils incorporating amendments and inorganic residues. Rev. Ceres. 2019;22:235–242. [Google Scholar]
- 18.Goulart L.M.L., Paiva H.N., Leite H.G., Xavier A., Duarte M.L. Produção de Mudas de Ipê-amarelo (Tabebuia serratifolia) em resposta a fertilização nitrogenada. Floresta e Ambiente. 2017;24:e00137315. [Google Scholar]
- 19.Silva D.G., Carvalho M.L.M., Nery M.C., Oliveira L.M., Caldeira C.M. Alterações fisiológicas e bioquímicas durante o armazenamento de sementes de Tabebuia serratifolia. CERNE. 2011;17:1–7. [Google Scholar]
- 20.Schulze M., Grogan J., Landis R.M., Vidal E. How rare is too rare to harvest?: management challenges posed by timber species occurring at low densities in the Brazilian Amazon. For. Ecol. Manag. 2008;256:1443–1457. [Google Scholar]
- 21.Herrero-Jáuregui C., Guariguata M.R., Cárdenas D., Vilanova E., Robles M., Licona J.C., Nalvarte W. Assessing the extent of “conflict of use” in multipurpose tropical forest trees: a regional view. J. Environ. Manag. 2013;130:40–47. doi: 10.1016/j.jenvman.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 22.Machado D.F.M., Tavares A.P., Lopes S.J., Silva A.C.F. Trichoderma spp. na emergência e crescimento de mudas de cambará (Gochnatia polymorpha (Less.) Cabrera) Rev. Árvore. 2015;39:167–176. [Google Scholar]
- 23.Silva R.F., Eitelwein M.T., Cherubin M.R., Fabbris C., Weirich S., Pinheiro R.R. Produção de mudas de Eucalyptus grandis em substratos orgânicos alternativos. Ciênc. Florest. 2014;24:609–619. [Google Scholar]
- 24.Gasparin E., Avila A.L., Araujo M.M., Cargnelutti Filho A., Dorneles D.U., Foltz D.R.B. Influência do substrato e do volume de recipiente na qualidade das mudas de Cabralea canjerana (Vell.) Mart. em viveiro e no campo. Ciênc. Florest. 2014;24:553–563. [Google Scholar]
- 25.Menten J.O., Moraes M.H.D. Avanços no tratamento e recobrimento de sementes tratamento de sementes: histórico, tipos, características e benefícios. Informativo ABRATES. 2010;20:52–53. [Google Scholar]
- 26.Lucon C.M.M. Artigo em Hypertexto; 2009. Promoção de crescimento de plantas com o uso de Trichoderma spp.http://www.infobibos.com/Artigos/2009_1/trichoderma/index.htm Avaliable in. [Google Scholar]
- 27.Cavero P.A.S., Hananda R.E., Gasparotto L., Coelho Neto R.A., Souza J.T. Biological control of banana black Sigatoka disease with Trichoderma. Ciência Rural. 2015;45:951–957. [Google Scholar]
- 28.Silva A.N., Azevedo G.B., Rocha Sobrinho G.G., Novaes Q.S. Efeito de produtos químicos e de Trichoderma spp. no controle de Fusarium solani do maracujazeiro. Interciencia. 2014;39:398–403. [Google Scholar]
- 29.Machado D.F.M., Parzianello F.R., Silva A.C.F., Antoniolli Z.I. Trichoderma no Brasil: o fungo e o bioagente. Rev. de Ciências Agrárias. 2012;35:274–288. [Google Scholar]
- 30.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species – opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 31.Reichert Júnior F.W., Scariot M.A., Forte C.T., Pandolfi L., Dil J.M., Weirich S., Carezia C., Mulinari J., Mazutti M.A., Fongaro G., Galon L., Treichel H., Mossi A.J. New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon. 2019;5:e01676. doi: 10.1016/j.heliyon.2019.e01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoresh M., Harman G., Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 33.Saba H., Vibhash D., Manisha M., Prashant K.S., Farhan H., Tauseef A. Trichoderma – a promising plant growth stimulator and biocontrol agent. Mycosphere. 2012;3:524–531. [Google Scholar]
- 34.Mukherjee P.K., Horwitz B.A., Herrera-Estrella A., Schmoll M., Kenerley C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013;51:105–129. doi: 10.1146/annurev-phyto-082712-102353. [DOI] [PubMed] [Google Scholar]
- 35.Fortes F.O., Silva A.C.F., Almança M.A.K., Tedesco S.B. Promoção de enraizamento de microestacas de um clone de Eucalyptus sp. por Trichoderma spp. Rev. Árvore. 2007;31:221–228. [Google Scholar]
- 36.Santos M.F., Costa D.L., Vieira T.A., Lustosa D.C. Effect of Trichoderma spp. fungus for production of seedlings in Enterolobium Schomburgkii (Benth.) Benth. Aust. J. Crop. Sci. 2019;13:1706–1711. [Google Scholar]
- 37.Lustosa D.C., Araújo A.J.C., Campo B.F., Vieira T.A. Trichoderma spp. and its effects on seeds physiological quality and seedlings development of African mahogany. Rev. Bras. Cienc. Agrar. 2020;15:e5843. [Google Scholar]
- 38.Kumar G., Maharshi A., Patel J., Mukherjee A., Singh H.B., Sarma B.K. Trichoderma: a potential fungal antagonist to control plant diseases. SATSA Mukhaptra - Ann. Tech. Iss. 2017;21:206–218. [Google Scholar]
- 39.Alvares C.A., Stape J.L., Sentelhas P.C., Gonçalves J.L.M., Sparovek G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2013;22:711–728. [Google Scholar]
- 40.Santarém Santarém. 2019. Meio Ambiente – Clima. Santarém: PMS. [Google Scholar]
- 41.Maguire J.D. Speed of germination - aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962;2:176–177. [Google Scholar]
- 42.Dickson A., Leaf A.L., Hosner J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960;36:10–13. [Google Scholar]
- 43.Ferreira E.B., Cavalcanti P.P., Nogueira D.A. ExpDes: an R package for ANOVA and experimental designs. Appl. Math. 2014;19:2952–2958. [Google Scholar]
- 44.Pereira F.T., Oliveira J.B., Muniz P.H.P.C., Peixoto G.H.S.P., Guimarães R.R., Carvalho D.D.C. Growth promotion and productivity of lettuce using Trichoderma spp. commercial strains. Hortic. Bras. 2019;37:69–74. [Google Scholar]
- 45.Melo I.S. Agentes microbianos de controle de fungos fitopatogênicos. In: Melo I.S., Azevedo J.L., editors. Controle Biológico. Jaguariúna: Embrapa. 1998. pp. 17–60. [Google Scholar]
- 46.Santos M.F., Costa D.L., Matos J.C.N., Silva G.B., Vieira T.A., Lustosa D.C. Tratamento biológico de sementes de cupuaçu para o controle de fitopatógenos e promoção da germinação. Cadernos de Agroecologia. 2018;13 [Google Scholar]
- 47.Oliveira L.M., Carvalho M.L.M., Silva T.T.A., Borges D.I. Temperatura e regime de luz na germinação de sementes de Tabebuia impetiginosa (Martius ex A. P. de Candolle) Standley e T. serratifolia Vahl Nich. – Bignoniaceae. Ciênc. agrotec. 2005;29:642–648. [Google Scholar]
- 48.Junges E., Menezes J.P., Manzoni C.G., Flores R., Garlet T.M.B., Menezes N.L., Muniz M.F.B., Blume E. Simpósio de Ensino Pesquisa e Extensão, 15. Anais. Unifra; Santa Maria: 2011. Microbiolização com Trichoderma sp. na germinação e vigor de sementes de soja. [Google Scholar]
- 49.Patekoski K.S., Pires-Zottarelli C.A. Patogenicidade de Pythium aphanidermatum a alface cultivada em hidroponia e seu biocontrole com Trichoderma. Pesq. agropec. bras. 2010;45:805–810. [Google Scholar]
- 50.Migliorini P., Kulczynki S.M., Silva T.A., Bellé C., Koch F. Efeito do tratamento químico e biológico na qualidade fisiológica e sanitária de sementes de canola. Enciclopédia Biosfera. 2012;8:788–801. [Google Scholar]
- 51.Pereira F.B. 2017. Fungos Promotores de Crescimento e Produção de mudas de Pinus taeda L. MSc. Thesis. [Google Scholar]
- 52.Amaral P.P., Steffen G.P.K., Maldaner J., Missio E.L., Saldanha C.W. Promotores de crescimento na propagação de caroba. Pesq. flor. bras. 2017;37:149–157. [Google Scholar]
- 53.Donoso E., Lobos G.A., Rojas N. Efecto de Trichoderma harzianum y compost sobre el crecimiento de plántulas de Pinus radiata em viveiro. Bosque. 2008;29:52–57. [Google Scholar]
- 54.Azevedo G.B., Novaes Q.S., Azevedo G.T.O.S., Silva H.F., Sobrinho G.G.R., Novaes A.B. Efeito de Trichoderma spp. no crescimento de mudas clonais de Eucalyptus camaldulensis. Sci. For. 2017;45:343–352. [Google Scholar]
- 55.Soldan A., Watzlawick L.F., Botelho R.V., Faria C.M.D.R., Maia A.J. Development of forestry species inoculated with Trichoderma spp. Fertilized with rock phosphate. Floresta Amb. 2018;25:1–8. [Google Scholar]
- 56.Carvalho Filho M.R., Mello S.C.M., Santos R.P., Menêzes J.E. Embrapa Recursos Genéticos e Biotecnologia; 2008. Avaliação de Isolados de Trichoderma na Promoção de Crescimento, Produção de Ácido Indolacético in vitro e Colonização Endofítica de Mudas de Eucalipto. Brasília. [Google Scholar]
- 57.Aguiar A.R., Aguiar D., Tedesco S.B., Silva A.C.F. Efeito de metabólitos produzidos por Trichoderma spp. sobre o índice mitótico em células das pontas de raízes de Allium cepa. Biosci. J. 2015;31:934–940. [Google Scholar]
- 58.Hoyos-Carvajal L., Orduz S.E., Bissett J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Contr. 2009;51:409–416. [Google Scholar]
- 59.Stewart A., Hill R. Applications of Trichoderma in plant growth promotion. In: Gupta V.K., Schmoll M., Herrera-Estrella A., Upadhyay R.S., editors. Druzhinina I, Tuohy MG. Biotechnology and Biology of Trichoderma. Elsevier USA; Boston: 2014. pp. 415–428. [Google Scholar]
- 60.Harman G.E. Myth and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000;84:377–393. doi: 10.1094/PDIS.2000.84.4.377. [DOI] [PubMed] [Google Scholar]
- 61.Santos H.A. 2008. Trichoderma spp. como promotores de crescimento em plantas e como antagonista a Fusarium oxysporum. MSc. Thesis. [Google Scholar]
- 62.Caldeira M.V.W., Spathelf P., Barichello L.R., Vogel H.L.M., Schumacher M.V. Effect of different doses of vermicompost on the growth of Apuleia leiocarpa (Vog) Macbr. seedlings. Revista Acadêmica: Ciência animal. 2005;3:11–17. [Google Scholar]
- 63.Caldeira M.V.W., Marcolin M., Moraes E., Schaadt S.S. Influência do resíduo da indústria do algodão na formulação de substrato para produção de mudas de Schinus terebinthifolius Raddi, Archontophoenix alexandrae Wendl. et Drude e Archontophoenix cunninghamiana Wendl. et Drude. Ambiência. 2007;3:1–8. [Google Scholar]
- 64.Eloy E., Caron B.O., Schmidt D., Behling A., Schwers L., Elli E.F. Avaliação da qualidade de mudas de Eucalyptus grandis utilizando parâmetros morfológicos. Floresta. 2013;43:373–384. [Google Scholar]
- 65.Chandra Nayaka S., Niranjana S.R., Uday Shankar A.C., Niranjan Raj S., Reddy M.S., Prakash H.S., Mortensen C.N. Seed biopriming with novel strain of Trichoderma harzianum for the control of toxigenic Fusarium verticillioides and fumonisins in maize. Arch. Phytopathol. Plant Protect. 2010;43:264–282. [Google Scholar]
- 66.Rosmana A., Taufik M., Asman A., Jayanti N.J., Hakkar A.A. Dynamic of vascular Streak dieback disease incidence on Susceptible cacao treated with composted plant residues and Trichoderma asperellum in field. Agronomy. 2019;9:650. [Google Scholar]
- 67.Promwee A., Issarakraisila M., Chamswarng C., Yenjit P. Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma Strains. J. Agric. Sci. 2014;6:8–20. [Google Scholar]
- 68.Rosmana A., Sakrabani R., Sjam S., Nasaruddin N., Asman A., Pandin B.Y.S. Plant residue based-composts applied in combination with Trichoderma asperellum improve cacao seedling growth in soil derived from nickel mine area. J. Anim. Plant Sci. 2019;29:291–298. [Google Scholar]