Abstract
SARS-CoV-2, SARS and MERS are all enveloped viruses that can cause acute respiratory syndrome. Arachidonic acid (AA) and other unsaturated fatty acids (especially eicosapentaenoic acd, EPA and docosahexaenoic acid DHA) are known to inactivate enveloped viruses and inhibit proliferation of various microbial organisms. The pro-inflammatory metabolites of AA and EPA such as prostaglandins, leukotrienes and thromboxanes induce inflammation whereas lipoxins, resolvins, protectins and maresins derived from AA, EPA and DHA not only suppress inflammation but also enhance would healing and augment phagocytosis of macrophages and other immunocytes and decrease microbial load. In view of these actions, it is suggested that AA and other unsaturated fatty acids and their metabolites may serve as endogenous anti-viral compounds and their deficiency may render humans susceptible to SARS-CoV-2, SARS and MERS and other similar viruses’ infections. Hence, oral or intravenous administration of AA and other unsaturated fatty acids may aid in enhancing resistance and recovery from SARS-CoV-2, SARS and MERS infections.
Key Words: SARS-CoV-2, SARS, MERS, Polyunsaturated fatty acids, Arachidonic acid, Prostaglandins, Lipoxin A4, Resolvins, Protectins and maresins, Inflammation
Introduction
The current epidemic of COVID-19 (coronavirus) has been declared by WHO as a global epidemic. This COVID-19 epidemic is somewhat similar to the previous severe acute respiratory syndrome (SARS-CoV-2 or SARS; 2002-2003) and Middle East respiratory syndrome (MERS; 2012-ongoing) outbreaks. All these infections could be traced to zoonotic transmission. All 3 viral infections have similar clinical presentation with fever and cough leading to lower respiratory tract disease with significant mortality especially in the elderly and those with underlying health conditions. These infections can be confirmed by nucleic acid testing of respiratory tract samples (eg, throat swabs) though initial clinical diagnosis is based on symptoms, exposures, and chest imaging. Since no specific effective antiviral drugs are available for these diseases, supportive care is of paramount importance (1).
SARS-CoV-2, SARS and MERS Infections are Similar
Studies showed that the SARS-CoV-2 and the current coronavirus disease 2019 (COVID-19) share similar genome. For instance, genome of SARS-CoV-2 shares about 80% identity with that of SARS-CoV and is about 96% identical to the bat coronavirus BatCoV RaTG13 (2).
It is interesting to note that angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for SARS coronavirus (SARS-CoV) and the new coronavirus (SARS-CoV-2) that is responsible for the current epidemic COVID-19 (3).
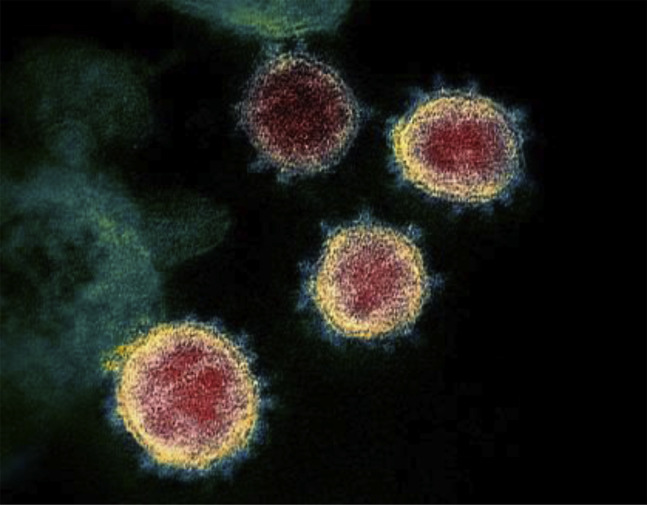
Like other coronaviruses, SARS-CoV-2 particles are spherical and have proteins called spikes protruding from their surface that can latch onto human cells, which then undergo a structural change allowing them to fuse with the cell membrane (Figure 1 ). This facilitates the viral genes to enter the host cell to be copied, producing more viruses. It was reported that the SARS-CoV-2 spike was 10–20 times more likely to bind ACE2 on human cells than the spike from the SARS virus from 2002, which enable SARS-CoV-2 to spread more easily from person to person than the earlier virus (4). Despite similarities in sequence and structure between the spikes of the two viruses, three different antibodies against the 2002 SARS virus could not successfully bind to the SARS-CoV-2 spike protein, suggesting that potential vaccine and antibody-based treatment strategies need to be more specific to SARS-CoV-2.
Figure 1.
Transmission electron microscope image shows SARS-CoV-2. The spikes on the outer edge of the virus particles give coronaviruses their name, crown-like.
Potential Drugs for SARS-CoV-2, SARS and MERS
SARS-CoV-2 is an enveloped, positive-sense, single-stranded RNA β-coronavirus similar to the SARS and MERS viruses. Some of the potential antiviral targets encoded by the viral genome include non-structural proteins (e.g., 3-chymotrypsin-like protease, papain-like protease, RNA-dependent RNA polymerase and its helicase), structural proteins (e.g., the capsid spike glycoprotein) and accessory proteins. Several clinical trials are underway, either alone or with various combinations of interferons, guanosine-analog RNA synthesis inhibitors, reverse transcriptase inhibitors or influenza drugs, such as baloxavir marboxil, oseltamivir and umifenovir. Investigational drug remdesivir, a nucleotide analog antiviral, that blocks the RNA polymerase of the Ebola virus is under investigation for its potential action against SARS-CoV-2.
It is noteworthy that at least ten clinical trials are testing chloroquine, an antimalarial and autoimmune disease drug for its effectiveness against SAES-CoV-2. It is believed that the endosomal acidification fusion inhibitory action of chloroquine can possibly block infection by SARS-CoV-2.
Some of the strategies employed to test the efficacy of most of the drugs in clinical trials against SARS-CoV-2 depend on their ability to inhibit key components of the coronavirus infection lifecycle including viral entry into the host cell (blocked by umifenovir, chloroquine or interferon), viral replication (blocked by lopinavir/ritonavir, ASC09 or darunavir/cobicistat, which inhibit the 3C-like protease [3Clpro]) and viral RNA synthesis (inhibited by remdesivir, favipiravir, emtricitabine/tenofovir alafenamide or ribavirin). To a large extent these clinical trials are depending on the high level of genomic sequence similarity between the SARS-CoV-2, SARS and MERS proteins involved in the replication cycle.
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) and the cellular protease transmembrane protease serine 2 (TMPRSS2) to enter target cells (Figure 2 ). TMPRSS2 inhibitor camostat mesylate seems to have the ability to block cellular entry of the SARS-CoV-2 virus. Janus-associated kinase (JAK) inhibitor Olumiant (baricitinib), approved for rheumatoid arthritis, is being tested on the basis of its capacity to inhibit ACE2-mediated endocytosis. JAK inhibitor, Jakafi (ruxolitinib), is now under clinical trials (combined with mesenchymal stem cell infusion) for COVID-19. Despite all these efforts, the development of a suitable and effective drug(s) against SARS-CoV-19 will take time.
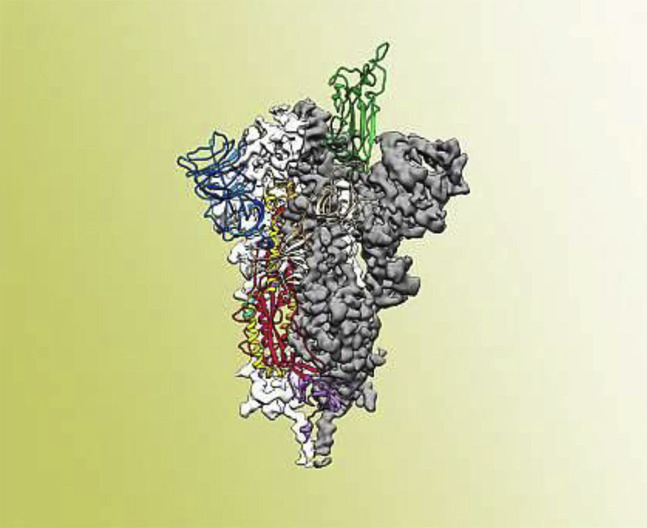
Figure 2.
Atomic-level structure of the SARS-CoV-2 spike protein. The receptor binding domain, the part of the spike that binds to the host cell, is colored green. (4).
Bioactive Lipids Inactive Enveloped Viruses, Possess Anti-inflammatory and Wound Healing Actions and Decrease Microbial Load
Some of the mechanisms by which the invading microorganisms are eliminated or neutralized include generation of specific antibodies by the immunocytes that aid in their elimination by elaborating various cytokines, reactive oxygen species (ROS), activation of complement system and other specific and non-specific means to trap and kill them with little or no injury to various tissues. In this process of protecting the body against the onslaught by the microorganisms, there could occur some amount of inevitable inflammation, injury and cell/tissue damage. In order to repair the tissue damage as a consequence of attack by the invading organisms and the resultant inflammatory process, resolution of inflammation and wound healing process need to occur in a coordinated and orderly fashion to restore homeostasis. Thus, the infection induced by the invading microorganisms could be local or systemic whereas the resolution of inflammation and wound healing and restoration of homeostasis is more a local process. For all these events to occur, there need to be a cross-talk among invading organisms, tissues that they attack, immune system, inflammatory events associated cells and resolution initiating and inducing cells/tissues and their molecules.
In this context, it is noteworthy that polyunsaturated fatty acids and their metabolites (termed as bioactive lipids) seem to have a crucial role. It is known that Staphylococcus aureus and coagulase-negative staphylococci, group A streptococci present on normal human skin are rendered ineffective to cause any infection by the skin surface lipids, especially unsaturated fatty acids. Group A streptococcus exposed to oleic acid (OA, 18:1n-9) die within 5 minutes of exposure as a result of an alteration in the integrity of the cell membrane (5). In addition, oleic acid enriched mouse peritoneal macrophages showed 3–4 fold greater erythrophagocytic capacity compared to palmitic acid-enriched macrophages (6). Staphylococci in the lung alveoli are killed mainly outside alveolar macrophages that seem to reside in the highly unsaturated arachidonic acid (AA, 20:4n-6) present in the surfactant (7, 8, 9, 10, 11). Other unsaturated fatty acids: linoleic, oleic, and palmitoleic also showed anti-bacterial activity but were less potent compared to AA that is effective against gram-positive and gram-negative bacteria, fungi and enveloped viruses, including influenza and HIV (8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21). The mechanism of the antimicrobial action of AA and other fatty acids may include their ability to induce leakage and even lysis of microbial cell membranes (including disruption of viral protein envelopes), as well as various cellular metabolic effects, including but not limited to inhibition of respiratory activity, effects on transportation of amino acids, and uncoupling of oxidative phosphorylation (21, 22, 23, 24). Based on these evidences, it is reasonable to suggest that alveolar macrophages, leukocytes, T and B cells, NK cells and other immunocytes release AA and other unsaturated fatty acids into their surrounding milieu when challenged by various microorganisms including viruses such as SARS-CoV-2, SARS and MERS, in turn, inactivate these invading organisms and thus, protect lungs and other tissues. This could be one of the fundamental mechanisms employed by human body to protect itself from various microbes. This implies that a deficiency of AA and other unsaturated fatty acids may render a person more susceptible to various infections including SARS-CoV-2, SARS and MERS.
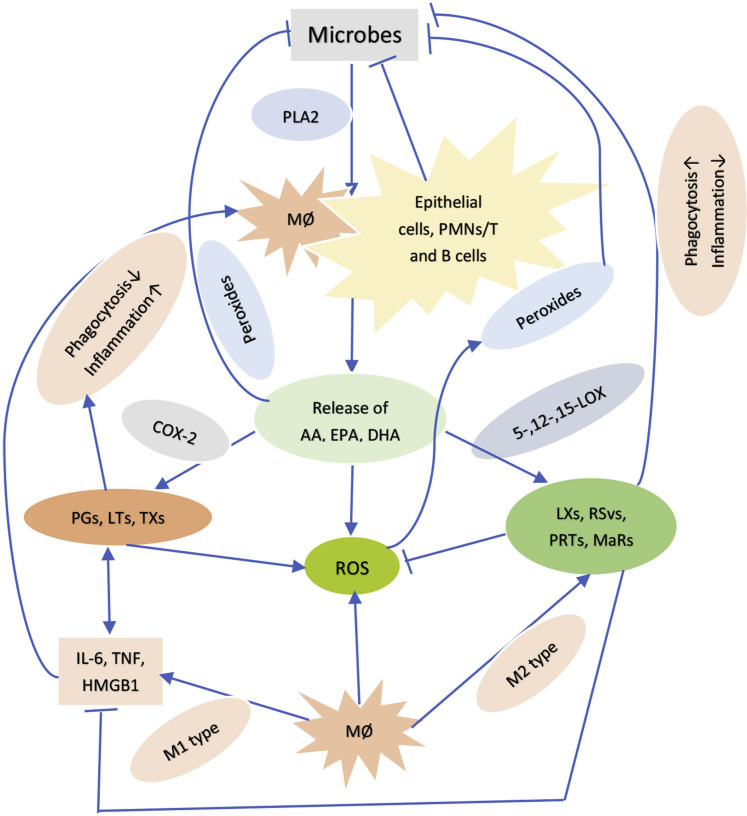
It is noteworthy that prostaglandins, leukotrienes and thromboxanes formed from AA have pro-inflammatory actions whereas lipoxins (from AA), resolvins from EPA (eicosapentaenoic acid, 20:5 n-3) and DHA (docosahexaenoic acid, 22:6 n-3) and protectins and maresins (derived from DHA) have potent anti-inflammatory actions, resolve inflammation and aid in wound healing and at the same time augment phagocytic capacity of macrophages and other cells to remove debris from the site(s) of infection and injury and enhance microbial clearance (25, 26, 27, 28, 29, 30, 31) (Figure 3 ). In view of the fact that SARS-CoV-2, SARS and MERS are enveloped viruses, they can be easily inactivated by AA and other unsaturated fatty acids. This implies that oral or intravenous administration of these fatty acids may enhance recovery from these infections and when present in adequate amounts in immunocytes and body fluids (especially in the alveolar fluid) may actually prevent these infections.
Figure 3.
Scheme showing how bioactive lipids could inhibit microbial proliferation.
Conclusions
It is evident from the preceding discussion that AA and other unsaturated fatty acids can inactivate enveloped viruses including SARS-CoV-2, SARS and MERS. The metabolites of AA, EPA and DHA have both pro- and anti-inflammatory actions and participate in resolution of inflammation and wound healing and regulate phagocytic action of macrophage and other immunocytes and have the ability to reduce microbial load. This suggests that AA, EPA and DHA and their anti-inflammatory metabolites such as lipoxin A4, resolvins, protectins and maresins function as endogenous anti-microbial molecules and so their appropriate use may aid in decreasing the morbidity and mortality due to SARS-CoV-2, SARS and MERS.
(ARCMED_2020_258)
Supplementary Data
References
- 1.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan R., Zhang Y., Li Y. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020 doi: 10.1126/science.abb2762. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 doi: 10.1126/science.abb2507. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speert D.P., Wannamaker L.W., Gray E.D. Bactericidal effect of oleic acid on group A streptococci: mechanism of action. Infect Immun. 1979;26:1202–1210. doi: 10.1128/iai.26.3.1202-1210.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokesh B.R., Wrann M. Incorporation of palmitic acid or oleic acid into macrophage membrane lipids exerts differential effects on the function of normal mouse peritoneal macrophages. Biochim Biophys Acta. 1984;792:141–148. doi: 10.1016/0005-2760(84)90215-7. [DOI] [PubMed] [Google Scholar]
- 7.Juers J.A., Rogers R.M., McCurdy J.B. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976;58:271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman C. Influence of trace amounts of fatty acids on the growth of microorganisms. Bacteriol Rev. 1954;18:147–162. doi: 10.1128/br.18.2.147-163.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabara J.J., Swieczkowski D.M., Conley A.J. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson K., Noren B., Odham G. Antimicrobial effect of simple lipids with different branches at the methyl end group. Antimicrob Agents Chemother. 1975;8:742–750. doi: 10.1128/aac.8.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heczko P.B., Lutticken R., Hryniewicz W. Susceptibility of Staphylococcus aureus and Group A, B, C, and G streptococci to free fatty acids. J Clin Microbiol. 1979;9:333–335. doi: 10.1128/jcm.9.3.333-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyss O., Ludwig B.J., Joiner R.R. The fungistatic and fungicidal action of fatty acids and related compounds. Arch Biochem. 1945;7:415–424. [Google Scholar]
- 13.Stock C.C., Francis T., Jr. The inactivation of the virus of epidemic influenza by soaps. J Exp Med. 1940;71:661–681. doi: 10.1084/jem.71.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands J., Auperin D., Snipes W. Extreme sensitivity of enveloped viruses, including herpes simplex, to long chain unsaturated monoglycerides and alcohols. Antimicrob Agents Chemother. 1979;15:67–73. doi: 10.1128/aac.15.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohn A., Gitelman J., Inbar M. Unsaturated free fatty acids inactivate animal enveloped viruses. Arch Virol. 1980;66:301–306. doi: 10.1007/BF01320626. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz B., Piët M.P., Prince A.M. Inactivation of lipid-enveloped viruses in labile blood derivatives by unsaturated fatty acids. Vox Sang. 1988;54:14–20. doi: 10.1111/j.1423-0410.1988.tb01606.x. [DOI] [PubMed] [Google Scholar]
- 17.Das U.N. Anti-biotic-like action of essential fatty acids. Can Med Assoc J. 1985;132:1350. [PMC free article] [PubMed] [Google Scholar]
- 18.Das U.N. Do unsaturated fatty acids function as endogenous anti-bacterial and anti-viral molecules? Am J Clin Nutr. 2006;83:390–391. doi: 10.1093/ajcn/83.2.390. [DOI] [PubMed] [Google Scholar]
- 19.Das U.N. Can essential fatty acid deficiency predispose to AIDS? Can Med Assoc J. 1985;132:900–902. [PMC free article] [PubMed] [Google Scholar]
- 20.Das U.N. Essential fatty acids and acquired immunodeficiency syndrome. Med Sci Monit. 2005;11:RA206–RA211. [PubMed] [Google Scholar]
- 21.Das U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J Adv Res. 2018;11:57–66. doi: 10.1016/j.jare.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu C.W., Konings W.N., Freese E. Effects of acetate and other short chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972;111:525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram L.O., Eaton L.C., Erdos G.W., Tedder T.F., Vreeland N.L. Unsaturated fatty acid requirement in Escherichia coli. Mechanism of palmitate-induced inhibition of growth by strain WNl. J Membr Biol. 1982;1982:31–40. doi: 10.1007/BF01870466. [DOI] [PubMed] [Google Scholar]
- 24.Fay J.P., Farias R.N. Inhibitory action of a nonmetabolizable fatty acid on the growth of Escherichia coli. Role of metabolism and outer membrane integrity. J Bacteriol. 1977;1977:790–795. doi: 10.1128/jb.132.3.790-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris P.C., Arnardottir H., Sanger J.M. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2016;22 doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramon S., Dalli J., Sanger J.M. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am J Pathol. 2016;186:962–973. doi: 10.1016/j.ajpath.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalli J., Winkler J.W., Colas R.A. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B., Walker J., Spur B. Effects of Lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fatty Acids. 2015;94:55–64. doi: 10.1016/j.plefa.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Walker J., Dichter E., Lacorte G. Lipoxin A4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 30.Wu B., Capilato J., Pham M.P. Lipoxin A4 augments host defense in sepsis and reduces Pseudomonas aeruginosa virulence through quorum sensing inhibition. FASEB J. 2016;30:2400–2410. doi: 10.1096/fj.201500029R. [DOI] [PubMed] [Google Scholar]
- 31.Poorani R., Bhatt A.N., Dwarakanath B.S. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol. 2016;785:116–132. doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.