Abstract
Yersinia pestis is the causative agent of plague and is considered one of the most likely pathogens to be used as a bioweapon. In humans, plague is a severe clinical infection that can rapidly progress with a high mortality despite antibiotic therapy. Therefore, early treatment of Y. pestis infection is crucial. This review provides an overview of its clinical manifestations, diagnosis, treatment, prophylaxis, and protection requirements for the use of clinicians.
We discuss the likelihood of a deliberate release of plague and the feasibility of obtaining, isolating, culturing, transporting and dispersing plague in the context of an attack aimed at a westernized country. The current threat status and the medical and public health responses are reviewed. We also provide a brief review of the potential prehospital treatment strategy and vaccination against Y. pestis. Further, we discuss the plausibility of antibiotic resistant plague bacterium, F1-negative Y. pestis, and also the possibility of a plague mimic along with potential strategies of defense against these. An extensive literature search on the MEDLINE, EMBASE, and Web of Science databases was conducted to collate papers relevant to plague and its deliberate release. Our review concluded that the deliberate release of plague is feasible but unlikely to occur, and that a robust public health response and early treatment would rapidly halt the transmission of plague in the population. Front-line clinicians should be aware of the potential of a deliberate release of plague and prepared to instigate early isolation of patients. Moreover, front-line clinicians should be weary of the possibility of suicide attackers and mindful of the early escalation to public health organizations.
Keywords: Plague, Y. pestis, Deliberate release, Bioweapon, Biowarfare, Antibiotic resistant plague, Plague mimic
1. Introduction
Yersinia pestis is the causative agent of plague and is considered one of the most likely pathogens to be used as a bioweapon. In humans, plague is a severe clinical infection that can progress rapidly despite antibiotic therapy and is associated with a high mortality rate. Plague manifests itself in three main clinical syndromes: 1) bubonic plague, 2) septicemic plague and 3) pneumonic plague, which is transmissible from human-to-human. In this review paper we will discuss clinically relevant aspects of pneumonic plague, including diagnosis, treatment and prophylaxis, history and current epidemiology of Y. pestis.
A deliberate or intentional release of plague is the act of using it as a weapon to infect a healthy population with the intention to cause harm. To understand the likelihood of such an attack, questions, such as, “who would do such a thing and why?”, “who would be capable?”, “how would they go about obtaining plague or transporting it around the world covertly?”, need to be answered.
Furthermore, this review will explore how someone could release plague, including the potential use of a “suicide attacker”, the feasibility of antibiotic resistant plague, the danger of a plague mimic (including a theoretical mechanism to defend against it), explain the public health response and evaluate the likelihood of a deliberate release of plague.
2. Methodology
A search of the MEDLINE, EMBASE and Web of Science databases was undertaken during the period of April to May 2019, with the most recent search being performed on 2nd May 2019. For a detailed account of the search criteria used in these databases please see Appendix 1. The general search criteria used to find relevant information in these databases were as follows:
2.1. (Plague OR Y. pestis) AND (Deliberate release OR Biowarfare OR Bioterrorism OR Terrorism)
Inclusion criteria: Any papers relevant to Y. pestis and its deliberate release, or protection from deliberate release within the previous 10 years.
Exclusion criteria: non-English language, any papers relating to other than the inclusion criteria.
A total of 502 papers were found and handled using Endnote online. After manual deletion of duplicates 294 papers remained, of which 192 had met the inclusion and exclusion criteria based on a screening performed by title and abstract. These papers were used for full text review. Moreover, to give a more up-to-date review of the literature and current climate, only papers published in the past 10 years were used. A review of the references of these papers also allowed further identification of relevant papers for inclusion according to the inclusion criteria. Furthermore, separate literature searches were performed for each of the discussion subheadings in order to identify papers not “captured” in the initial preliminary background searches. Finally, any papers suggested for inclusion by experts in the field including chemical, biological, radiological, nuclear, and explosive (CBRNE) experts, Emergency Medicine and Prehospital Medicine Consultants, were reviewed and added accordingly. If a piece of information in a paper was from a referenced paper, the original paper has been cited in most cases, unless it was not found.
3. Background - Y. pestis
3.1. Biology
Y. pestis, a gram-negative bacterium of the Enterobacteriaceae family, is a zoonotic pathogen and the causative agent of plague.1 The Enterobacteriaceae family consists of 11 species of bacteria, of which 3 are pathogenic in humans, namely; Y. pestis, Y. pseudotuberculosis and Y. enterocolitica.2 While Y. pseudotuberculosis and Y. enterocolitica cause a self-limiting gastrointestinal illness, Y. pestis causes a severe, acute and rapidly progressing febrile illness with high mortality rates.2 Y. pestis is a nonmotile, non-spore-forming coccobacillus, that exhibits bipolar staining. The organism can grow in a wide range of temperatures from 4 to 40 °C, and can tolerate a wide range of pH, from 5 to 9.6.1 This demonstrates how Y. pestis is somewhat resistant to environmental conditions, furthermore, when contained in small droplets at least one hour of sunlight is needed to kill the organism, and it can resist drying for many days.3
Plague is primarily a vector-borne illness transmitted by fleas to a variety of wild rodents in many habitats in the world – representing a natural reservoir for the disease.4 The classical vector of Y. pestis is the oriental rat flea, Xenopsylla cheopis, but 30 different flea species have been shown to be vectors of Y. pestis. 1, 5 With the use of next-generation DNA sequencing and the recovery of Y. pestis DNA from the teeth of prehistoric individuals, it has been estimated that Y. pestis evolved from Y. pseudotuberculosis around 5700–6000 years ago with the introduction of some virulence-associated plasmids.2 This new knowledge about the above-mentioned close genetic similarity between Y. pestis and Y. pseudotuberculosis, the plasmids (pCD1, pPCP1 and pMT1) and the F1 capsular antigen, plays an important role in recent vaccine development efforts. However, currently there is no worldwide license for any vaccine against Y. pestis. 2, 6, 7, 8, 9, 10
3.2. History
Historically, plague is a well-known infectious disease, with 3 major pandemics. There are also reports of earlier occurrences of plague, including the Plague of Athens (430–427 BCE) and the Antonine Plague (165–180 CE), although these are disputed due to a lack of genetic evidence.5, 11, 12, 13 This is also the case with the earliest report of the plague which is found in the Bible occurring around 1000 BCE, in the city of Ashdod.13 The first great pandemic, the 6th century Justinian Plague, which is thought to have originated in Egypt in 542 AD and lasted until approximately 750 AD, resulted in the deaths of an estimated 100 million people.13, 14 The second great pandemic is what we refer to as “The Black Death” or in some places in Europe as “The Great Pestilence,” and it is probably the most well-known of the three pandemics. Lasting from 1347–1351, it is one of the best historical examples of an emerging infection with rapid dissemination and a high mortality.15 The Black Death is thought to have led to the death of 30 – 50% of Europe’s population at that time.16 The third and final great pandemic originated in China in the 1860 s, but exploded across the globe after a major outbreak in Hong Kong 1894 and rapidly spread worldwide–primarily through trade routes on steamships and railways,14 leading to the death of more than 10 million people.13
3.3. Epidemiology
Plague is thought to be a disease of the past; however, in recent times outbreaks have occurred on every continent apart from Antarctica,17 and it is endemic in parts of Africa, Asia and The Americas.18 Since the 1990s plague has been considered a re-emerging disease by the World Health Organization (WHO).19 In nature, plague is transmitted between rodents and other mammals through fleas and feeding, with natural foci of Y. pestis existing without the need of human hosts.18 Typically, Y. pestis infection causes the death of large numbers of rodents. As a result, the fleas which were feeding on them lose their main food supply and start feeding on humans spreading the plague-causing bacteria through their bites.14
Currently, the three most endemic countries are Madagascar, and The Democratic Republic of Congo and Peru.17 From 1957–1997, internationally, there were 80,163 confirmed cases1 notified to WHO with 6578 deaths in 38 countries.20 More recently, from 2010–2015 there have been 3248 confirmed cases of plague in humans resulting in 584 deaths.18 Madagascar now accounts for around 75% of the global plague cases reported to WHO.19 During the most recent urban plague epidemic, which occurred in late 2017 in Madagascar, 2414 plague cases were reported leading to 221 deaths. This was a particularly important outbreak as, unusually, the majority (78%) of cases were of primary pneumonic plague, the deadliest form of plague, with a human-to-human transmission, leading to a large international multisectoral response to prevent international spread.19 The index case of this outbreak was one gentleman who had spread his infection to others travelling alongside him on public transport, eventually spreading the disease throughout the capital.19 Through epidemiological studies we can understand that plague is endemic in many areas of the world but is still very uncommon.17, 18
3.4. Clinical manifestations of natural infection
Plague is a severe clinical infectious disease that can rapidly progress to death if not diagnosed and treated early.21 As plague is not commonly encountered in clinics, it can be difficult for clinicians who do not have experience diagnosing and treating plague to correctly diagnose the disease.22 There are many forms of plague infection; the three major forms namely bubonic plague, septicemic plague, and pneumonic plague, are explored in further detail below. It should be kept in mind that other forms of plague, including exceptionally rare forms, such as gastrointestinal plague (from consumption of uncooked contaminated meats), plague pharyngitis and plague meningitis have also been reported.21
3.4.1. Bubonic plague
Most cases of plague today are of the bubonic form, which has a distinctive clinical picture, recognizable to clinicians and patients – especially in endemic areas.5 Bubonic plague results from the bite of a flea infected with Y. pestis that had previously fed on an infected organism, such as a rodent.23 In the flea, Y. pestis forms a biofilm that blocks access to the flea’s midgut and, as a result, the flea regurgitates the obstructive biofilm into the mammalian host while feeding2 leading to the inoculation of thousands of organisms into a patient’s skin.24 Bubonic plague may also develop from exposure of open wounds to infected material.1 The onset of bubonic plague is sudden and characterized by malaise, high temperature and a severe lymphadenitis.23
The bacteria deposited from the flea bite migrate rapidly to a regional lymph node and multiply during an incubation period of 2–6 days.25 Alongside the onset of sudden fever and chills, a recognizable and extremely painful bubo 2 appears from the significant bacterial proliferation and inflammation, in as early as up to one day.24 Within the lymph node they are phagocytosed but evade destruction, and subsequently cause necrosis of the lymph node architecture.24 The bubo most typically develops in the inguinal region but can also be distributed anywhere there is a lymph node in the body, commonly in the cervical and axillary regions24 (Fig. 1 ). Often the buboes are so painful they completely restrict movement of the affected limb, and range between 1–10 cm in diameter.24 Rarely, the primary lymph node affected is retroperitoneal, or even intraperitoneal giving the impression of an acute surgical abdomen on examination.26 From the bubo, in untreated patients especially, bacteria then disseminate throughout the body resulting in secondary septicemic plague, and/or secondary pneumonic plague.27
Fig. 1.
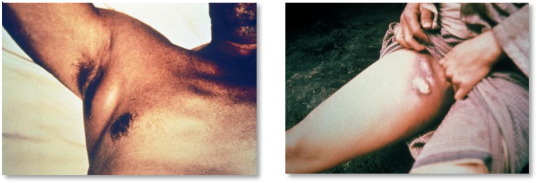
Axillary and inguinal buboes in patients with bubonic plague.
The mortality of untreated bubonic plague can be as high as 60%, but it is largely reduced to less than 5% if detected early and treated with effective antimicrobial therapy.25
3.4.2. Septicemic plague
Primary septicemic plague occurs from a flea bite directly into the vasculature,1, 2 or when bacteria bypass the regional lymph node and multiply directly in the blood.5 Secondary septicemic plague occurs from the spread from a focus of bacteria in the body, such as hematological spread from a bubo in bubonic plague, or from pneumonic plague.2
Primary septicemic plague is the second most common form of plague,21 beginning with no evidence of any palpable lymph nodes but with bacteremia. The disease can be rapidly fatal within a few days, with high fevers, rapidly developing sepsis and multiorgan failure from hypotension and shock.24 Once the infection reaches the end stages it leads to disseminated intravascular coagulation and vasculitis, leading to gangrene particularly in the extremities resulting in the need for amputation.1 This black discoloration of the gangrenous necrotic tissues is where “The Black Death” obtained its name from, as patients would turn black and then soon die24 (Fig. 2 ).
Fig. 2.
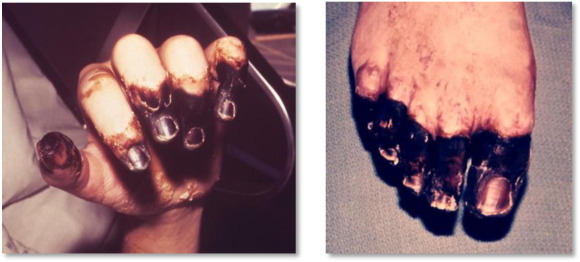
Digital gangrene and gangrene of the right foot.
Without appropriate treatment, septicemic plague is invariably fatal with a mortality of 100%. Even with treatment the mortality rate can be as high as in the range of 30–50%.25
3.4.3. Pneumonic plague
Pneumonic plague is the third most common and also the most virulent form of plague, representing the most severe manifestation of plague.2 Primary pneumonic plague occurs from direct inhalation of aerosolized droplets of Y. pestis, originating from expelled respiratory droplets of mammals, with many reports from cats and dogs, and importantly human-to-human spread.28, 29 Spread by respiratory droplets through an infected person’s coughs or sneezes requires a distance of less than 2 meters.1 Secondary pneumonic plague occurs from hematological spread of bacteria in bubonic or septicemic plague to the lungs and can be the first step of transmission of primary pneumonic plague in a population, as seen in Madagascar in 2017.30
This disease progresses rapidly with an acute onset within 1–4 days of inoculation, beginning with non-specific symptoms and signs as seen in humans and non-human primates.31 The initial disease is very similar to influenza with coryzal symptoms, fever, headache, chills and malaise.5, 31 Within one day this progresses to significant lower respiratory tract symptoms such as dyspnea, tachypnoea, cough and haemoptysis25 with bilateral pulmonary infiltrates developing rapidly, as observed on chest x-ray (Fig. 3 ). The initial purulent sputum may become tinged with blood or be acutely haemorrhagic.27 Over time the cough becomes increasingly productive and in the final stages patients produce vast volumes of bright red sputum containing “an enormous number of plague bacilli in almost pure culture”.32 In pneumonic plague, if antibiotics are not started within 24 h, the mortality rates can reach 100%.2, 25 Even with proper antibiotic treatment up to 50% of patients can still die due to the severity of the infection and many would require admission to an intensive care facility.21
Fig. 3.
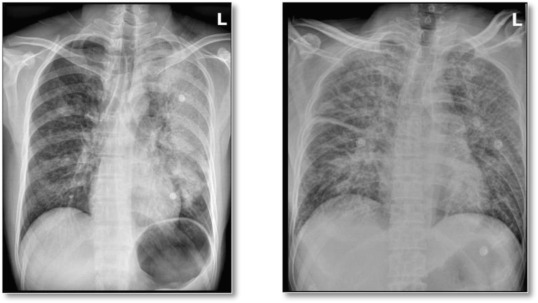
Chest radiographs of two patients with pneumonic plague.
As pneumonic plague is transmissible from person to person, many studies have been undertaken to ascertain the basic reproduction number3 34, with many quoting an average secondary infection rate of around 1.3.25, 34 Further modelling has developed our understanding of super spreading events (SSE) where a certain individual may infect many more individuals, while the majority of individuals would fail to transmit to anyone else. If early in a disease outbreak there are many SSEs, the rate of transmission could be much higher than the often quoted R0 of 1.3. This could also have been the case in the recent epidemic in Madagascar in 2017 as the reproductive number quoted was 2.4 (1.6–3.6) despite Madagascar’s relative preparedness from previous (although smaller) plague outbreaks.35
3.5. Diagnosis, treatment and prophylaxis of Y. Pestis infection
The clinical diagnosis of Y. pestis infection in its three clinical syndromes is described extensively in Table 1 below.
Table 1.
| Syndrome | Typical time course | Symptoms | Physical findings | Laboratory findings | Radiographic findings |
|---|---|---|---|---|---|
| Bubonic | Incubation period 2–8 days Early 1–2 d
|
Early:
|
Early - Fever, Buboes; 1–10 cm diameter, firm, non-fluctuant and exquisitely tender Late - SIRS, sepsis |
Leukocytosis Presumptive - Direct Gram-stain; Wright or Giemsa stain; Culture colony morphology and biochemical testsConfirmatory - Isolation of Y. pestis from clinical specimen (bubo aspirate); 4-fold or greater increase in serum antibody to F1 antigen |
CXR - Bilateral pulmonary infiltrates with a nodular appearance Develops to be similar to ARDS |
| Septicemic | As above | As above | As above, but no buboes; Ischaemia and necrosis of digits (black death) | As above | As above |
| Pneumonic | Short incubation period (24 h-3 d) followed by sudden onset of symptoms and rapid deterioration and death as early as 18–24 h after the onset of symptoms |
|
High fever Respiratory failure Desaturation Coma | As above plusLactic acidosisHypoxemia on ABGCulture or specimen can be from sputum | CXR - Bilateral alveolar and parenchymal infiltrates, may have bilateral pleural effusionsCT - Focal or lobular consolidationNuclear imaging - lymphadenitis or meningeal inflammation |
The treatment, prophylaxis, protection and supportive therapy for infection with Y. pestis is outlined in Table 2 below, describing some of the nuances of treatment between adults, children and pregnant women.
Table 2.
| Patient Group | Treatment Treatment course 10–14 days, can be switched to oral therapy when improving. Begin within 24 h of onset of symptoms. |
Prophylaxis Indicated for asymptomatic individuals in close contact (<2m), exposure to bodily fluids, or aerosolized deliberate release. |
Protection and supportive treatment For all patients |
|---|---|---|---|
| Adults | 1st line; Streptomycin 1 g IM BD or Gentamicin 5 mg/kg IV/IM once daily and/or Doxycycline IV 200 mg once daily 2nd line; Ciprofloxacin, Levofloxacin, Moxifloxacin, Chloramphenicol |
1st line; Doxycycline 100 mg oral BD or Ciprofloxacin 500 mg oral BD 2nd line; Chloramphenicol 25 mg/kg orally QDS For a minimum of 7 days Prevent flea bites with insect repellents such as N,N-diethyl-meta-toluamide (DEET) |
Tissue perfusion and oxygenation in septic patients should be maintained by fluid resuscitation and vasopressors if required. With the possibility of ECLS if needed. Patients with pneumonic plague may require ventilatory support. Isolate patients with pneumonic plague (48 h minimum) and take precautions against droplet transmission in the form of surgical face masks. Report all cases of plague per local procedure. Procedures that may aerosolize plague should be avoided, such as bone sawing in amputation. |
| Children | 1st line; Streptomycin 15 mg/kg IM BD (2 g max) or Gentamicin 2.5 mg/kg IV/IM TD 2nd line; Doxycycline, Ciprofloxacin, Chloramphenicol - (maintain concentration between 5–20 µg/ml) |
1st line; Doxycycline if ≥ 45 kg give adult dose, if ≤ 45 kg give 2.2 mg/kg orally BD or Ciprofloxacin 20 mg/kg orally BD 2nd line; Chloramphenicol 25 mg/kg orally QDS (maintain concentration between 5–20 µg/ml) Prevent flea bites with insect repellents such as N,N-diethyl-meta-toluamide (DEET) |
|
| Pregnant women | 1st line; Gentamicin 5 mg/kg IV/IM OD 2nd line; Doxycycline or Ciprofloxacin Avoid Streptomycin For breastfeeding women and their infants use Gentamicin |
As for non-pregnant adults |
4. Discussion – Deliberate release of Y. pestis
Y. pestis is classed as a category A (tier 1) biological agent by the Centers for Disease Control and Prevention (CDC)40, 41 as it is a high priority agent that could pose a risk to national security. It has been selected to be on this list for its dangerous qualities, as seen in Table 3 . This discussion will review the potential for Y. pestis to be used as a biological weapon aimed at harming “westernized countries” such as the United Kingdom (UK). It will encompass the history of the deliberate release of plague, the likelihood of an attack, who would have the potential to undertake it, for what motives, the feasibility of creating a plague bioweapon, the mechanisms of deliberate release, and the possibilities of antibiotic resistance, F1-negative plague and plague mimic. Furthermore, we will discuss the public health response and evaluate the possible effects on our population.
Table 3.
| Category A biological agents | Quality for selection by CDC |
|---|---|
|
|
4.1. History of deliberate release of plague
Alongside the deadly history of plague as a natural infection,4,13–14,16 it has also been used in the past as a biological weapon to wreak havoc during war times. Two highly reported incidents of these heinous acts are reviewed.
4.1.1. Siege of caffa
In 1346, Caffa16 (now Feodosia, Ukraine) was besieged by the Mongols (Tartars) for approximately 3 years. Towards the end of the siege, the Tartars were struck with an epidemic of plague that ravaged their forces.44 According to Gabriele De Mussis45 (a 14th century lawyer) account at the time, “thousands upon thousands of Tartars died each day… as soon as the signs of disease showed on their body as swellings in the armpits or groins caused by coagulating humours.” After selecting the most putrid smelling corpses, the Tartars then proceeded to catapult those bodies infected with plague into the city, raining bodily fluids into the population and drawing huge nests of rodents to fester in the city walls.44 This led to huge numbers of people infected and killed within the city (“mountains of dead”45) and is a powerful reminder of the horrific consequences of successfully using a disease as a weapon.44
4.1.2. Japanese Unit 731
During the second Sino-Japanese war and World War II, a secret branch of the Imperial Japanese Army – Unit 73146, 47 conducted huge amounts of research, development and deployment of biological weapons on the population of Manchuria and Eastern China.48 They used plague-infected fleas as their main dissemination technique, initially by dropping hollow clay vessels filled with infected fleas which opened on impact. This first method was relatively ineffective, as they failed to bring enough people in contact with the fleas this way. Unit 731 then moved to restrict the target population’s food sources and then proceeded to airdrop flea-infested wheat and other food sources. The unsuspecting famished inhabitants then rushed to gather the infested food and as a result they were bitten by the fleas initiating an outbreak of plague.49, 50, 51
The most well-documented of these numerous attacks, in particular in a 2019 historical reconstruction, was the Ningbo outbreak that occurred on the October 27, 1940.48 All of the cases reported were of bubonic plague, apart from one case of pneumonic plague in a health worker. The reported aim of Unit 731 was to kill 1,450 Ningbo citizens, but the outbreak killed only 112 people despite a case fatality of 68%. This was mostly attributed to the robust public health response of the population and officials, despite unfamiliarity with plague.47, 48, 52 The community acted together to quarantine those infected and return those who were infected and had fled back to isolation hospitals, they erected 14-foot concrete walls around infected buildings and burned them to the ground, rapidly identified and isolated suspected human cases, destroyed all rodents, and killed all cats, dogs and any other companion animals.48, 53 This evasion of social disintegration and panic, and unified response by the local population provides an example that even in the pre-antibiotic era public health response could mitigate the disruptive societal effects of a biological weapon, including plague. However, this occurred in a population that was trained to be under attack for many years, and as a result was able to develop coping mechanisms to high threat situations – quite different to our populations in the west now.47, 48
4.2. Prerequisites for a biological agent for bioterrorism use
Bioterrorism is defined as “the deliberate release of viruses, bacteria or other agents used to cause illness or death in people, animals or plants. It is aimed at creating casualties, terror, societal disruption, or economic loss, inspired by ideological, religious or political beliefs.”42 Whoever was to undertake an attack would select a pathogen that was easy to obtain, multiply (retaining its high virulence), and have a dispersal method which could allow the agent to reach a significant number of humans in significant enough quantities to cause significant harm.42, 46 We will now review the ability of plague to be used as a biological weapon in these domains.
4.2.1. Obtaining, isolating, culturing and transporting Y. Pestis
As described in the epidemiology of Y. pestis above, it is known that it is endemic in many areas of the world,1, 24, 54 with the majority of cases occurring in Madagascar and the Dominican Republic of Congo.18 There is also a wide distribution of foci of plague across the world including many areas of the west of North America, and a large proportion of Asia.17
It could be relatively easy to access, although time consuming to find, one of these foci of plague in nature and obtain a small sample of Y. pestis to take away and then culture.55 A simple method could include the capture of animals infected with plague and their transportation to another location for isolation of the bacteria, but this can have personal safety concerns.55 Another method that has been described involves capturing the infected fleas from infected rodent burrows, which can be found in nature, grinding up the fleas and then culturing the mixture.55 The use of Cefsulodin-Irgasan-Novobiocin (CIN) agar,56 which was initially developed for the isolation of Y. pseudotuberculosis, is inhibitory to other bacteria that compete with Y. pestis, so for 48 h the only bacteria that grow are Y. pestis.55 Then, standard culture procedures can be undertaken using sheep blood agar. However, for plague aerosolization, a high bacterial concentration is needed, which may require more advanced techniques.57 Moreover, as the CDC recommends, the use of a Biosafety level 2 laboratory is required for prevention of harm to the scientist performing the experiments.57 The presence of Y. pestis could be confirmed through PCR, or from inoculation of mice and specimen culture of those that die within 21 days.58, 59
The question then arises, once obtained, “how would one covertly transport the bacteria across the world?,” for example, from the location obtained to a “laboratory” in another country. Through experimental data it is understood that some strains of Y. pestis are still culturable up to 138 days when stored in bottled water at 26 °C,60 and most strains are culturable until 21 days in sterilized tap water stored at 4 °C.61 Therefore, it is theoretically possible that Y. pestis can be surreptitiously transported across the world by camouflaging it using perfume bottles or bottled water to be cultured elsewhere to multiply it to larger quantities.
Therefore, it is feasible that Y. pestis can be obtained, isolated, cultured, and transported from a natural focus. Although it is important to remember that this would require a fairly good understanding of biology, at least to a postgraduate level, and a considerable amount of equipment to be completed safely, all of that can be purchased online.
However, all these steps can theoretically be bypassed by infiltrating the stockpiles of Y. pestis reserved in relatively safe laboratories across the world for research purposes. That could take a much more considerable effort to be completed covertly, though, unless the attacker already had access to such stockpiles, through a nations current or past biological warfare program – making an attack from those nations much easier.
4.2.2. Potential mechanisms of dispersal of Y. Pestis
There could be an almost infinite number of mechanisms of release of a biological weapon such as plague. The most likely form of plague to be used as biological weapon would be in the form of pneumonic plague; however, there have been previous uses of infected fleas to spread bubonic plague during war time that we should be aware of. Both these mechanisms have the potential to enter a human in sufficient quantities to trigger a severe infection, a prerequisite for a useful biological weapon.
4.2.2.1. Pneumonic plague – Mechanical aerosol release and suicide attacker
The most feared and studied use of plague as a biological weapon would be a deliberate release of pneumonic plague as an aerosol.2 Aerosolized biological warfare agents, in particulate form between 0.3–5 µm in diameter pose a huge risk to the public in a bioweapons attack46, 62 as they can penetrate directly into the alveoli with normal inhalation. Y. pestis has the potential, in an aerosol form, to be a true silent killer; the particles could be delivered into the lungs without any warning.62 As an aerosol it would be odorless and invisible to susceptible and unknowing humans of all ages and sexes.17, 62 Therefore, the deployment of medical countermeasures would only be undertaken once cases were found from the emergence of signs and symptoms – for pneumonic plague this could take up to 4 days for some people, although most commonly signs and symptoms occur within 2 days.25
Aerosol generators such as a Collison nebulizer, or other makeshift devices if needed, mounted on any form of vehicle, including aircraft, would enable mechanical aerosol release of Y. pestis.3 The WHO estimated that an aerosol release of 50 kg of plague over a city of 5 million people would result in 150,000 initial clinical cases, and 36,000 deaths. Even more dangerous they reported, would be the transmission to secondary cases leading to a total of up to 500,000 hospitalizations and up to 100,000 deaths spread over a number of weeks, not including the spread to other cities.3 Aerosolization involves a complicated process and can spread plague in either dry or liquid form. In liquid form it would be easy to disseminate with commercially available sprayers; however, it would be difficult to prevent the bacteria from dying during this process. Although release of a freeze-dried powder would be much easier, its production would be a very dangerous process. The major difficulty in aerosolization would be producing particles less than 5 µm in diameter, as any larger particles would not be able to reach the alveoli and hence be useless in propagating the disease. Therefore, the most difficult step in the process of creating a plague bioweapon would likely be its aerosolization.63
Another possible release strategy could include air conditioning units and the use of the London Underground as a commuter hub.46 This is a preferred location for an attack as it offers the opportunity to infect the large numbers of people who use the trains, who are in close proximity to one another (within the necessary two meters) making it easy to transmit to secondary cases. Moreover, in this transmission process, locating the source of the release would probably prove more difficult as passengers would be traveling to and from many locations. Furthermore, being underground there would be no direct sunlight to degrade the bacteria.14 There would be multiple surfaces for Y. pestis to seed onto and survive on, as it can survive on glass and stainless steel for up to 72 h,64 and theoretically the air currents in the tube network could then re-aerosolize the pathogen from these surfaces causing further dissemination.
The possibility of a “suicide attacker” is another potential mechanism of deliberate release of pneumonic plague. A human (or more than one) would be purposefully infected with the pneumonic plague, then undertake the mission of infecting as many people as possible, in a similar mechanism of a super spreading event (SSE). This could be modelled in a very similar way to the recent plague outbreak in Madagascar in 2017,19, 34 where we saw an explosion in cases of pneumonic plague originating from a single source with multiple secondary infections. Computer modelling of the transmission dynamics of pneumonic plague demonstrated an R0 of around 1.0, but described that in the beginning of the disease if there are super spreading events then the rate of secondary infection can be much higher (R0 ≥ 1.6) and propagate the disease in the population rapidly before public health interventions are initiated.33, 34, 65 These models were described with the SSE expected to be like that in the natural transmission of pneumonic plague, but a suicide attack could theoretically infect many more cases than estimated in the model and spread the disease much faster. Furthermore, with advancements in the understanding of transmission of pneumonic plague we know that mammal-to-mammal transmission involves the transfer of organisms that have been growing at 37 °C to an environment which is also at 37 °C.2, 66 We know that many virulence factors and genes in Y. pestis are activated once the bacteria move from 26 °C to 36 °C, from flea midgut to mammalian tissues.67 Therefore, the bacteria when travelling from human to human, as would occur in a suicide attack, would transmit a more virulent and already adapted form of Y. pestis probably leading to a more severe clinical syndrome.2, 67 These suicide attackers could also wreak havoc in the public health response by refusing to wear protective face masks and aim to purposefully spread disease in hospitals and other health care centers and maliciously infect healthcare workers before the threat of the disease is realized.
Therefore, pneumonic plague is amenable to various methods of dispersion leading to a silent and deadly disease in humans when used as a biological weapon and would be the likely preferred mechanism of release.
4.2.2.2. Bubonic plague – Release of infected fleas
Similar to the attacks undertaken by the Japanese Unit 731 in 1940,48 a release of fleas infected with Y. pestis could be a viable method of releasing plague as a biological weapon.21, 53 The fleas could be infected by allowing them to feast on mammals infected with the plague that could be found in nature or given the infection by direct inoculation.58, 68 Depending on the mechanism of releasing the fleas, for example by direct release from an aircraft by dropping non-explosive missiles, there could be a location-based release which would have a more acute response than a covert attack.46 Then, the initial outbreak could be identified as the casualties travel directly to emergency departments similar to what would be expected in a chemical weapons attack.69
Assuming it was successful, an attack with fleas would primarily cause bubonic plague21 and rarely primary septicemic plague, as the fleas feast on the humans as their primary source of blood and inoculate them with the bacteria. There would be no human-to-human transmission except by secondary pneumonic plague, which only 3% of bubonic plague cases develop into.25 With the development and use of insecticides the contagion could be contained rapidly, so an attack like this is rendered unlikely.48, 70 Furthermore, as bubonic plague is less virulent than pneumonic plague,2 and antibiotics are an effective treatment – particularly if initiated early as they seemingly would be here, then mortality could be kept low – possibly even less than the estimated 5% mortality with early intervention.25
Hence, a deliberate release of plague by using infected fleas as vectors is a feasible attack option, as it has been done to a moderate effect before, but is unlikely to be used since the response would be particularly robust, resulting in low mortality rates.
4.2.3. Current threat
In order to evaluate the current threat of use of biological weapons, particularly using Y. pestis, we must consider who would use them, if they would be able to do so, and for what motive. We have now established that the most likely form of deliberate release of plague would be aerosol dispersal of pneumonic plague.
4.2.3.1. Motives for using plague as a weapon
The main motivation for using a biological weapon such as plague would be to terrify the population, rather than to kill a large number of people. If the primary intention was to kill a large number of people, then other means of attack such as the use of bombs would be more effective, cheaper and easier. The success of a deliberate release of Y. pestis would be quantified by the fear, societal disruption and panic rather than the number of deaths, by the attackers.42
A deliberate release of pneumonic plague would be particularly likely to create fear in the public for a number of reasons. The pathology of pneumonic infection, which is silent in the beginning with seemingly innocent symptoms, can develop rapidly to a fatal syndrome within 24 hours,21 so those who feel they might have symptoms may rush anxiously to seek medical attention. Also, the possibility of a relatively slower death compared to an incendiary device could cause more terror. As plague is transmissible between humans, unlike anthrax and botulinum, it could create fears of a spreading pandemic across the nation, particularly since people would know little about this modern-day infection with Y. pestis.34 What the public are more aware of is the history of plague, particularly “The Black Death” and its devastating effects on past civilizations – reminding them of the severity of the disease, and so increasing fear more so than other potential biological weapons. Depending on the mechanism of release it could be difficult to identify the perpetrator, or the location of the initial release, increasing fear of a continuing release infecting more people, although it is likely to be controlled quickly.
4.2.3.2. Who would deliberately release plague?
With the tremendous technological advancements in science and medicine over the last few decades, the major restrictions of using biological weapons are formed by international opinion, rather than capability.42, 63 Possible aggressors who could have a role in the deliberate release of biological weapons would include individuals, rogue terrorist states, and there is also the possibility of biological warfare from state actors.46 Biological weapons have been known as “the poor man’s nuclear bomb” as they are relatively cheap for the damage they cause, and can cause significant economic disruption, but they can be difficult to produce and disseminate on a large enough scale.46
Individuals and rogue states would likely have difficulty in producing and dispersing pneumonic plague due to technical constraints related to cell culture and dispersal of plague, but it would not be impossible.63
According to the Biological Weapons Convention (BWC) of 1972, nations are prohibited to undertake research to produce biological weapons or to produce and stockpile them.71 However, the BWC has no inspection mechanism and a biological weapons research and production program would be relatively easy to hide in a nation’s biotechnological infrastructure.42 Furthermore, it does not describe which biological agents are not to be developed or what quantities would go beyond defensive research.42 Notwithstanding those who have signed and ratified the BWC, it is fairly certain that a number of rogue nations or those willing to risk international disgrace are secretly carrying out their own biological weapons research. There are numerous allegations that many countries have biological weapons programs including China, Egypt, Iran, Israel, North Korea, Russia, Syria and the USA, but all deny these allegations.72 The most recent chemical weapons attack on British soil was almost certain to have been undertaken by the Russian state using Novichok.73, 74 This preposterous action could be a defining moment in a change in warfare to make state on state violence more likely, and the states would probably be able to use plague.
Therefore, Y. pestis could potentially be used as a biological weapon and would be an excellent choice if the aim of the attack would be to incite fear. It is unlikely to be used by any actors other than nations due to the technical difficulties of a clandestine release.
4.3. Medical response
4.3.1. Initial outbreak, medical treatment and identification of deliberate release
In consideration of a covert deliberate release of pneumonic plague, the most likely form of attack of plague, the natural history is likely to follow along these lines:
-
1.
A number of patients who are infected sit with a silent infection for around 24 hours to 3 days.21
-
2.
The first of these people will begin to have coryzal symptoms, headache, malaise and fever, which is to be expected to lead them to their General Practitioner or local Emergency Department. It is likely that they will be diagnosed with a viral upper respiratory tract infection and be discharged home with a normal chest x-ray at such an early stage.75, 76
-
3.
Within the next 24 hours (with the length of time depending on their initial bacterial challenge and their immune status) a significant number of patients will suddenly deteriorate and begin to develop respiratory failure and have hemoptysis.21
-
4.
Blood and sputum cultures of the bacteria causing the pneumonia will be taken and antibiotics would begin and according to national guidance, the severity of the disease would be calculated, and the treatment delivered relative to this. As we know the early and accurate initiation of antibiotics is vital in treating pneumonic plague and reducing mortality. The first line antibiotic for mild, moderate and severe community acquired pneumonia is a penicillin (in many locations) which would be ineffective against plague, but the addition of a macrolide would provide some limited coverage. The 2nd line, or for those with penicillin allergies, is doxycycline (or an alternative tetracycline) which is highly active and recommended against Y. pestis. Ideally, respiratory precautions would be undertaken with any suspicion of Influenza or Mycobacterium tuberculosis, infections these patients would be highly likely suspected of having.77
-
5.
Either with the arrival of many, especially including young, fit and healthy patients or return of culture results (taken between 24–48 h) we would become aware of the presence of Y. pestis.
-
6.
Public health organizations would be alerted rapidly, and a huge and robust response would ensue. The total time taken to alert these organizations from the beginning of the release would most likely be around 2–3 days, the time necessary for symptoms to develop and cultures to be identified. Moreover, this time could be considerably reduced if a huge number of patients were infected initially, as this would have ignited front-line clinician suspicions.
The danger of the time lapse of identifying plague in such a scenario would be that, if the release was ongoing, more patients would be infected from the release source, so it would need to be quashed quickly. Another thing to consider is that there could be human-to-human transmission in this time, spreading plague to secondary cases, but this is unlikely to be on a large scale as those with a pneumonic Y. pestis infection generally need to progress in their disease process before they become very contagious,2 but since the inoculation challenge is relatively low (100 – 2,000 organisms),42 it is still possible.
4.3.2. Public health response
Once the public health services have been notified, a robust and powerful response would occur.78 In order to break the human-to-human transmission of pneumonic plague, infected patients must wear simple surgical face masks, or even cover their face with their jackets to prevent droplet spread, and normal infectious respiratory precautions need to be followed for at least the first three days of antibiotic therapy to prevent spread.2, 42 Once face masks have been distributed to the general population along with other hygiene advice including hand washing, isolation and social distancing over 2 meters to prevent inhalation of droplets, the rate of transmission would drop rapidly.1, 2 The response could be initiated in a similar way to that of a response to the Coronavirus pandemic or a pandemic of influenza, for which the UK has prepared to a relatively high degree. The recent testing of the international public health response from the COVID-19 pandemic has primed all populations to understand the requirements of a robust response, and be practised in implementing self-isolation techinques and social distancing. Therefore, we would likely be able to prevent the transmission of plague through the population quickly, as seen by the 'primed' population of Ningbo in 1940.79, 48
However, due to the nature of pneumonic plague infection, those who do not receive antibiotics within 24 hours of their symptoms beginning, will have almost 100% mortality rates.2 Those who are treated quickly are likely to do much better but still will have mortality rates as high as up to 40–60%, and some will require significant supportive therapy including ITU support.21
Post-exposure-prophylaxis would also assist in preventing the disease developing in asymptomatic patients potentially exposed to Y. pestis. 2, 36 We would also probably need to cull some domestic animals in the homes of infected patients to prevent plague persisting and causing subsequent infections.48
4.3.3. Prehospital treatment strategy and identification
In the scenario of a deliberate release of pneumonic plague, it is likely there would be huge media coverage and heightened fear in the public. In terms of treating patients, and especially for distributing prophylactic antibiotics, we could employ a number of strategies to try to avoid swathes of patients attending local emergency departments. For example, we could request all patients who suspect that they are symptomatic to come to a mass treatment center (such as a local sports hall) and evaluate all patients there for convenience and ease. However, it could have drawbacks, including increasing transmission between patients with plague and those with either no disease or with the flu – this is particularly risky in the possibility of a suicide attack event with plague.80
Another potential treatment strategy could include a method similar to the influenza plan, with patients remaining at home and calling a phone number to be assessed over the phone and having a “flu friend” to go to the local pharmacy to obtain antivirals or antibiotics in the case of plague. A flu friend being someone who does not have any symptoms at all.79 Therefore, maintaining some level of isolation of patients from each other.
One potential strategy could include pre-hospital health care workers utilizing newer rapid diagnostic technology: either driving to patients or evaluating them at a mass treatment center. Developments of rapid identification systems for Y. pestis could provide a much faster diagnosis and identify those who require escalation to hospital for definitive treatment, and could be utilized in the prehospital setting to triage patients.81, 82, 83, 84 A dipstick system has been developed for detection of the F1 antigen in humans, and another immuno-strip (PLA-dipstick) that can detect F1-negative plague rapidly in asymptomatic humans under field conditions.85, 86 This could prove extremely valuable in the prehospital and hospital settings to guide management, and requirement for post exposure prophylaxis.
4.3.4. Vaccine
Y. pestis is one of the most virulent human pathogens, and to this date there are no licensed plague vaccines available.7 Historically, there was a formalin-killed whole cell Y. pestis vaccine developed and used by the American military in the Vietnam war.87, 88 Unfortunately, the vaccine was highly reactogenic and only conveyed some protection to bubonic plague and no protection to pneumonic plague and has since been discontinued.87, 89
A vaccine would be the ideal preventative treatment against a deliberate release of plague and could protect the population from this dangerous disease in the circumstances of a deliberate release. The WHO proposes two potential strategies for vaccination: a reactive vaccine to prevent plague during an outbreak and to interrupt the chains of transmission, and a prophylactic vaccine used primarily in endemic areas. A reactive vaccine would be most ideal for use in deliberate release but would require a number of “critical” qualities, as defined by WHO, to make it effective. It would need to provide safe and long-lasting protection, be effective against all strains and all forms of plague including pneumonic, and be easily delivered to large volumes of the population – preferably through oral or mucosal routes.90
There are currently 17 potential plague vaccines in the pipeline, of which a number show very promising results – such as oral vaccination with Y. pseudotuberculosis as the two pathogens have surprisingly similar genetic information.6, 7, 14, 90 Other positive recent advancements include bacteriophage vaccines91 and another vaccine which protects against both plague and anthrax.8 Furthermore, the food and drug administration (FDA) has granted “orphan drug status”92 for a plague vaccine for marketing in 2020 after successfully completing phase 2 trials; although this is a recombinant vaccine against only the F1 capsular antigen and the low-calcium-response V antigen (LcrV) of Y. pestis.7, 10 This, however, will not provide protection against F1 negative strains of plague, which exist in nature and could be used as a bioweapon, rendering the vaccine useless.
Other future treatments could include monoclonal antibodies as therapeutic vaccines, for example antibodies against the F1 subunit of plague, or the LcrV, have been created and used to passively protect mice from challenges of pneumonic plague and could be used to rapidly protect populations.7, 93, 94 However, these treatments also run into problems similar to those of the vaccine when individuals are challenged with F1-negative strains of plague.
The ideal long-term prevention of any disease is its eradication usually undertaken by vaccination, in a similar fashion to the eradication of smallpox.95 Y. pestis, however, cannot be feasibly eradicated from the world because of its reserves in nature and persistence in soil.96, 97
In summary, a vaccine would be the gold standard used for protection and as a deterrent for deliberate release of plague. At this moment in time we have no vaccine that could be used safely, but hopefully one will be on its way soon and offer protection to populations against a deliberate release of plague.7 Theoretically, if an attack did occur at this moment we could expedite the production process of a vaccine rapidly, but this would be associated with significant risks and probably could not be done within a useful timeframe, unless the outbreak was sustained over a long period.
4.4. Potential future considerations
4.4.1. Antibiotic resistant plague
The primary treatment for infection with Y. pestis is the use of antibiotics.36 The emergence of antibiotic resistant strains of plague is a large public health concern,24 particularly with the possibility of a deliberate release of Y. pestis.
In nature, a number of antibiotic resistant strains of Y. pestis have been identified,98 including two human cases in Madagascar in 1995 where one was found to be resistant to streptomycin and the other had multidrug resistance (MDR). These strains obtained their resistance from the transfer of conjugative plasmids, most likely transmitted from MDR enterobacteria.99 Since then, investigations have been undertaken to identify the distribution of these antibiotic resistant strains, and extensive screening has shown us that they are very rare in nature.100 However, all the identified resistant strains demonstrate that Y. pestis has the potential to be resistant to ampicillin, chloramphenicol, kanamycin, streptomycin, spectinomycin, sulfonamides, tetracycline, minocycline, and doxycycline.98, 100
The question then arises, “how would someone malevolently use antibiotic resistant plague in a deliberate release?”. One could potentially obtain some of these MDR strains from nature; however they are exceptionally rare and would be extremely difficult to find.101 From our understanding of the biology of antibiotic resistance in Y. pestis, which originated from plasmid transfer, there is significant potential for artificial creation of MDR plague. Nevertheless, this would require a high, probably post-doctorate, level of understanding and the use of equally advanced equipment. This would make MDR plague unlikely unless formed and used by state level biological weapons programs, as has been reported in the past.51 Although, as recent development of CRISPR technology has demonstrated that genetic modification and optimization of Y. pestis is possible, it may become easier for those with less understanding to do what has previously been much more difficult.102
Although unlikely, we should evaluate how we could respond to such an attack, which would surely bring about even more fear, in terms of the medical treatment. Significant amounts of research have been undertaken into alternative therapies against Y. pestis, including, immunotherapy, immunomodulatory therapy, phage therapy, bacteriocin therapy, and the use of inhibitors of virulence factors. These have shown some promise in mouse studies, but they are a long way away from being used in clinical practice, yet hope remains they will provide a new line of defense against MDR plague.103, 104
In summary, an attack of MDR plague, although possible, is very unlikely due to technical limitations. The public health response would be even more important in this situation, the primary aim would be the reduction in the number of people infected to begin with, then the simultaneous treatment of those infected with a wide range of antibiotics (for example trimethoprim-sulfamethoxazole has successfully been used in a single case of MDR plague98) and the consideration of alternative therapies. Furthermore, thoughtful consideration should be taken when treating plague in the mass casualty setting, as massive administration of singular antimicrobial medications may lead to the preferential selection of antibiotic resistant strains.105
4.4.2. F1-negative plague and genetically modified plague
As briefly discussed above in regard to vaccines against Y. pestis, those currently in the later stages of coming to market are aimed at the F1 antigen of plague.7, 8 Also, many rapid diagnostic tests aim to detect the F1 antigen, along with the antibody detection for the definitive diagnosis of Y. pestis infection,106 so it may be difficult to detect and protect against F1-negative plague. There have previously been allegations that some nation’s biological warfare programs created F1-negative strains of Y. pestis and stored them.51 Therefore, we must be convinced of the coverage of the vaccine we roll out before we do so and rely on clinical judgement for the treatment of patients rather than solely on tests. It is also possible that a genetically modified plague is developed, depending on the creator of the bioweapon and their potential capabilities. Probably, most post-graduate level microbiologists or geneticists would have no difficulty in doing this successfully, developing already deadly bacteria into something even more evil. However, we must remember that the public health response, specifically the use of modest measures such as social distancing and simple face masks can completely eliminate the transmission of pneumonic plague.
4.4.3. Plague mimic
A possible tactic that could be used in a biological weapons attack is that of a mimic. The initial symptoms of pneumonic plague are almost identical to those at the beginning of many other respiratory illnesses, including in particular those of influenza and the common cold.36 Furthermore, there are other diseases that could mimic plague in the later stages such as Streptococcus pneumoniae, Staphylococcus aureus, Francisella tularensis, and a severe viral pneumonia from influenza A or B viruses.27
If a deliberate release of plague occurred alongside a deliberate release of influenza or, released alongside the normal seasonal variations of influenza, then there would be a significantly higher number of people having the initial symptoms of both, including cough, fevers, and coryzal symptoms. This could lead to a huge number of patients presenting to many healthcare centers with fear of having the symptoms of pneumonic plague, overwhelming the services. A prehospital treatment model with the usage of rapid diagnostic dipstick could potentially aid in the triage of patients and prioritize the treatment of those having Y. pestis infection.85, 107
5. Conclusion
To use plague as a bioweapon, one must be able to obtain, isolate, culture and transport plague, and use a number of feasible mechanisms of plague dispersal to infect a significant number of humans. Pneumonic plague in particular, having a high mortality, is the most likely form of plague to be used as a bioweapon, although this does come with significant difficulties. Assuming the primary aim of an attacker was to incite fear, plague would be an excellent choice of weapon, no matter how many people were killed. There is potential for a number of actors to be able to deliberately release plague in a clandestine attack, the most likely being state actors, primarily due to technological capabilities.
Our medical and public health response would be robust, quickly preventing the spread of pneumonic plague transmission in the population as soon as the first case was identified but would be under great stress from the casualties – a significant proportion of those initially infected may still die rapidly. There could be a number of strategies to the public health response, use of prehospital Y. pestis dipsticks may be a preferable option, particularly in the event of a plague mimic. A vaccine against Y. pestis would be ideal for reactive or prophylactic immunization. We are relatively close to producing a potential vaccine but at this moment there are no available plague vaccines, particularly against F1-negative plague, which is an additional potential danger. Genetically modified and antibiotic resistant plague could be an avenue to increase the mortality of plague in those infected, although some novel therapies could provide a solution, but the basic public health strategies such as the use of surgical face masks would contain the spread.
Therefore, a deliberate release of Y. pestis is feasible but unlikely to occur due to technical constraints, particularly of release, and the strong public health response would prevent the spread of pneumonic plague. The next steps would be to create a viable vaccine, continue research on novel therapies, distribute rapid diagnostic tests, and raise the awareness of clinicians to the possibility of an attack. Moreover, it is vital to acknowledge the importance of early isolation of patients and timely initiation of public health mechanisms.
Footnotes
A confirmed case of Y. pestis infection, according to the World Health Organization, is one which has been confirmed by the gold standard test for plague–laboratory isolation of Y. pestis from a clinical specimen.
A bubo is an acutely swollen, exquisitely tender lymph node.
Basic reproductive number, R0, represents the expected number of secondary infections following introduction of a single infected individual into a completely susceptible population.
Appendix 1. Literature search strings
| MEDLINE/PUBMED | |
|---|---|
| Search String | Number of papers found |
| Plague | 10,413 |
| Y. Pestis | 4887 |
| Plague OR Y. Pestis | 12,359 |
| Deliberate release | 490 |
| Intentional release | 548 |
| Biowarfare | 7913 |
| Bioterrorism | 6249 |
| Terrorism | 14,734 |
| ((((Deliberate Release) OR Intentional release) OR Biowarfare) OR Bioterrorism) OR Terrorism | 20,004 |
| (((Plague) OR Y. Pestis)) AND (((((Deliberate Release) OR Intentional release) OR Biowarfare) OR Bioterrorism) OR Terrorism) | 500 |
| Search (((Plague) OR Y. Pestis)) AND (((((Deliberate Release) OR Intentional release) OR Biowarfare) OR Bioterrorism) OR Terrorism) Sort by: Best Match Filters: Publication date from 2010/01/01 to 2019/12/31 | 166 |
| EMBASE | |
|---|---|
| Search String | Number of papers found |
| Plague OR “Y. Pestis” | 16,026 |
| “Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism | 15,321 |
| (Plague OR “Y. Pestis”) AND (“Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism) | 571 |
| (Plague OR “Y. Pestis”) AND (“Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism) AND [2010–2019]/py | 173 |
| Web of Science | |
|---|---|
| Search String | Number of papers found |
| Plague OR “Y. Pestis” | 21,623 |
| “Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism | 25,877 |
| (Plague OR “Y. Pestis”) AND (“Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism) | 350 |
| (Plague OR “Y. Pestis”) AND (“Deliberate release” OR “intentional release” OR Biowarfare OR Bioterrorism OR Terrorism)Refined by: PUBLICATION YEARS: (2019 OR 2013 OR 2018 OR 2012 OR 2017 OR 2011 OR 2016 OR 2010 OR 2015 OR 2014 ) | 163 |
References
- 1.Perry R.D., Fetherston J.D. Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pechous R.D., Sivaraman V., Stasulli N.M., Goldman W.E. Pneumonic Plague: The Darker Side of Yersinia pestis. Trends Microbiol. 2016;24(3):190–197. doi: 10.1016/j.tim.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 3.GoW Consultants. WHO; WHO: 1970. Health aspects of chemical and biological weapons. [Google Scholar]
- 4.Demeure C.E., Dussurget O., Mas Fiol G., Le Guern A.S., Savin C., Pizarro-Cerdá J. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Genes Immun. 2019 doi: 10.1038/s41435-019-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson J. Combating Plague: Fighting a War on Two Fronts Through Vaccine Design and New Therapeutic Intervention. Doctoral Dissertation. UTMB Health: UTMB. Health. 2017 [Google Scholar]
- 6.Demeure C.E., Derbise A., Carniel E. Oral vaccination against plague using Yersinia pseudotuberculosis. Chem Biol Interact. 2016 doi: 10.1016/j.cbi.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Sun W., Singh A.K. Plague vaccine: recent progress and prospects. npj Vaccines. 2019;4. doi: 10.1038/s41541-019-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao P., Mahalingam M., Zhu J. A Bivalent Anthrax-Plague Vaccine That Can Protect against Two Tier-1 Bioterror Pathogens, Bacillus anthracis and Yersinia pestis. Front Immunol. 2017;8:687. doi: 10.3389/fimmu.2017.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta G., Khan A.A., Rao D.N. Cell-Mediated Immune Response and Th-1/Th-2 Cytokine Profile of B-T Constructs of F1 and V Antigen of Yersinia pestis. Scand J Immunol. 2010;71(3):186–198. doi: 10.1111/j.1365-3083.2009.02365.x. [DOI] [PubMed] [Google Scholar]
- 10.Moore B.D., New R.R.C., Butcher W. Dual route vaccination for plague with emergency use applications. Vaccine. 2018;36(34):5210–5217. doi: 10.1016/j.vaccine.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Papagrigorakis M.J., Synodinos P.N., Stathi A., Skevaki C.L., Zachariadou L. The plague of Athens: an ancient act of bioterrorism? Biosecur Bioterror. 2013;11(3):228–229. doi: 10.1089/bsp.2013.0057. [DOI] [PubMed] [Google Scholar]
- 12.Campbell G.D. The plague of Athens. Proc R Coll Physicians Edinb. 1997;27(2):263–264. [PubMed] [Google Scholar]
- 13.Khan I.A. Plague: the dreadful visitation occupying the human mind for centuries. Trans R Soc Trop Med Hyg. 2004;98(5):270–277. doi: 10.1016/S0035-9203(03)00059-2. [DOI] [PubMed] [Google Scholar]
- 14.Andam C.P., Worby C.J., Chang Q., Campana M.G. Microbial Genomics of Ancient Plagues and Outbreaks. Trends Microbiol. 2016;24(12):978–990. doi: 10.1016/j.tim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bos K.I., Schuenemann V.J., Golding G.B. A draft genome of Yersinia pestis from victims of the Black Death. Nature. 2011;478:506. doi: 10.1038/nature10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict O.J. Boydell Press; 2004. The Black Death 1346–1353: The Complete History. [Google Scholar]
- 17.Organisation W.H. Plague Fact Sheet. 2017 [Google Scholar]
- 18.Organisation WH. Plague around the world 2010-2015. WHO - weekly epidemiological review; 2016.
- 19.Randremanana R., Andrianaivoarimanana V., Nikolay B. Epidemiological characteristics of an urban plague epidemic in Madagascar, August–November, 2017: an outbreak report. Lancet Infect Dis. 2019;19(5):537–545. doi: 10.1016/S1473-3099(18)30730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organisation WH. WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases - Plague. WHO; 2000.
- 21.Yang R. Plague: Recognition, Treatment, and Prevention. J Clin Microbiol. 2018;56(1) doi: 10.1128/JCM.01519-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove S.E., Perl T.M., Song X., Sisson S.D. Ability of physicians to diagnose and manage illness due to category A bioterrorism agents. Arch Intern Med. 2005;165(17):2002–2006. doi: 10.1001/archinte.165.17.2002. [DOI] [PubMed] [Google Scholar]
- 23.Prentice M.B., Rahalison L. Plague. The Lancet. 2007;369(9568):1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- 24.Inglesby T.V., Dennis D.T., Henderson D.A. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama. 2000;283(17):2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 25.Narayanan N., Lacy C.R., Cruz J.E. Disaster preparedness: biological threats and treatment options. Pharmacotherapy. 2018;38(2):217–234. doi: 10.1002/phar.2068. [DOI] [PubMed] [Google Scholar]
- 26.Mollaret H.H. A personal view of the history of the genus Yersinia. Contrib Microbiol Immunol. 1987;9:1–13. [PubMed] [Google Scholar]
- 27.Kwon E.H., Reisler R.B., Cardile A.P., Cieslak T.J., D”Onofrio MJ, Hewlett AL, Distinguishing Respiratory Features of Category A/B Potential Bioterrorism Agents from Community-Acquired Pneumonia. Health Secur. 2018;16(4):224–238. doi: 10.1089/hs.2018.0017. [DOI] [PubMed] [Google Scholar]
- 28.Hu Wang YC, Zuyun Wang, Xiaoyi Wang, Zhaobiao Guo, Yanfeng Yan,, Chao Li BC, Xiao Xiao, Yonghai Yang, Zhizhen Qi, Guojun Wang, Baiqing Wei,, Shouhong Yu DH, Hongjian Chen, Gang Chen, Yajun Song and Ruifu Yang. A Dog-Associated Primary Pneumonic Plague in Qinghai Province, China. Clinical infectious disease. 2010; Volume 52, Issue 2. [DOI] [PubMed]
- 29.John M. Doll † PSZ, Paul Ettestad, Ann L. Bucholtz, Ted Davis, Kenneth Gage. Cat-Transmitted Fatal Pneumonic Plague in a Person who Traveled from Colorado to Arizona. The American Journal of Tropical Medicine and Hygiene , Volume 51, Issue 1, 1 Jul 1994, p 109 - 114. 1994. [DOI] [PubMed]
- 30.Rabaan A.A., Al-Ahmed S.H., Alsuliman S.A., Aldrazi F.A., Alfouzan W.A., Haque S. The rise of pneumonic plague in Madagascar: current plague outbreak breaks usual seasonal mould. J Med Microbiol. 2019;68(3):292–302. doi: 10.1099/jmm.0.000915. [DOI] [PubMed] [Google Scholar]
- 31.Layton R.C., Mega W., McDonald J.D. Levofloxacin cures experimental pneumonic plague in African green monkeys. PLoS Negl Trop Dis. 2011;5(2) doi: 10.1371/journal.pntd.0000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y.-F., Li D.-B., Shao H.-S., Li H.-J., Han Y.-D. Plague in China 2014-All sporadic case report of pneumonic plague. BMC Infectious Diseases. 2016;16:85-. doi: 10.1186/s12879-016-1403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kool J.L., Weinstein R.A. Risk of Person-to-Person Transmission of Pneumonic Plague. Clin Infect Dis. 2005;40(8):1166–1172. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 34.Hinckley A.F., Biggerstaff B.J., Griffith K.S., Mead P.S. Transmission dynamics of primary pneumonic plague in the USA. Epidemiol Infect. 2012;140(3):554–560. doi: 10.1017/S0950268811001245. [DOI] [PubMed] [Google Scholar]
- 35.Majumder MS, Cohn EL, Santillana M, Brownstein JS. Estimation of Pneumonic Plague Transmission in Madagascar, August-November 2017. PLoS Curr. 2018;10. [DOI] [PMC free article] [PubMed]
- 36.Christian M.D. Biowarfare and bioterrorism. Crit Care Clin. 2013;29(3):717–756. doi: 10.1016/j.ccc.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson A.K., Ellington S., Nelson C., Treadwell T., Jamieson D.J., Meaney-Delman D.M. Preparing for Biological Threats: Addressing the Needs of Pregnant Women. Birth Defects Research. 2017;109(5):391–398. doi: 10.1002/bdr2.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organisation W.H. Plague manual: epidemiology, distribution, surveillance and. control. 1999 [PubMed] [Google Scholar]
- 39.Practice BB. Yersnia Infection, Management Algorithm, BMJ Best Practice. In: England PH, editor. 2017.
- 40.Darling R.G., Catlett C.L., Huebner K.D., Jarrett D.G. Threats in bioterrorism. I: CDC category A agents. Emerg Med Clin North Am. 2002;20(2):273–309. doi: 10.1016/s0733-8627(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 41.Centre for Disease Control U. Bioterrorism Agents/Diseases: CDC; 2019 [Available from: https://emergency.cdc.gov/agent/agentlist-category.asp.
- 42.Jansen H.J., Breeveld F.J., Stijnis C., Grobusch M.P. Biological warfare, bioterrorism, and biocrime. Clin Microbiol Infect. 2014;20(6):488–496. doi: 10.1111/1469-0691.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong T., Gurian P.L., Huang Y., Haas C.N. Prioritizing risks and uncertainties from intentional release of selected Category A pathogens. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0032732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mark W. Biological Warfare at the 1346 Siege of Caffa. Em Infectious Disease J. 2002;8(9):971. doi: 10.3201/eid0809.010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derbes V.J. De Mussis and the great plague of 1348. A forgotten episode of bacteriological warfare. JAMA. 1966;196(1):59–62. [PubMed] [Google Scholar]
- 46.Beeching N.J., Dance D.A., Miller A.R., Spencer R.C. Biological warfare and bioterrorism. BMJ. 2002;324(7333):336–339. doi: 10.1136/bmj.324.7333.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keiichi T. Unit 731 and the Japanese Imperial Army's Biological Warfare Program. The Asia-Pacific Journal, Japan. Focus. 2005;Volume;3(Issue:11). [Google Scholar]
- 48.Wilson J.M., Daniel M. Historical reconstruction of the community response, and related epidemiology, of a suspected biological weapon attack in Ningbo, China (1940) Intelligence and National Security. 2019;34(2):278–288. [Google Scholar]
- 49.Jacobs M.K. The history of biologic warfare and bioterrorism. Dermatol Clin. 2004;22(3):231–246. doi: 10.1016/j.det.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Sabbatani S. The experiments conducted by Japanese on human guinea pigs, and the use of biological weapons during the Sino-Japanese war (1937–1945) Infez Med. 2014;22(3):255–266. [PubMed] [Google Scholar]
- 51.Carus W.S. The history of biological weapons use: what we know and what we don”t. Health Security. 2015;13(4):219–255. doi: 10.1089/hs.2014.0092. [DOI] [PubMed] [Google Scholar]
- 52.Metcalfe N. A short history of biological warfare. Med Confl Surviv. 2002;18(3):271–282. doi: 10.1080/13623690208409635. [DOI] [PubMed] [Google Scholar]
- 53.Lockwood J.A. Insects as weapons of war, terror, and torture. Annu Rev Entomol. 2012;57:205–227. doi: 10.1146/annurev-ento-120710-100618. [DOI] [PubMed] [Google Scholar]
- 54.Cobbs C.G., Chansolme D.H. Plague. Dermatol Clin. 2004;22(3):303–312. doi: 10.1016/j.det.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Sarovich D.S., Colman R.E., Price E.P. Selective isolation of Yersinia pestis from plague-infected fleas. J Microbiol Methods. 2010;82(1):95–97. doi: 10.1016/j.mimet.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasoamanana B., Rahalison L., Raharimanana C., Chanteau S. Comparison of Yersinia CIN agar and mouse inoculation assay for the diagnosis of plague. Trans R Soc Trop Med Hyg. 1996;90(6):651. doi: 10.1016/s0035-9203(96)90420-4. [DOI] [PubMed] [Google Scholar]
- 57.Agar S.L., Sha J., Foltz S.M. Characterization of a mouse model of plague after aerosolization of Yersinia pestis CO92. Microbiology. 2008;154(Pt 7):1939–1948. doi: 10.1099/mic.0.2008/017335-0. [DOI] [PubMed] [Google Scholar]
- 58.Engelthaler D.M., Gage K.L., Montenieri J.A., Chu M., Carter L.G. PCR detection of Yersinia pestis in fleas: comparison with mouse inoculation. J Clin Microbiol. 1999;37(6):1980–1984. doi: 10.1128/jcm.37.6.1980-1984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safari Foroshani N., Karami A., Pourali F. Simultaneous and Rapid Detection of Salmonella typhi, Bacillus anthracis, and Yersinia pestis by Using Multiplex Polymerase Chain Reaction (PCR) Iran Red Crescent Med J. 2013;15(11) doi: 10.5812/ircmj.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torosian S.D., Regan P.M., Taylor M.A., Margolin A. Detection of Yersinia pestis over time in seeded bottled water samples by cultivation on heart infusion agar. Can J Microbiol. 2009;55(9):1125–1129. doi: 10.1139/w09-061. [DOI] [PubMed] [Google Scholar]
- 61.Gilbert S.E., Rose L.J. Survival and persistence of nonspore-forming biothreat agents in water. Lett Appl Microbiol. 2012;55(3):189–194. doi: 10.1111/j.1472-765X.2012.03277.x. [DOI] [PubMed] [Google Scholar]
- 62.Corriveau JL. Aerobiological aspects of biological warfare. 2016. p. 330-44.
- 63.Carus W, S. Bioterrorism and Biocrimes: The Illicit Use of Biological Agents Since 1900. National defense Univ Washington DC. 2001.
- 64.Rose L.J., Donlan R., Banerjee S.N., Arduino M.J. Survival of Yersinia pestis on environmental surfaces. Appl Environ Microbiol. 2003;69(4):2166–2171. doi: 10.1128/AEM.69.4.2166-2171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casman E.A., Fischhoff B. Risk communication planning for the aftermath of a plague bioattack. Risk Anal. 2008;28(5):1327–1342. doi: 10.1111/j.1539-6924.2008.01080.x. [DOI] [PubMed] [Google Scholar]
- 66.Li B., Tan Y., Guo J. Use of protein microarray to identify gene expression changes of Yersinia pestis at different temperatures. Can J Microbiol. 2011;57(4):287–294. doi: 10.1139/w11-007. [DOI] [PubMed] [Google Scholar]
- 67.Han Y., Zhou D., Pang X. Microarray analysis of temperature-induced transcriptome of Yersinia pestis. Microbiol Immunol. 2004;48(11):791–805. doi: 10.1111/j.1348-0421.2004.tb03605.x. [DOI] [PubMed] [Google Scholar]
- 68.Engelthaler D.M., Gage K.L. Quantities of Yersinia pestis in fleas (Siphonaptera: Pulicidae, Ceratophyllidae, and Hystrichopsyllidae) collected from areas of known or suspected plague activity. J Med Entomol. 2000;37(3):422–426. doi: 10.1093/jmedent/37.3.422. [DOI] [PubMed] [Google Scholar]
- 69.Adalja A.A. Biothreat Agents and Emerging Infectious Disease in the Emergency Department. Emerg Med Clin North Am. 2018;36(4):823–834. doi: 10.1016/j.emc.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weir E. Plague: a continuing threat. CMAJ : Canadian Medical Association journal = journal de l”Association medicale canadienne. 2005;172(12):1555-. [DOI] [PMC free article] [PubMed]
- 71.Affairs UNOfD. The Biological and Toxins Weapons Convention, Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction. United Nations2019.
- 72.Association AC. Chemical and Biological weapons fact sheet update June 2018. 2018.
- 73.Prime minister Theresa May HoC. Salisbury Update. In: House of Commons UG, editor. 05 September 2018.
- 74.Peter Wilson UPRttO. OPCW Executive Council meeting: 18 April update on the use of a nerve agent in Salisbury In: House of Commons UG, editor. 18 April 2018.
- 75.Yang R.F. Plague: Recognition, Treatment, and Prevention. J Clin Microbiol. 2018;56(1) doi: 10.1128/JCM.01519-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adalja A.A., Toner E., Inglesby T.V. Clinical management of potential bioterrorism-related conditions. N Engl J Med. 2015;372(10):954–962. doi: 10.1056/NEJMra1409755. [DOI] [PubMed] [Google Scholar]
- 77.Practice BB. Community Acquired Pneumonia diagnosis, treatment and managment overview. 2019.
- 78.Shoaf K.I., Rottman S.J. The Role of Public Health in Disaster Preparedness, Mitigation, Response, and Recovery. Prehospital and Disaster Medicine. 2000;15(4):18–20. [PubMed] [Google Scholar]
- 79.Health UDo. UK Influenza Pandemic Preparedness Strategy. In: office Npf, editor. 2011.
- 80.Rubin G.J., Dickmann P. How to reduce the impact of “low-risk patients” following a bioterrorist incident: lessons from SARS, anthrax, and pneumonic plague. Biosecur Bioterror. 2010;8(1):37–43. doi: 10.1089/bsp.2009.0059. [DOI] [PubMed] [Google Scholar]
- 81.Banada P.P., Deshpande S., Banik S. Multiplex detection of three select agents directly from blood using the GeneXpert system. J Clin Microbiol. 2019 doi: 10.1128/JCM.00036-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jauset-Rubio M., Tomaso H., El-Shahawi M.S., Bashammakh A.S., Al-Youbi A.O., O'Sullivan C.K. Duplex Lateral Flow Assay for the Simultaneous Detection of Yersinia pestis and Francisella tularensis. Anal Chem. 2018;90(21):12745–12751. doi: 10.1021/acs.analchem.8b03105. [DOI] [PubMed] [Google Scholar]
- 83.Cho M., Chung S., Kim Y.T., Jung J.H., Kim D.H., Seo T.S. A fully integrated microdevice for biobarcode assay based biological agent detection. Lab Chip. 2015;15(13):2744–2748. doi: 10.1039/c5lc00355e. [DOI] [PubMed] [Google Scholar]
- 84.Batra S.A., Krupanidhi S., Tuteja U. A sensitive & specific multiplex PCR assay for simultaneous detection of Bacillus anthracis, Yersinia pestis, Burkholderia pseudomallei & Brucella species. Indian J Med Res. 2013;138:111–116. [PMC free article] [PubMed] [Google Scholar]
- 85.Simon S., Demeure C., Lamourette P. Fast and simple detection of Yersinia pestis applicable to field investigation of plague foci. PLoS ONE. 2013;8(1)::e54947-e. doi: 10.1371/journal.pone.0054947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chanteau S., Rahalison L., Ralafiarisoa L. Development and testing of a rapid diagnostic test for bubonic and pneumonic plague. The Lancet. 2003;361(9353):211–216. doi: 10.1016/S0140-6736(03)12270-2. [DOI] [PubMed] [Google Scholar]
- 87.Cavanaugh D.C., Elisberg B.L., Llewellyn C.H. Plague Immunization. V. Indirect Evidence for the Efficacy of Plague Vaccine. J Infect Dis. 1974;129(Supplement_1):S37–S40. doi: 10.1093/infdis/129.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- 88.Meyer K.F. Effectiveness of live or killed plague vaccines in man. Bull World Health Organ. 1970;42(5):653–666. [PMC free article] [PubMed] [Google Scholar]
- 89.Lutwick L.I., Nierengarten M.B. Vaccines for Category A bioterrorism diseases. Expert Opin Biol Ther. 2002;2(8):883–893. doi: 10.1517/14712598.2.8.883. [DOI] [PubMed] [Google Scholar]
- 90.Organisation TWH. The WHO Plague vaccine Target Product Profile (TPP) WHO2018 [Available from: https://www.who.int/blueprint/what/norms-standards/Plague_vaccines_workshop-23-april-2018/en/.
- 91.Tao P., Mahalingam M., Zhu J., Moayeri M., Sha J., Lawrence W.S. . A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague. MBio. 2018;9(5) doi: 10.1128/mBio.01926-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Administration FaD. FDA grants Orphan Drug Designation for Plague vaccine 2017 [Available from: https://globalbiodefense.com/2017/03/10/fda-grants-orphan-drug-designation-plague-vaccine/.
- 93.Avril A. Therapeutic antibodies for biodefense. 2017. p. 173-205. [DOI] [PubMed]
- 94.Froude J.W., Stiles B., Pelat T., Thullier P. Antibodies for biodefense. MAbs. 2011;3(6):517–527. doi: 10.4161/mabs.3.6.17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strassburg M.A. The global eradication of smallpox. Am J Infect Control. 1982;10(2):53–59. doi: 10.1016/0196-6553(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 96.Eisen R.J., Petersen J.M., Higgins C.L. Persistence of Yersinia pestis in soil under natural conditions. Emerg Infect Dis. 2008;14(6):941–943. doi: 10.3201/eid1406.080029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun W., Roland K.L., Curtiss R., 3rd. Developing live vaccines against plague. J Infect Dev Ctries. 2011;5(9):614–627. doi: 10.3855/jidc.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Galimand M., Guiyoule A., Gerbaud G. Multidrug Resistance in Yersinia pestis Mediated by a Transferable Plasmid. N Engl J Med. 1997;337(10):677–681. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 99.Welch T.J., Fricke W.F., McDermott P.F. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS ONE. 2007;2(3):e309-e. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cabanel N., Bouchier C., Rajerison M., Carniel E. Plasmid-mediated doxycycline resistance in a Yersinia pestis strain isolated from a rat. Int J Antimicrob Agents. 2018;51(2):249–254. doi: 10.1016/j.ijantimicag.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 101.Urich S.K., Chalcraft L., Schriefer M.E., Yockey B.M., Petersen J.M. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob Agents Chemother. 2012;56(1):555–558. doi: 10.1128/AAC.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang T., Wang M., Zhang Q. Reversible Gene Expression Control in Yersinia pestis Using an Optimized CRISPRi System. Appl Environ Microbiol. 2019 doi: 10.1128/AEM.00097-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anisimov A.P., Amoako K.K. Treatment of plague: promising alternatives to antibiotics. J Med Microbiol. 2006;55(Pt 11):1461–1475. doi: 10.1099/jmm.0.46697-0. [DOI] [PubMed] [Google Scholar]
- 104.Sulakvelidze A. Phage therapy: an attractive option for dealing with antibiotic-resistant bacterial infections. Drug Discov Today. 10. England. 2005:807–809. doi: 10.1016/S1359-6446(05)03441-0. [DOI] [PubMed] [Google Scholar]
- 105.Navas E. Problems associated with potential massive use of antimicrobial agents as prophylaxis or therapy of a bioterrorist attack. Clin Microbiol Infect. 2002;8(8):534–539. doi: 10.1046/j.1469-0691.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- 106.Glynn A., Freytag L.C., Clements J.D. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine. 2005;23(16):1957–1965. doi: 10.1016/j.vaccine.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 107.Safari Foroshani N., Karami A., Pourali F., Aeidi A., Fatemeh Tavana B.S. Simultaneous detection of Salmonella typhi, Bacillus antheracis, and Yersinea pestis by multiplex PCR. Journal of Zanjan University of Medical Sciences and Health Services. 2013;21(85):93–107. [Google Scholar]


