Graphical abstract
Keywords: Whey, Lactose, Succinic acid, Batch fermentation, Actinobacillus succinogenes
Highlights
-
•
A productivity of 0.9 g L−1 h−1 and a yield of 65% were obtained with 25 g L-1 of lactose.
-
•
A productivity of 0.61 g L−1 h−1 and a yield of 61.1% were obtained with 35 g L-1 of whey.
-
•
The best productivity and yield was achieved lactose in comparison with whey.
-
•
The adequate rate of CO2 suitable for the batch results was 0.4 ppm.
Abstract
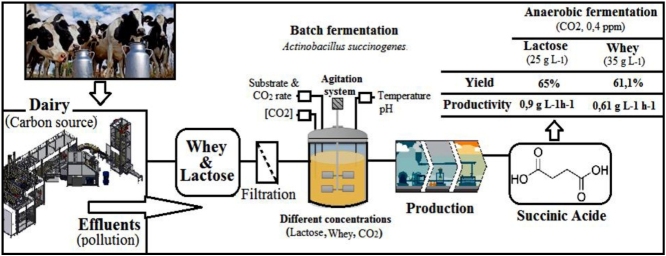
This study focuses on succinic acid production by Actinobacillus succinogenes in batch fermentation from whey and lactose widely encountered in dairy effluents. The effects of initial whey and lactose concentration, CO2 rate on succinic acid production were investigated.
The optimal succinic acid production was obtained with 25 g L−1 of lactose and 35 g L−1 of whey with yields and productivities respectively of 65% and 0.9 g L−1 h−1 for lactose and 62.1%, 0.81 g L−1 h−1 for whey. The maximum yield and productivity of succinic acid was obtained with lactose in comparison with whey.
Productivity and yield decreased when the amount of initial lactose was increased. Biomass, acetic acid and formic acid increased when whey was used as a substrate compared to lactose.
Succinic acid production by anaerobic fermentation is a green biotechnology alternative to valorize whey and lactose from dairy effluent and to reduce their impact on the environment.
1. Introduction
Dairy industry is a one of the highest water consumers producing large quantities of liquid effluents. Water is used in all stages of milk processing including cleaning, cooling and washing [[1], [2], [3]]. It uses about 0.2–10 liters of water per liter of processed milk [4] producing about 2.5 L of wastewater per liter of processed milk [4,5]. Because of their relatively high organic load with a range of BOD (biological oxygen demand) and COD (chemical oxygen demand) of 0.1−100 g L−1 [6], the dairy effluent are causing pollution of aquatic and terrestrial ecosystems and an imbalance of flora and fauna and other serious problems in receiving environments.
The environmental impact of dairy effluents is mainly due to their richness in whey [7], which is produced in large quantities as a by-product in the cheese and milk industry [8]. The worldwide whey production is estimated at around 190–200 Mt year−1 [9,10].
Whey is the residual liquid remaining after coagulation of milk during cheese manufacture and milk processing [11].
It is saturated in organic matter mainly presented by lactose (4.5–6% w/v) [[11], [12], [13]], proteins (0.6−0.8% w/v), lipids (0.4–1% w/v) [6,11,13] and mineral salts (8–10% of dried extract) [11].
This organic load is the cause of high COD and BOD, which can reach a range of 50−102 g L−1 and 27−60 g L−1 respectively [6,11].
Several studies have focused on the treatment or valorization of whey or these compounds. Whey can be treated not only by physicochemical and / or biological processes [[14], [15], [16], [17]], but also by recovery and valorization of its constituents. For its undeniable importance, whey can be valorized by bioethanol production [[18], [19], [20]], as well as the use of whey protein as a health promoter [10].
This work concerns the valorization of whey and lactose from dairy effluents by succinic acid production by Actinobacillus succinogenes.
Succinic acid (SA) is a product of cellular metabolism; it is the end product of anaerobic metabolism in the Krebs cycle [21]. Given the great demand of SA in various fields such as food processing or chemical and pharmaceutical industry [22], succinic acid production is done in high yield and purity by chemical technologies like catalytic hydrogenation or electrolytic reduction of maleic anhydride [22,23].
However, the chemical production of SA is expensive and generates greenhouse gas, which exacerbates the impact on the environment further aggravating climate change. This is why several studies have focused on the fermentation of numerous carbon sources by specific microorganisms in order to produce SA. As an example, we cite pure simple sugars like xylose [24] or glucose [21,25], glycerol [26], lignocellulosic biomass [27], or whey [28,29]. Some sources matter require pretreatment to facilitate the assimilation of simple sugars by bacteria [30].
This study focuses on succinic acid production using lactose and whey from dairy effluent by batch anaerobic fermentation. Several parameters like CO2 rate, substrate concentration are studied in order to illustrate their influence on SA production.
2. Material and methods
2.1. Chemicals and strain cultivation
All chemicals used in this study were purchased from Sigma-Aldrich. The strain used for the fermentation and succinic acid production from whey and lactose is Actinobacillus succinogenes 130Z (ATCC 55618).
The strain was stored at −80 °C in 2 mL cryogenic tubes containing a mixture of tryptone soy broth (TSB) sterile medium supplemented with 20% (w/w) sterile glycerol.
The inoculum was activated by reviving a frozen culture of A. succinogenes in TSB sterile medium at 1% and was anaerobically incubated at 38 °C and 200 rpm [31] for 16−24 h until late exponential phase was reached and confirmed.
2.2. Preparation of lactose and whey
2.2.1. Whey sampling
Whey was collected directly from the rejects of a milk-processing unit. Its physicochemical characterization shows that it is mainly composed by 64.20% of lactose, 6.5% of proteins and 2.8% of fats.
Lactose was obtained by ultrafiltration/discontinuous diafiltration of whey (supplementary material).
2.2.2. Whey drying and concentration
Whey contains approximately 92% of water, its concentration before lyophilization is extremely important to reduce the drying time. Therefore, whey samples are sterilized for 10 min at 110 °C, then the whey was concentrated in a vacuum rotary evaporator type IGNOS at a temperature of 60–70 °C and a pressure of 0.2 to 0.3 bar. Immediately after each concentration, the samples were placed into porcelain capsules of the same thickness less than 10 mm. The samples were frozen for five hours until complete solidification of the liquid.
2.2.3. Whey lyophilization and powder recovery
The whey samples were placed in the chamber of a TELESTAR Cryodos-50 lyophilizer type at a temperature between -45 °C and−55 °C and at a reduced pressure of 10−2 to 10-1 mbar. Then lyophilization was started after activation of the vacuum pump and completed when the vacuum level is below 10−2 mbar. Finally, the powder was recovered in sterile glass bottles with hermetic lid. Concentration, freezing and lyophilization processes were performed throughout the period of our study. Lyophilization was performed in order to obtain whey powder for reconstitute it at the desired concentrations.
2.3. Batch fermentation conditions
Batch fermentation was carried out in a 1 L benchtop bioreactor containing 500 ml of growth medium formulated according to wan et al. [31] with some modifications.
The growth medium composed per 1 L: 3 g K2HPO4, 2 g NaCl, 0.2 g CaCl2 H2O, 0.2 g MgCl2 6H2O and 5 g yeast extract. The medium was sterilized for 15 min at 121 °C.
In order to determine the amount of whey and lactose to use, preliminary batch fermentation experiments were performed in duplicate with different concentrations of sterilized whey ranging from 5, 15, 25, 35, 45–55 g L−1.
Preliminary results have shown the ability of Actinobacillus succinogenes to produce SA from whey. SA yields and productivities were too low with 5, 15, 25, 45 and 55 g L−1 of whey, (data not shown). Maximum yield and productivity was obtained with 35 g L−1. This is why the initial concentration of whey 35 g L−1 was adopted in the experiments of this work. Since in physicochemical characterization, lactose was present in the whey at 64% (w/w) (data not shown), so a whey concentration of 35 g L−1 corresponds approximately to 22.5 g L−1, so 25 g L−1 and also 50 g L−1 of lactose have been tested.
So, the lactose was added to the medium at 25 g L−1 or 50 g L−1, while the whey was previously sterilized at 110 °C for 10 min and mixed with the medium at 35 g L−1.
The inoculation rate used for all experiments was 2% (v/v). The fermentation was started with a temperature of 38 °C, a pH of 6.8 and stirring speed at 200 rpm. When whey was used as substrate, the sparging of CO2 rate was varied from 0.2, 0.4 and 0.8 vvm to elucidate its effect on SA production.
2.4. Analytical methods
Lactose and fermentation products (succinic, acetic and formic acids) were quantified by high-performance liquid chromatography (HP 1050 series HPLC Hewlett-Packard) using a ION- 300 column and a waters 410 differential refractometer detector. The mobile phase is a 0.0065 N H2SO4 solution at a flow rate of 0.4 mL/min. Column and detector temperatures are maintained at 35 °C.
The yield of succinic acid / substrate was calculated as the quantity of succinic acid produced from 1 g of lactose consumed, it is expressed as a percentage.
The bacterial growth was determined by optical density (OD) measurements at a wavelength of 660 nm using the spectrophotometer.
Biomass is expressed by measuring the dry weight, the samples were centrifuged at 10000 rpm for 5 min, and the solid phase was washed thrice with deionized water and dried at 100 °C to a constant weight.
The statistical significance was evaluated by means of a one-way analysis of variance (ANOVA). Fisher's Least Significant Difference (LSD) test was used to separate significant means from non-significant means at α = 0.05. The data were analyzed by SPSS statistical software for Windows® (version 21.0).
3. Results and discussion
3.1. Batch fermentation of SA from lactose
3.1.1. Variation of bacterial growth and pH during fermentation
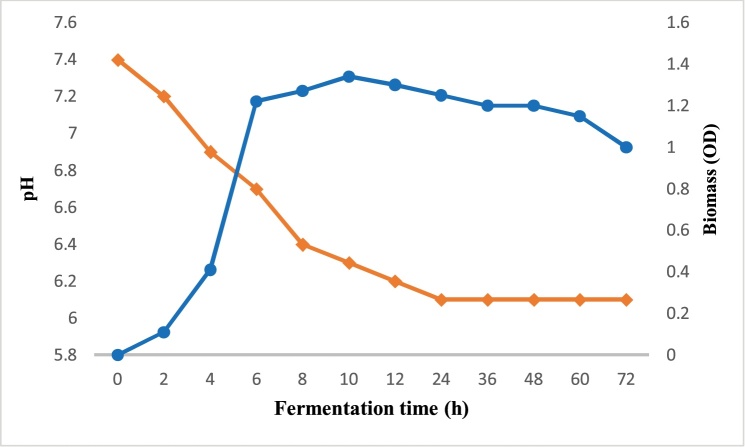
Fig. 1 illustrates the results of pH and the bacterial growth during the anaerobic fermentation of lactose by Actinobacillus succinogenes. The optical density increases during the first 6 h of fermentation to reach a maximum value of 1.29, in parallel; the pH decreased from 7.44 at the start the fermentation to 6.4 at 72 h. The decrease in pH is due to acidification of the medium, which is a consequence of the production of succinic acid as a major fermentation product and other organic acids such as acetic and formic acids (Fig. 2).
Fig. 1.
pH variation and the biomass growth during the anaerobic fermentation of lactose by Actinobacillus succinogenes.
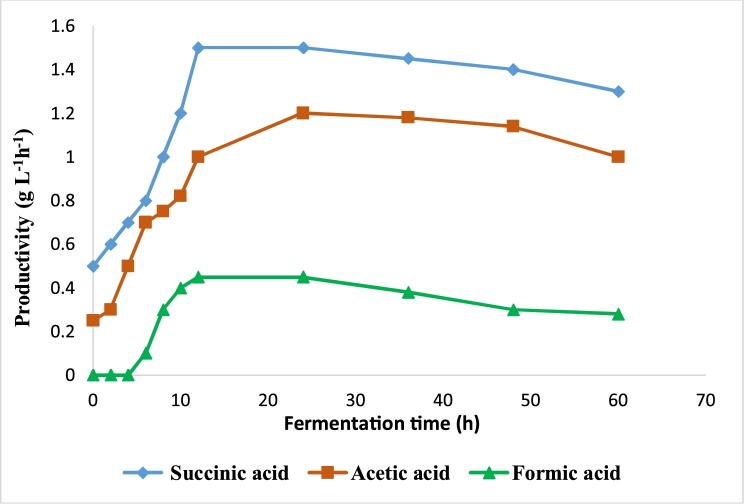
Fig. 2.
Profile of organic acids production during fermentation.
3.1.2. Organic acids production
During lactose fermentation, A. succinogenes produced succinic, acetic and formic acids. No other organic acids, in particular lactic acid, was produced.
The principal product in the fermentation was succinic acid followed by acetic acid and formic acid (Fig. 2). This result shows the capacity of A. succinogenes to ferment lactose after its hydrolysis to glucose and galactose; which are used as carbon source for synthesized and produce these organic acids. Longanesi et al. [29] reports that A. succinogenes prefers to consume glucose, but it is also able to metabolize galactose.
On the other hand, the short duration of the exponential growth phase of A. succinogenes (Fig. 1) can be explained by the acidification of the medium after production of these organic acids.
Indeed, the decrease of pH is a limiting factor on the growth of A. succinogenes and consequently of the production of succinic acid [32,21,33]. Lin et al. [33] was reported that production of only 5 g L−1 of formic acid decreased cell growth of A. succinogenes by 30%. Also Corona-González et al. [21] revealed a reduction in the biomass concentration immediately after 22 g L−1 of acids produced in batch fermentation.
A pH neutralization during fermentation guarantees a longer exponential phase and accordingly an increase in succinic acid production [21,31,34].
3.1.3. Effect of lactose concentration on succinic acid production
In order to determine the influence of lactose concentration on succinic acid production, both concentrations of lactose were used; 25 and 50 g L−1. The CO2 rate used in two cases was 0.4 vvm.
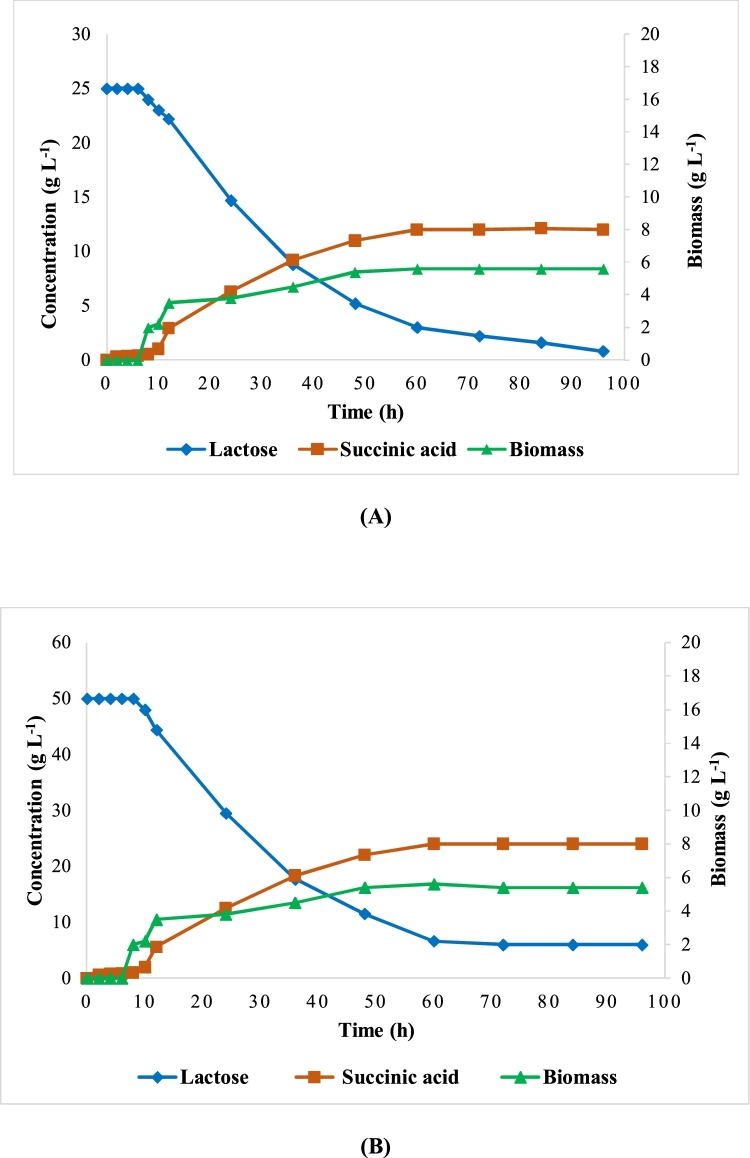
Succinic acid production increases with the increase of lactose consumption to reach high values in SA corresponding to the maximum concentration of lactose consumed at 60 h of fermentation (Fig. 3A and B).
Fig. 3.
Fermentation profiles of succinic acid production by A. succinogenes. Fermentations started with two initial lactose concentration; 25 (A) or 50 g L−1(B).
The increase in the initial concentration of lactose did not induce a change in the latency phase which is 6 h in both cases. Biomass increases simultaneously with lactose consumption and succinic acid production to achieve a maximum value at 60 h of fermentation (Fig. 3A and B).
The plateau started from 60 h and even if the fermentation was followed, there was no change in the values of biomass, SA or lactose.
The maximum SA production is not associated with total lactose consumption, while the production of succinic acid begins to slow down at 48 h even if lactose still exists in the medium. In addition, the amount of lactose remaining at the end of each fermentation is greater when the initial concentration of lactose increases. An inhibitory effect of organic acids and substrate on bacterial growth and succinic acid production can be suggested.
The average values of productivity and yield SA as well as SA/AA and SA/FA ratios are shown in Table 1.
Table 1.
Effect of initial lactose concentration on the production of succinic acid by Actinobacillus succinogenes.
| Initial lactose (g L−1) |
Final lactose (g L−1) |
SA (g L−1) |
Productivity (g L−1 h−1) |
Yield (%) |
SA/AA | SA/FA | Maximal biomass (g L−1) |
|---|---|---|---|---|---|---|---|
| 25 | 0.103±0.006a | 21,23 ± 0.5a | 0,903 ± 0.03a | 65.14 ± 1.69a | 2.56 ± 0.02a | 5.62 ± 0.08a | 7.1 ± 0.04a |
| 50 | 6.64±0.023b | 24.02 ± 0.12b | 0.5 ± 0.009b | 48.4 ± 1.11b | 3.45 ± 0.05b | 6.02 ± 0.03b | 6.52 ± 0.08b |
SA : Succinic Acid. AA : Acetic acid, FA : Formic acid.
Data are means ± SEM of three replications.
The means in a column with different letters are significantly different (α = 0.05).
The increase in the initial concentration of lactose results in a significant increase in succinic acid production with also a significant decrease in the yield and the productivity of SA (Table 1). Thus, the maximum SA yield and productivity are obtained with 25 g L−1 of lactose, which are respectively 65% and 0.9 g L-1 h-1. These values are significantly important compared to 50 g L-1 of lactose where the SA yield and productivity reached only 48.4% and 0.5 g L-1 h-1 respectively.
SA/AA and SA/FA ratios increase with increasing initial concentration of lactose used. With 25 g L−1 of lactose, the SA/AA and SA/FA ratios are respectively 2.56 and 5.61; these values are significantly lower when the concentration of lactose is 50 g L-1, which are respectively 3.4 and 6.01. Indeed, high concentrations of lactose favor the production of succinic acid at the expense of other organic acids (acetic and formic acid) which are fermentation by-products [35].
3.2. Batch fermentation of SA from whey
Actinobacillus succinogenes ATCC55618 has shown its ability to ferment lactose to produce succinic acid with good yield and productivity, the objective of this part is to compare the performance of this strain to produce succinic acid directly from whey.
Several batch fermentations were carried out directly using whey at 35 g L−1, this concentration is equivalent to 25 g L−1 of lactose, and the effect of CO2 sparging rate was is evaluated (Table 2).
Table 2.
Kinetic parameters of succinic acid production by A. succinogenes from whey with different CO2 sparging rates.
| CO2 sparging rates (vvm) |
Final lactose (g L−1) |
SA (g L−1) |
Productivity (g L−1 h−1) |
Yield (%) | SA/AA | SA/FA | Maximal biomass (g L−1) |
|---|---|---|---|---|---|---|---|
| 0.2 | 0 ± 0.0a | 13.98 ± 0.1a | 0.84 ± 0.03 a | 60.67 ± 0.45a | 2.88 ± 0.07a | 4.66 ± 0.04a | 9.2 ± 0.015 a |
| 0.4 | 0 ± 0.0a | 13.46 ± 0.3b | 0.81 ± 0.02a | 62.1 ± 0.26b | 2.49 ± 0.04b | 4.12 ± 0.03b | 10.11 ± 0.0b |
| 0.8 | 0 ± 0.0a | 13.22 ± 0.04b | 0.54 ± 0.04b | 63.2 ± 0.31c | 2.18 ± 0.03c | 3.1 ± 0.052c | 10.23 ± 0.06c |
SA : Succinic Acid. AA : Acetic acid, FA : Formic acid.
Data are means ± SEM of three replications.
In the same column, letters of similar alphabets are not statistically significant (P < 0.05 for LSD).
Whatever the CO2 rate; the succinic acid concentrations obtained from whey is approximately similar (13.96, 13.46 and 13.22 g L−1); in addition, at the end of the fermentation, there is no more lactose in the medium. This result demonstrates the ability of A. succinogenes ATCC55618 to consume lactose from whey to produce SA without any enzymatic treatment or chemical hydrolysis.
Indeed, the study carried out with Wan et al. [31] with a whey concentration equal to 50 g L−1, revealed that A. succinogenes 130Z could directly ferment lactose from cheese whey with less single sugar such as galactose and glucose produced due to the degradation of lactose.
However, succinic acid yields and productivities obtained with the three rate of CO2 are characterized by statistically significant differences (Table 2).
Succinic acid productivities (0.84 and 0.81 g L−1 h−1) obtained respectively with the two CO2 rates (0.2 and 0.4 vvm) are approximately similar, whereas with 0.8 vvm of CO2, succinic acid productivity decreases significantly to 0.54 g L−1 h−1. On the other hand, the SA yield increases significantly from 60.67 to 63.2%, with the increase in CO2 rate from 0.2 to 0.8 vvm. However, even if the maximum SA yield (63.2%) is obtained with 0.8 vvm rate of CO2, it is correlated with the minimum value of productivity (0.54 g L−1 h−1) (Table 2).
The statistically significant increase in biomass, from 9.2–10.23, as a function of rate of CO2 reveals that CO2 is a factor promoting cell growth.
Under the same conditions, the yield and productivity values of SA in batch fermentation of whey are lower than those obtained with lactose (Table 1, Table 2).
For 0.4 vvm CO2 rate, the maximal biomass obtained in batch fermentation using whey is equal to 10.11 (Table 2), this value is higher than that obtained with lactose 7.1 (Table 1). This result confirms that whey allows the development of biomass at the expense of SA production in comparison with lactose. Indeed, whey contains some complex sources of nitrogen and also some vitamins and minerals, which further favors bacterial growth and biomass development at the expense of succinic acid production. These results were also confirmed by other works [8,36].
Thus, for a CO2 rate equal to 0.4 vvm, the values of the mass ratios SA/AA and SA/FA (respectively 2.49 and 4.12) obtained during the batch fermentation of whey by A. succinogenes are lower than those obtained in the case of lactose (respectively 2.56 and 5.61). In comparison with lactose, the batch fermentation of whey promotes the development of biomass and the production of acid by-products of fermentation; acetic and formic acids.
Therefore, the most appropriate CO2 rate to have both optimal yield and productivity in SA from whey is 0.4 vvm.
The yield and productivity obtained in our work with pure lactose are more important than those obtained with whey (65% and 0.90 g L−1 h−1 versus 62.1% and 0.81 g L−1 h−1 respectively). Compared to other studies that have used lactose or whey to produce SA with A succinogenes ; when lactose is used, the average values of yield obtained by our study (65%) is more important than that reported by Longanesi et al. (61%) [29], the SA productivity are approximately in agreement (0.90 g L−1 h−1 vs 0.99 g L−1 h−1). Concerning the whey, the average values of yield obtained by our study (62.1%) is lower than the yield achieved by Longanesi et al. (68%) [29], but the SA productivity in our case (0.81 g L−1 h−1) greatly exceeds the value obtained by Longanesi et al. [29] (0.46 g L−1 h−1).
The study of Wan et al. [31] was interested in the production of SA from cheese whey in batch fermentation with an initial whey concentration equal to 50 g L−1, the yield and productivity correspond respectively to 0.57% and 0.44 g L−1 h−1, these values are lower than those obtained in our study. This variation may be due to the effect of the initial concentration of whey, which in our case is 35 g L−1. We can suggest that the increase in the concentration of whey induces a decrease in the productivity and the yield of SA. This result is already demonstrated in our study in the case of pure lactose where the increase in the concentration of lactose induces a reduction in productivity and yield (Table 1).
4. Conclusion
This study elucidates that succinic acid can be successfully produced from whey and lactose by A. succinogenes in batch fermentation. The production of SA is more important with lactose than with whey, however yields and productivities in the both cases agree or exceed those obtained by other studies under the same conditions of batch fermentation. The optimal results were obtained with 35 g L−1 of whey or 25 g L−1 lactose at CO2 rate 0.4 vvm, and pH 6.8.
The efficiency of SA production from lactose depend of initial concentration of lactose, thus, excess substrate is a limiting factor in SA production.
Given its availability and its richness in lactose, whey is a suitable feedstock for succinic acid production; however, further research on the fermentation mode (fed-batch, continuous, biofilm), the bacterial strain used and other parameters such as pH and CO2 level are needful in order to help develop a bio-production of succinic acid more cost-effective.
CRediT authorship contribution statement
Bouchra Louasté: Validation, Writing - original draft, Writing - review & editing, Visualization. Noureddine Eloutassi: Conceptualization, Methodology, Investigation, Supervision, Data curation, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sarkar B., Chakrabarti P.P., Vijaykumar A., Kale V. Wastewater treatment in dairy industries-possibility of reuse. Desalination. 2006;195:141–152. doi: 10.1016/j.desal.2005.11.015. [DOI] [Google Scholar]
- 2.Kumar A.S., Karunakar S. Utilization of whey for the production of instant energy beverage by using response surface methodology. Adv. J. Food Sci. Technol. 2012;4(2):103–111. [Google Scholar]
- 3.Louasté B., Boudine L., Allali A., Chaouch M. Physico-chemical and biological treatment of a dairy liquid effluent. J. Appl. Sci. Environ. Stud. 2018;1(2):45–52. doi: http://revues.imist.ma/index.php?journal=jases. [Google Scholar]
- 4.Shete S., Bharati, Shinkar N.P. Comparative study of various treatments for dairy industry wastewater. IOSR J. Eng. (IOSRJEN) 2013;3(8):42–47. doi: 10.9790/3021-03844247. [DOI] [Google Scholar]
- 5.Kolev Slavov A. Dairy wastewater treatment review. Food Technol. Biotechnol. 2017;55(1):14–28. doi: 10.17113/ftb.55.01.17.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prazeres A.R., Carvalho F., Rivas J. Cheese whey management: a review. J. Environ. Manage. 2012;110:48–68. doi: 10.1016/j.jenvman.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Rajeshwari K.V., Balakrishnan M., Kansal A., Lata K., Kishore V.V.N. State-of- the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000;4(2):135–156. doi: 10.1016/S1364-0321(99)00014-3. [DOI] [Google Scholar]
- 8.Lee P.C., Lee S.Y., Hong S.H., Chang H.N. Batch and continuous cultures of Mannheimia succiniciproducens MBEL55E for the production of succinic acid from whey and corn steep liquor. Bioprocess Biosyst. Eng. 2003;26:63–67. doi: 10.1007/s00449-003-0341-1. [DOI] [PubMed] [Google Scholar]
- 9.Baldasso C., Barros T.C., Tessaro I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination. 2011;278:381–386. doi: 10.1016/j.desal.2011.05.055. [DOI] [Google Scholar]
- 10.Ryan M.P., Walsh G. The biotechnological potential of whey. Rev. Environ. Sci. Bio/Tehnology. 2016;15(3):479–498. doi: 10.1007/s11157-016-9402-1. [DOI] [Google Scholar]
- 11.Guimarães P.M., Teixeira J.A., Domingues L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010;28(3):375–384. doi: 10.1016/j.biotechadv.2010.02.002. Epub 2010 Feb 11. [DOI] [PubMed] [Google Scholar]
- 12.Ghaly A.E., Kamal M.A. Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res. 2004;38(3):631–644. doi: 10.1016/j.watres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Ozmihci S., Kargi F. Comparison of yeast strains for batch ethanol fermentation of cheese-whey powder (CWP) solution. Lett. Appl. Microbiol. 2007;44:602–606. doi: 10.1111/j.1472-765X.2007.02132.x. [DOI] [PubMed] [Google Scholar]
- 14.Baroudi M., Kabbout R., Bakkour H., Dabboussi F., Taha S., Halwani J. Characterization, physicochemical and biological treatment of sweet whey (Major pollutant in dairy effluent) Asian J. Water Environ. Pollut. 2012;9(4):11–15. [Google Scholar]
- 15.Louaste B., Boudine L., Eloutassi N., Chaouch M. Biological treatment of liquid effluent resulting from the dairy industry by exogenous fungi. Int. J. Innov. Appl. Stud. 2014;7(4):1551–1559. http://www.ijias.issr-journals.org/abstract.php?article=IJIAS-14-196-05 [Google Scholar]
- 16.Bosco F., Carletto R.A., Marmo L. An integrated cheese whey valorization process. Chem. Eng. Trans. 2018;64:379–384. doi: 10.3303/CET1864064. [DOI] [Google Scholar]
- 17.Torres E.F., González G., Klotz B. Effect of the addition of liquid whey from cheese making factory on the physicochemical properties of whey protein isolate gels made by high hydrostatic pressure. J. Food Sci. Technol. 2019;56:245–252. doi: 10.1007/s13197-018-3483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen A.D., Kádár Z., Oleskowicz-Popiel P., Thomsen M.H. Production of bioethanol from organic whey using Kluyveromyces marxianus. J. Ind. Microbiol. Biotechnol. 2011;38(2):283–289. doi: 10.1007/s10295-010-0771-0. [DOI] [PubMed] [Google Scholar]
- 19.Deshpande D.P., Patil P.J., Anekar S.V. Biomethanation of dairy waste. Res. J. Chem. Sci. 2012;2(4):35–39. https://pdfs.semanticscholar.org/78f3/1ad9ca7ccd3141ebca6283d0cff454ec6725.pdf [Google Scholar]
- 20.Eloutassi N., Louasté B., Boudine L., Chaouch M. Valorisation of whey: bioethanol production by free and immobilized yeasts. Int. J. Innov. Appl. Stud. 2014;6(3):493–498. http://www.ijias.issr-journals.org/abstract.php?article=IJIAS-14-132-06 [Google Scholar]
- 21.Corona-González R.I., Bories A., González-Álvarez V., Pelayo-Ortiz C. Kinetic study of succinic acid production by Actinobacillus succinogenes ZT-130. Process Biochem. 2008;43:1047–1053. doi: 10.1016/j.procbio.2008.05.011. [DOI] [Google Scholar]
- 22.Cok B., Tsiropoulos I., Roes A.L., Patel M.K. Succinic acid production derived from carbohydrates: an energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod. Bioref. 2014;8:16–29. doi: 10.1002/bbb.1427. [DOI] [Google Scholar]
- 23.Muzumdar A.V., Sawant S.B., Pangarkar V.G. Reduction of maleic acid to succinic acid on titanium cathode. Org. Process Res. Dev. 2004;8:685–688. doi: 10.1021/op0300185. [DOI] [Google Scholar]
- 24.Li J., Jiang M., Chen K., Shang L., Wei P., Ying H., Ye Q., Ouyang P., Chang H. Enhanced production of succinic acid by Actinobacillus succinogenes with reductive carbon 3source. Process Biochem. 2010;45:980–985. doi: 10.4236/jsbs.2012.22003. [DOI] [Google Scholar]
- 25.Krige A., Nicol W. Continuous succinic acid fermentation by Escherichia coli KJ122 with cell recycle. Process. Biochem. 2015;50(12):2004–2011. doi: 10.1016/j.procbio.2015.09.023. [DOI] [Google Scholar]
- 26.Gao C., Yang X., Wang H., Rivero C.P., Li C., Cui Z., Qi Q., Sze C., Lin K. Robust succinic acid production from crude glycerol using engineered Yarrowia lipolytica. Biotechnol. Biofuels. 2016;9(1):179. doi: 10.1186/s13068-016-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan J.P., Jahim J.Md., Wu T.Y., Harun S., Kim B.H., Mohammad A.W. Insight into biomass as a renewable carbon source for the production of succinic acid and the factors affecting the metabolic flux toward higher succinate yield. Ind. Eng. Chem. Res. 2014;53:16123–16134. doi: 10.1021/ie502178j. [DOI] [Google Scholar]
- 28.Domingos J.M.B., Martinez G.A., Scoma A., Fraraccio S., Kerckhof F.-M., Boon N. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities and microbial community structure. ACS Sustain. Chem. Eng. 2016;5:1400–1407. doi: 10.1021/acssuschemeng.6b01901. [DOI] [Google Scholar]
- 29.Longanesi L., Frascari D., Spagni C., DeWeverb H., Pinellia D. Succinic acid production from cheese whey by Actinobacillus succinogenes biofilms. J. Chem. Technol. Biotechnol. 2018;93:246–256. doi: 10.1002/jctb.5347. [DOI] [Google Scholar]
- 30.Thuy N., Thi H., Kongkaew A., Flood A., Boontawan A. Fermentation and crystallization of succinic acid from Actinobacillus succinogenes ATCC55618 using fresh cassava root as the main substrate. Bioresour. Technol. 2017;233:342–352. doi: 10.1016/j.biortech.2017.02.114. [DOI] [PubMed] [Google Scholar]
- 31.Wan C., Li Y., Shahbazi A., Xiu S. Succinic acid production from cheese whey using Actinobacillus succinogenes 130 Z. Appl. Biochem. Biotechnol. 2008;145(1-3):111–119. doi: 10.1007/s12010-007-8031-0. [DOI] [PubMed] [Google Scholar]
- 32.Samuelov N.S., Datta R., Jain M.K., Zeikus J.G. Whey fermentation by Anaerobiospirillum succiniciproducens for production of a succinate-based animal feed additive. Appl. Environ. Microbiol. 1999;65(5):2260–2263. doi: 10.1128/AEM.65.5.2260-2263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S.K.C., Du C., Koutinas A., Wang R., Webb C. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem. Eng. J. 2008;41:128–135. doi: 10.1016/j.bej.2008.03.013. [DOI] [Google Scholar]
- 34.Dorado M.P., Lin S.K., Koutinas A., Du C., Wang R., Webb C. Cereal-based biorefinery development: utilisation of wheat milling by-products for the production of succinic acid. J. Biotechnol. 2009;143:51–59. doi: 10.1016/j.jbiotec.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.P., Zheng P., Sun Z.H., Dong J.J., Wei P. Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J. Chem. Technol. Biotechnol. 2008;83(5):722–729. doi: 10.1002/jctb.1862. [DOI] [Google Scholar]
- 36.Barrosa M., Freitas S., Padilha G.S., Alegrea R.M. Biotechnological production of succinic acid by Actinobacillus succinogenes using different substrate. Chem. Eng. Trans. 2013;32:985–990. doi: 10.3303/CET1332165. [DOI] [Google Scholar]