Highlights
-
•
Long-term overall survival of CTEPH patients receiving PH-specific medial therapy is very reasonable.
-
•
Despite worse baseline characteristics at baseline, combination therapy showed similar survival as monotherapy.
-
•
Combination therapy strategy showed no difference in survival outcome.
Keywords: Chronic thromboembolic pulmonary hypertension, Survival, Monotherapy, Combination therapy
Abbreviations: 6MWD, 6-minute walking distance; BPA, balloon pulmonary angioplasty; CI, cardiac index; CO, cardiac output; COPD, chronic obstructive lung disease; CT, computed tomography; CTEPH, chronic thromboembolic pulmonary hypertension; ERA(s), endothelin receptor antagonist(s); FC, functional class; HR, hazards regression; IQR, interquartile range; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal pro brain natriuretic peptide; PAH, pulmonary arterial hypertension; PEA, pulmonary endarterectomy; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RHC, right heart catheterisation; SD, standard deviation; WHO, World Health Organization
Abstract
Objective
The current experience with combination therapy in chronic thromboembolic pulmonary hypertension (CTEPH) is limited. We present the first survival results up to 5 years for dual combination therapy versus monotherapy in CTEPH.
Methods
All consecutive, non-operated CTEPH or residual PH after pulmonary endarterectomy patients treated with PH-specific medical therapy between January 2002 and November 2019 were included. We report and compare survival between monotherapy and (upfront or sequential) dual combination therapy until five years after medication initiation.
Results
In total, 183 patients (mean age 65 ± 14 years, 60% female, 66% WHO FC III/IV, 86% non-operated) were included, of which 83 patients received monotherapy and 100 patients received dual combination therapy. At baseline, patients receiving combination therapy had a higher NT-proBNP (p = 0.02) mean pulmonary artery pressure (p = 0.0001) and pulmonary vascular resistance (p = 0.02), while cardiac index was lower (p = 0.03). Total follow-up duration was 3.3 ± 1.8 years, during which 31 (17%) patients died. Estimated 1-, 3- and 5-year survival for monotherapy were 99%, 92% and 79%, respectively. For combination therapy percentages were 98%, 89% and 70%, respectively. Survival did not significantly differ between both groups (p = 0.22).
Conclusion
Survival up to 5 years for patients treated with combination therapy, regardless of the combination strategy, was similar as patients with monotherapy, despite worse clinical and haemodynamic baseline characteristics.
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a pulmonary vascular disease due to chronic thromboembolic obstruction. Most patients experienced an acute thromboembolic event, although a minority has no history of previous acute thromboembolism. Chronic thromboembolisms may lead to CTEPH and subsequent secondary distal vasculopathy [1]. The exact incidence of CTEPH after acute pulmonary embolism is unclear. Prospective studies report an incidence ranging from 0.4% to 6.2% [2], [3], [4].
Pulmonary endarterectomy (PEA) is the preferred treatment, as it greatly improves outcome and prognosis [5]. However, not all patients do have accessible lesions for PEA, may be inoperable due to comorbidities or decline PEA. Some may have persistent or recurrent pulmonary hypertension (PH) after PEA due to vasculopathy [6]. In these patients, both balloon pulmonary angioplasty (BPA) and PH-specific medical therapy may improve clinical outcomes [7], [8], [9], [10]. Riociguat is currently the only registered PH-specific medical therapy for CTEPH, although other PH-specific drugs are increasingly being used [8], [11].
In pulmonary arterial hypertension (PAH), the current clinical practice is to start with initial dual combination therapy to achieve a low-risk status and to reduce the risk of clinical failure [12]. Nevertheless, the current experience with early combination therapy in CTEPH is limited and is adapted from PAH treatment strategies. The aim of the current study is to provide clinical data about the usefulness of dual combination therapy in a large cohort of CTEPH patients and to identify the effect on patient-related clinical outcome in daily practice.
2. Methods
2.1. Study population and treatment strategies
All consecutive newly diagnosed CTEPH patients, either non-operated or with residual PH after PEA, treated with PH-specific medical therapy in the St. Antonius Hospital in Nieuwegein, the Netherlands between January 1, 2002 and November 1, 2019 were included. CTEPH diagnosis was established in our multidisciplinary team, consisting of cardiologists, pulmonologists, radiologists, cardiothoracic surgeons and specialised nurse practitioners. Patients received anticoagulation therapy for at least three months, had mismatched perfusion defects on lung scintigraphy and signs of chronic pulmonary embolism on multidetector CT angiography or conventional angiography. Right heart catheterisation (RHC) showed a mean pulmonary artery pressure (mean PAP) of ≥25 mmHg and a wedge pressure ≤15 mmHg.
Patients were classified as monotherapy if they received only one PH-specific medical therapy during the complete follow-up, although they were able to switch between different monotherapies. Patients who received dual combination therapy were classified as sequential combination therapy or upfront combination therapy. Upfront combination therapy was defined as concomitant initiation of two PH-specific drugs within 3 months, sequential therapy was defined as sequential initiation of two PH-specific drugs at least 3 months apart. Patients were able to switch between different PH-specific drug groups, but follow-up ended if patients switched to triple therapy. A flowchart of subgroups and patient numbers are shown in supplemental Fig. 1.
From 2002 onwards, patients were initiated on monotherapy and switched to sequential combination therapy in case of insufficient improvement or clinical worsening during follow-up. In case of severely symptomatic or hemodynamic impairment at baseline, upfront combination therapy was initiated. From 2015 onwards, mainly upfront combination therapy was initiated as standard treatment for CTEPH, in accordance with PAH recommendations in the ESC/ERS guideline [12].
The local ethical commission approved the study (number W17.132).
2.2. Baseline, follow-up and outcomes
The date of multidisciplinary team discussion was classified as moment of diagnosis; date of initiation of PH-specific medical therapy was considered as baseline. Time between diagnosis and baseline was noted. Start of PH-specific medication in patients with persistent PH after PEA was set as date of PEA, while this was the date of RHC confirming PH in patients with recurrent PH after PEA.
Patient characteristics, medical history and additional tests were collected from hospital records and databases if performed within three months of diagnosis. A baseline non-invasive risk score was calculated, with WHO FC, 6-min walking distance (6MWD) and NT-proBNP, to estimate 1-year mortality [13], [14]. Outpatient follow-up visits alternated between a pulmonologist and cardiologist every three months. Patients were followed for up to five years from baseline or last available information before death, start of BPA, ending of (dual) PH medical therapy or observation period (01-12-2019). Death was defined as all-cause mortality.
2.3. Statistical analyses
Statistical analyses were performed with SPSS (IBM SPSS statistics version 24). Tests were two-tailed and a p-value below 0.05 was considered statistically significant. Categorical data were presented as number and percentage. Continuous data were presented as mean and standard deviation (SD) or as median and interquartile range (IQR). Groups were compared with Chi-squared test and t-test or Mann-Whitney U test for categorical and continuous data respectively. Survival was analysed with Kaplan-Meier method and comparisons between two groups with log-rank test. Predictors for survival were assessed with Cox regression for univariate and multivariate analysis. Univariate variables with a p-value below 0.10 were included for multivariate analysis using backward stepwise elimination. Waiting time from diagnosis to baseline was corrected with a time-dependent covariate.
Additional analyses were performed to demonstrate effects of BPA and time period on the current data.
3. Results
3.1. Study population
3.1.1. Entire cohort
In total, 183 patients (mean age 65 ± 14 years, 60% female, 66% WHO FC III/IV, 45/32/16/7% risk score) were included for analyses in our cohort. Most patients were non-operated (86%), while a minority had residual PH after PEA (14%). Ninety-one percent of all patients used vitamin K antagonists; the remaining nine percent used direct oral anticoagulants (DOACs). Comorbidities were frequent (systemic hypertension 29%, chronic obstructive pulmonary disease 20%). There was a history of an acute pulmonary embolism and venous thrombosis in 78% and 26% of all patients, respectively. In total, 16% of all patients did not experience any acute thromboembolic event. NT-proBNP (662 (226-2151) pg/mL) was elevated; mean 6MWD was 312 ± 126 m. RHC showed a cardiac index (CI) of 2.6 ± 0.8 L/min/m2, with mean PAP 40.9 ± 10.4 mmHg resulting in pulmonary vascular resistance (PVR) of 6.7 ± 3.8 WU at baseline. Characteristics are shown in Table 1.
Table 1.
Patient baseline characteristics entire cohort, monotherapy and combination therapy.
| Entire cohort (n = 183) | Monotherapy (n = 83) | Combination therapy (n = 100) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 65 ± 14 | 65 ± 16 | 65 ± 13 |
| Female gender | 60 | 57 | 62 |
| Non-operated / Residual CTEPH | 86/14 | 82/18 | 90/10 |
| VKA/DOAC | 91/9 | 95/5 | 88/12 |
| Monotherapy | |||
| Riociguat | 7 | ||
| ERA | 58 | ||
| PDE5i | 34 | ||
| Prostacyclin | 1 | ||
| Combination therapy | |||
| Riociguat + ERA | 39 | ||
| PDE5i + ERA | 61 | ||
| Total follow-up duration (years) | 3.3 ± 1.8 | 3.4 ± 1.7 | 3.3 ± 1.8 |
| History taking | |||
| Smokers (ever) | 48 | 41 | 54 |
| COPD | 20 | 18 | 21 |
| Systemic hypertension | 29 | 19 | 36# |
| Diabetes | 11 | 8 | 13 |
| Hyperlipidaemia | 5 | 2 | 7 |
| Thyroid disorders | 7 | 7 | 7 |
| Inflammatory bowel disease | 1 | 0 | 1 |
| Hematologic disease | 14 | 17 | 13 |
| Malignancy | 15 | 19 | 12 |
| Splenectomy | 2 | 1 | 3 |
| Cardiac device | 3 | 3 | 3 |
| Venous thrombosis | 26 | 33 | 21 |
| Acute pulmonary embolism | 78 | 78 | 78 |
| Clinical characteristics | |||
| WHO FC I/II/III/IV | 2/32/63/3 | 1/38/57/4 | 2/27/69/2 |
| NT-proBNP (pg/mL) | 662 (226–2151) | 347 (108–1273) | 1341 (293–2641)# |
| 6MWD (m) | 312 ± 126 | 324 ± 135 | 302 ± 118 |
| Non-invasive risk score (0/1/2/3) | 45/32/16/7 | 36/31/21/12 | 51/33/11/5# |
| Right-sided heart catheterization | |||
| CO (L/min) | 5.0 ± 1.7 | 5.3 ± 1.9 | 4.7 ± 1.5# |
| CI (L/min/m2) | 2.6 ± 0.8 | 2.8 ± 0.9 | 2.5 ± 0.7# |
| RAP mean (mmHg) | 8.7 ± 4.8 | 8.4 ± 5.3 | 9.0 ± 4.3 |
| PAP mean (mmHg) | 40.9 ± 10.4 | 37.7 ± 9.9 | 43.4 ± 10.1# |
| PVR (WU) | 6.7 ± 3.8 | 5.9 ± 4.0 | 7.3 ± 3.5# |
Data are presented as %, mean ± SD, median (IQR). SD: standard deviation, IQR: interquartile range, CTEPH: chronic thromboembolic pulmonary hypertension, VKA: vitamin K antagonist, DOAC: direct oral anticoagulant, ERA: endothelin receptor antagonist, PDE5i: phosphodiesterase type 5 inhibitor, BPA: balloon pulmonary angioplasty, COPD: chronic obstructive pulmonary disease, WHO FC: World Health Organisation functional class, NT-proBNP: N-terminal pro brain natriuretic peptide, 6MWD: 6-min walking distance, CO: cardiac output, CI: Cardiac index, RAP: right arterial pressure, PAP: pulmonary arterial pressure, PVR: pulmonary vascular resistance.
p < 0.05 compared with monotherapy.
3.1.2. Monotherapy
Eighty-three patients (mean age 65 ± 16 years, 57% female, 61% WHO FC III/IV, 36/31/21/12% risk score) in our cohort used monotherapy. At baseline, six patients (7%) had riociguat, 28 (34%) phosphodiesterase type 5 inhibitors (PDE5i), 48 (58%) endothelin receptor antagonists (ERAs) and one (1%) prostacyclin. In total 7 patients (8%) switched between monotherapies: 2 patients from PDE5i to riociguat, 3 from ERA to PDE5i, 1 from ERA to riociguat and 1 from ERA to prostacyclin. Full subgroup characteristics are shown in Table 1.
3.1.3. Combination therapy
One hundred patients (mean age 65 ± 13 years, 62% female, 71% WHO FC III/IV, 51/33/11/5% risk score) received combination therapy. At baseline 39 patients (39%) received riociguat/ERA, while 61 patients (61%) received PDE5i/ERA. Patients receiving combination therapy had worse baseline characteristics compared to monotherapy patients: patients were more symptomatic and had lower 6MWD, although not statistically significant. However, the percentage of patients with systemic hypertension (p = 0.01), the level of NT-proBNP (p = 0.02), mean PAP (p = 0.0001) and PVR (p = 0.02) were significantly higher, while CI and risk score were significantly lower (p = 0.03 and p = 0.02 respectively).
3.1.4. Sequential combination therapy
In our cohort, there were 58 patients (mean age 65 ± 12 years, 55% female, 69% WHO FC III/IV, 46/42/6/6% risk score) receiving sequential combination therapy. Mean time till start of the second PH-specific drug was 1.8 ± 1.2 years. Eighteen patients (31%) had riociguat/ERA, while 40 patients (69%) had PDE5i/ERA. Ten patients (17%) switched between groups: 5 switched from riociguat/ERA to PDE5i/ERA, 1 from riociguat/ERA to prostacyclin/ERA, 2 from PDE5i/ERA to riociguat/ERA and 2 from PDE5i/ERA to prostacyclin/ERA.
3.1.5. Upfront combination therapy
The 42 patients (mean age 64 ± 13 years, 71% female, 74% WHO FC III/IV, 57/20/20/3% risk score) receiving upfront combination therapy were equally divided between riociguat/ERA and PDE5i/ERA. Four patients switched between medication groups: three switched from riociguat/ERA to PDE5i/ERA and one patient the other way around. Significant more patients received DOACs (p = 0.03) compared to the sequential combination therapy group. Baseline PVR was significantly higher in the upfront combination therapy group (p = 0.05) in comparison to the sequential combination therapy group. Full subgroup characteristics are shown in Table 2.
Table 2.
Patient baseline characteristics combination therapy subgroups.
| Sequential combination therapy (n = 58) | Upfront combination therapy (n = 42) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 65 ± 12 | 64 ± 13 | 0.78 |
| Female gender | 55 | 71 | 0.10 |
| Non-operated / residual CTEPH | 93/7 | 86/14 | 0.31 |
| VKA/DOAC | 95/5 | 79/21 | 0.03 |
| Combination therapy | 0.06 | ||
| Riociguat + ERA | 31 | 50 | |
| PDE5i + ERA | 69 | 50 | |
| Total follow-up duration (years) | 4.1 ± 1.3 | 2.2 ± 1.7 | 0.0001 |
| History taking | |||
| Smokers (ever) | 55 | 52 | 0.78 |
| COPD | 28 | 12 | 0.06 |
| Systemic hypertension | 35 | 39 | 0.64 |
| Diabetes | 9 | 19 | 0.13 |
| Hyperlipidaemia | 7 | 7 | 0.24 |
| Thyroid disorders | 7 | 7 | 0.94 |
| Inflammatory bowel disease | 0 | 0 | 1.00 |
| Hematologic disease | 16 | 10 | 0.38 |
| Malignancy | 9 | 15 | 0.52 |
| Splenectomy | 5 | 0 | 0.26 |
| Cardiac device | 2 | 5 | 0.39 |
| Venous thrombosis | 26 | 14 | 0.16 |
| Acute pulmonary embolism | 78 | 79 | 0.91 |
| Clinical characteristics | |||
| WHO FC I/II/III/IV | 2/29/67/2 | 2/24/71/3 | 0.66 |
| NT-proBNP (pg/mL) | 1288 (280–2145) | 1723 (322–3310) | 0.19 |
| 6MWD (m) | 300 ± 119 | 306 ± 119 | 0.80 |
| Non-invasive risk score (0/1/2/3) | 46/42/6/6 | 57/20/20/3 | 0.89 |
| Right-sided heart catheterization | |||
| CO (L/min) | 5.0 ± 1.7 | 4.4 ± 1.2 | 0.06 |
| CI (L/min/m2) | 2.5 ± 0.8 | 2.4 ± 0.7 | 0.42 |
| RAP mean (mmHg) | 8.6 ± 4.4 | 9.4 ± 4.2 | 0.41 |
| PAP mean (mmHg) | 43.1 ± 10.2 | 43.8 ± 10.1 | 0.75 |
| PVR (WU) | 6.6 ± 2.9 | 8.1 ± 4.0 | 0.05 |
Data are presented as %, mean ± SD, median (IQR). SD: standard deviation, IQR: interquartile range, CTEPH: chronic thromboembolic pulmonary hypertension, VKA: vitamin K antagonist, DOAC: direct oral anticoagulant, ERA: endothelin receptor antagonist, PDE5i: phosphodiesterase type 5 inhibitor, BPA: balloon pulmonary angioplasty, COPD: chronic obstructive pulmonary disease, WHO FC: World Health Organisation functional class, NT-proBNP: N-terminal pro brain natriuretic peptide, 6MWD: 6-min walking distance, CO: cardiac output, CI: Cardiac index, RAP: right arterial pressure, PAP: pulmonary arterial pressure, PVR: pulmonary vascular resistance.
3.2. Outcomes
3.2.1. Entire cohort
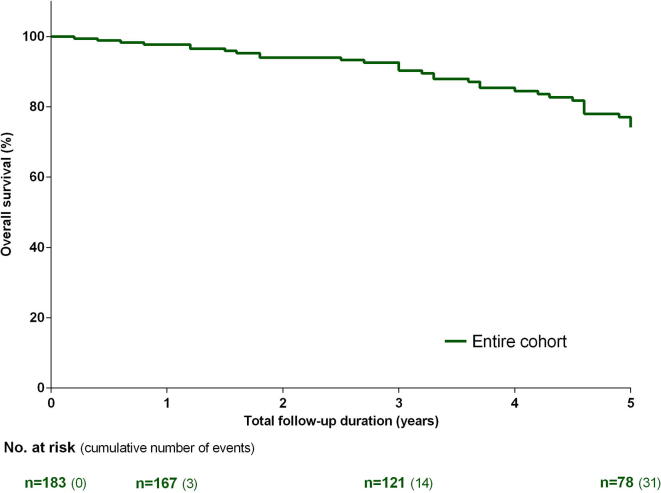
Total follow-up duration was 3.3 ± 1.8 years, during which 31 (17%) patients died. Seven patients died due to right ventricular failure, four due to sepsis and two due to malignancy, while for the remaining 18 patients the cause of death was unknown. Estimated 1-, 3- and 5-year survival were 98%, 90% and 74%, respectively. None of the patients underwent lung transplantation during the follow-up. Independent predictors at baseline of mortality in the entire cohort identified from multivariate analysis were absence of hematologic disease (HR 0.30, 95% CI 0.12-0.78), NT-proBNP (HR 3.80, 95% CI 1.68-8.60) and RAP (HR 1.15, 95% CI 1.06-1.24). Results are shown in Fig. 1 and supplemental Table 1.
Fig. 1.
Kaplan-Meier estimates of survival from baseline in the entire non-operated chronic thromboembolic pulmonary hypertension (CTEPH) cohort. Number of patients at risk and cumulative number of events are shown.
3.2.2. Monotherapy vs combination therapy
Patients receiving monotherapy had a mean follow-up duration of 3.4 ± 1.7 years, while this was 3.3 ± 1.8 years for patients receiving combination therapy. In the former group, 11 patients (13%) died, in the latter 20 patients (20%). Estimated 1-, 3- and 5-year survival for monotherapy were 99%, 92% and 79%, respectively. For combination therapy percentages were comparable, with an estimated 1-, 3- and 5-year survival of 98%, 89% and 70%. Survival did not significantly differ between both groups (p = 0.22). Results are shown in Fig. 2.
Fig. 2.
Kaplan-Meier estimates of survival from baseline in chronic thromboembolic pulmonary hypertension (CTEPH) monotherapy group and CTEPH combination therapy group. Number of patients at risk and cumulative number of events are shown.
A comparison between the different combination therapy strategies (riociguat + ERA and PDE5i + ERA) did not show any significant difference either (p = 0.52).
Independent predictors of mortality in the monotherapy group identified from multivariate analysis were 6MWD per 10 m (HR 0.87, 95% CI 0.79–0.96) and mean PAP (HR 1.08, 95% CI 1.01–1.17), all at baseline. For combination therapy this were absence of hematologic disease (HR 0.24, 95% CI 0.09–0.66) and RAP (HR 1.12, 95% CI 1.03–1.22). Results are shown in supplemental Tables 2 and 3.
3.2.3. Sequential combination therapy vs upfront combination therapy
A comparison between combination therapy strategies, showed a significant longer follow-up for sequential combination therapy than upfront combination therapy (4.1 ± 1.3 years vs 2.2 ± 1.7 years, p = 0.0001). Thirteen patients (22%) died in the sequential combination therapy group and seven patients (17%) in the upfront combination therapy group. Estimated 1-, 3- and 5-year survival for sequential therapy was 100%, 93% and 73%, respectively, and for upfront therapy 95%, 79% and 59%, respectively (p = 0.22). Results are shown in supplemental Fig. 2.
3.3. Additional analyses
For this manuscript, patient follow-up was censored when BPA treatment was initiated. Outcomes and data without censoring for BPA are described hereafter.
Thirteen percent of the patients receiving monotherapy underwent BPA during follow-up, while this was 27% in the patients receiving combination therapy (p = 0.02). Significantly more patients with upfront combination therapy than sequential combination therapy received BPA (p = 0.002).
Total follow-up duration increased to 3.6 ± 1.6 years, while two patients, one using monotherapy and the other using sequential combination therapy, died after start of BPA due to non-procedure related sepsis and right heart failure respectively. Estimated 1-, 3- and 5-year survival without censoring for BPA were 98%, 90% and 74%, respectively. Patients receiving monotherapy had a mean follow-up duration of 3.5 ± 1.7 years, while this was 3.6 ± 1.5 years for patients receiving combination therapy. Estimated 1-, 3- and 5-year survival for monotherapy were 98%, 90% and 79%, respectively. For combination therapy percentages were similar, except 71% survival at year 5. Survival did not significantly differ between both groups (p = 0.31). A comparison between combination therapy strategies, showed a significant longer follow-up for sequential combination therapy than upfront combination therapy (4.3 ± 1.1 years vs 2.7 ± 1.6 years, p = 0.0001). Estimated 1-, 3- and 5-year survival for sequential therapy was 100%, 91% and 73%, respectively, and for upfront therapy 95%, 87% and 65%, respectively (p = 0.48).
The inclusion period was long and management may have changed during the time span of this study. Additional analysis to compare survival before and after 2015 (start of macitentan and riociguat use) was performed. There was no significant difference in survival for mono- and combination therapy (p = 0.18) or monotherapy alone (p = 0.93) before and after 2015.
4. Discussion
The current study is the first to investigate the effect of dual combination therapy versus monotherapy in patients with non-operated CTEPH or residual PH after PEA on survival up to 5 years.
Despite worse clinical and haemodynamic characteristics at baseline in the combination therapy group, survival was similar compared to patients receiving monotherapy, regardless of the combination therapy strategy used.
The pathophysiology of CTEPH is currently not completely known. It is assumed that CTEPH is usually a consequence of prior acute pulmonary embolism [1]. Seventy-eight percent of all patients in our cohort had a history of an acute pulmonary embolism, similar as reported in an international CTEPH registry [15]. Common risk factors for CTEPH, such as thyroid disorders, malignancy, splenectomy and hematologic disorders, were present in our cohort. Patients also frequently had cardiovascular comorbidities. The prevalence of risk factors was comparable with results from the CTEPH registry [15].
Operable CTEPH patients treated with PEA have the best prognosis, with an estimated 1-, 3- and 5-year survival of >90%, >84% and >80%, respectively [5], [16]. However, not all patients are operable. In addition, operated patients may reveal residual PH after PEA, what leads to a decreased prognosis especially when pulmonary hemodynamics after PEA are severely impaired [16]. These two groups do probably suffer from vasculopathy [1]. This vasculopathy in CTEPH shows similarities in pathological features with PAH, what makes it likely that PAH therapy may also be useful in CTEPH [8].
A large, prospective European registry showed an estimated 1- and 3-year survival of 88% and 70% in non-operated patients [17]. In this registry, 61% of the patients received PH-specific medical therapy at any time, with 18% receiving dual combination therapy with sildenafil and ERA [17]. In our cohort of medically treated patients, we report higher survival percentages: an estimated 1-, 3- and 5-year survival of 98%, 90% and 74%, respectively. A comparison of our baseline characteristics with the European registry showed less symptomatic patients and a slightly lower mean PAP and PVR in our cohort [17], this might partly explain the difference in survival as the pulmonary hemodynamics are predictors for mortality [18]. It is also likely that the different percentage of patients receiving PH-medical (combination) therapy is very important, probably explaining the survival difference between both cohorts.
Historically, most CTEPH patients receive monotherapy [19]. In our study, 45% of patients received monotherapy, predominantly ERA or PDE5i. Most of these patients (75%) were diagnosed before the introduction of riociguat/macitentan/combination therapy, and received bosentan or sildenafil as monotherapy. Statistical analysis did not show a significant survival difference between the two time periods (before and after 01/01/2015).
Long-term survival data of patients treated with monotherapy in randomised controlled trials is scarce: the CHEST-2 study reported a survival of 97% at 1-year follow-up, comparable with our percentage [20]. Cohort studies showed a 1-year survival of 96% with bosentan and 100% survival with sildenafil monotherapy [21], [22]. A large cohort study in the UK, with 72% of technically-operable-not-operated patients and 86% of nonsurgical-disease-distribution patients treated with PH-specific therapy of whom most received PDE5i, showed a 5-year survival of 55% and 60% respectively [18]. CTEPH registries in Spain and Switzerland showed similar results [23], [24]. However, our estimated 5-year survival of 79% is higher; all of our patients received PH-specific medical therapy, were less symptomatic and had better baseline hemodynamics, probably explaining the difference [18].
The concept of combination therapy is more established in PAH compared with CTEPH. To achieve a low-risk status and to improve outcomes, the initial use of combination therapy in PAH is advised [13], [14], [25], [26], [27]. Studies directly assessing this concept in CTEPH are limited. A review of the available literature shows the use of background PAH-therapy combined with macitentan in 61% of all patients in the MERIT-1 trial, although these patients did predominantly receive PDE5i and follow-up duration was limited [28]. Cohort studies using combination therapy showed a reduction in PVR with sildenafil and inhaled prostacyclin [29], [30]. A case report described improved hemodynamics, WHO FC and 6MWD in one CTEPH patient receiving riociguat and treprostinil [31]. Currently, most of our patients are initiated on combination therapy and more than half of the patients in the current study received combination therapy. At baseline, these patients had worse clinical characteristics, risk score and hemodynamics compared to patients treated with monotherapy. Despite this, survival was similar between both groups, indicating the importance and potential of combination therapy in CTEPH.
Combination therapy can be given upfront or sequential. For PAH treatment, upfront combination therapy is preferred over sequential combination therapy [12], [27]. Nevertheless, both combination therapy strategies were never compared directly, but only with monotherapy.
We show in our CTEPH cohort a comparable survival between upfront and sequential combination therapy, despite higher baseline PVR in the upfront combination therapy group. More (randomised) research is necessary to compare both strategies.
4.1. Limitations
We described outcomes of different therapy strategies in a single-centre CTEPH population. In The Netherlands we are able to initiate PAH therapy in CTEPH patients. However, this may not always be possible in other countries. The number of events in the monotherapy group was low, what may lead to overfitting in the regression analyses. The period of patient inclusion was long and the preferred treatment (strategy) has changed over time. However, analyses did not show a significant difference between survival outcomes before and after 2015. Although all patients were discussed in the multidisciplinary team, bias for treatment strategy may be present. The group receiving combination therapy is heterogeneous, with a significant higher baseline PVR in the upfront combination therapy group. Nevertheless, PVR was not a predictor for mortality. It may be interesting for future research to differentiate therapy strategies results in a standardised population with risk stratification.
5. Conclusion
Survival up to five years for patients treated with dual combination therapy, regardless of the combination strategy, was similar as compared with patients receiving monotherapy, despite worse clinical and haemodynamic characteristics at baseline.
6. Take home message
Chronic thromboembolic pulmonary hypertension patients treated with combination therapy had, despite worse clinical and haemodynamic baseline characteristics, similar survival as patients treated with monotherapy.
7. Compliance with ethical standards
M. van Thor, J. Mager and M. Post report grants from Actelion Pharmaceuticals. J. Kelder has nothing to disclose. R. Snijder reports grants from Pfizer and Actelion Pharmaceuticals.
8. Funding
The authors declare that no funding was received for the current manuscript.
9. Publication
The authors declare that neither the work nor any part of its essential substance, tables or figures have been or will be published or submitted to another scientific journal or are being considered for publication elsewhere. There are no simultaneous submissions of similar or related manuscripts at the point of submission.
Acknowledgments
MvT, JK and MP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. MvT, RS, JK, JM and MP contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. All authors approve the final version to be submitted.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100544.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Simonneau G., Torbicki A., Dorfmüller P., Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017;26(143) doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., van Kralingen K.W., van Dijk A.P.J., Heyning F.H., Vliegen H.W., Huisman M.V. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95:970–975. doi: 10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pengo V., Lensing A.W.A., Prins M.H., Marchiori A., Davidson B.L., Tiozzo F. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N. Engl. J. Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 4.Becattini C., Agnelli G., Pesavento R., Silingardi M., Poggio R., Taliani M.R. Incidence of chronic thromboembolic pulmonary hypertention after a first episode of pulmonary embolism. Chest. 2006;130:172–175. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins D., Madani M., Fadel E., D’Armini A.M., Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017;26(143) doi: 10.1183/16000617.0111-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim N.H.S., Fesler P., Channick R.N., Knowlton K.U., Ben-Yehuda O., Lee S.H. Preoperative Partitioning of Pulmonary Vascular Resistance Correlates with Early Outcome after Thromboendarterectomy for Chronic Thromboembolic Pulmonary Hypertension. Circulation. 2004;109(1):18–22. doi: 10.1161/01.CIR.0000111841.28126.D4. [DOI] [PubMed] [Google Scholar]
- 7.Lang I., Meyer B.C., Ogo T., Matsubara H., Kurzyna M., Ghofrani H.-A. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017;26(143) doi: 10.1183/16000617.0119-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepke-Zaba J., Ghofrani H.-A., Hoeper M.M. Medical management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017;26(143):1–12. doi: 10.1183/16000617.0107-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepke-Zaba J., Jais X., Channick R. Medical Therapy in Chronic Thromboembolic Pulmonary Hypertension. Annals ATS. 2016;13(Supplement_3):S248–S254. doi: 10.1513/AnnalsATS.201512-802AS. [DOI] [PubMed] [Google Scholar]
- 10.van Thor M.C.J., ten Klooster L., Snijder R.J., Post M.C., Mager J.J. Long-term clinical value and outcome of riociguat in chronic thromboembolic pulmonary hypertension. IJC Hear Vasc. 2019;28(22):163–168. doi: 10.1016/j.ijcha.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Thor M.C.J., ten Klooster L., Snijder R.J., Kelder J.C., Mager J.J., Post M.C. Bosentan or Macitentan Therapy in Chronic Thromboembolic Pulmonary Hypertension? Lung. 2019;197(6):753–760. doi: 10.1007/s00408-019-00274-9. [DOI] [PubMed] [Google Scholar]
- 12.Galie N., Humbert M., Simon G., Lang I., Torbicki A., Simmoneau G. 2015 ESC / ERS Guidelines for the diagnosis and treatment of pulmonary hypertension – web addenda The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and of the European Respiratory. Eur. Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 13.Boucly A., Weatherald J., Savale L., Jaïs X., Cottin V., Prevot G. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017;50:1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M., Farber H.W., Ghofrani H.A., Benza R.L., Busse D., Meier C. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019;53:1802004. doi: 10.1183/13993003.02004-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepke-Zaba J., Delcroix M., Lang I., Mayer E., Jansa P., Ambroz D. Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Circulation. 2011;124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 16.Cannon J.E., Su L., Kiely D.G., Page K., Toshner M., Swietlik E. Dynamic risk stratification of patient long-term outcome after pulmonary Endarterectomy: Results from the United Kingdom national cohort. Circulation. 2016;133(18):1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delcroix M., Lang I., Pepke-Zaba J., Jansa P., D’Armini A.M., Snijder R. Long-Term Outcome of Patients with Chronic Thromboembolic Pulmonary Hypertension : Results from an International Prospective Registry. Circulation. 2016;133(9):859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 18.Quadery S.R., Swift A.J., Billings C.G., Thompson A.A.R., Elliot C.A., Hurdman J. The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2018;52(3) doi: 10.1183/13993003.00589-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gall H., Preston I.R., Hinzmann B., Heinz S., Jenkins D., Kim N.H. An international physician survey of chronic thromboembolic pulmonary hypertension management. Pulm Circ. 2016;6(4):472–482. doi: 10.1086/688084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonneau G., D’Armini A.M., Ghofrani H.A., Grimminger F., Hoeper M.M., Jansa P. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: A long-term extension study (CHEST-2) Eur. Respir. J. 2015;45(5):1293–1302. doi: 10.1183/09031936.00087114. [DOI] [PubMed] [Google Scholar]
- 21.Hughes R.J., Jais X., Bonderman D., Suntharalingam J., Humbert M., Lang I. The efficacy of bosentan in inoperable chronic thromboembolic pulmonary hypertension: A 1-year follow-up study. Eur. Respir. J. 2006;28:138–143. doi: 10.1183/09031936.06.00135905. [DOI] [PubMed] [Google Scholar]
- 22.Reichenberger F., Voswinckel R., Enke B., Rutsch M., El Fechtali E., Schmehl T. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2007;30(5):922–927. doi: 10.1183/09031936.00039007. [DOI] [PubMed] [Google Scholar]
- 23.Escribano-Subías P., Del Pozo R., Román-Broto A., Domingo Morera J.A., Lara-Padrón A., Elías Hernández T. Management and outcomes in chronic thromboembolic pulmonary hypertension: From expert centers to a nationwide perspective. Int. J. Cardiol. 2016;203:938–944. doi: 10.1016/j.ijcard.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Mueller-Mottet S., Stricker H., Domeninghetti G., Azzola A., Geiser T., Schwerzmann M. Long-term data from the swiss pulmonary hypertension registry. Respiration. 2015;89:127–140. doi: 10.1159/000370125. [DOI] [PubMed] [Google Scholar]
- 25.Lajoie A.C., Lauzière G., Lega J.C., Lacasse Y., Martin S., Simard S. Combination therapy versus monotherapy for pulmonary arterial hypertension: A meta-analysis. Lancet Respir Med. 2016;4(4):291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 26.Sitbon O., Gaine S. Beyond a single pathway: Combination therapy in pulmonary arterial hypertension. Eur. Respiratory Rev. 2016:408–417. doi: 10.1183/16000617.0085-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galiè N., Channick R.N., Frantz R.P., Grünig E., Jing Z.C., Moiseeva O. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghofrani H.A., Simonneau G., D’Armini A.M., Fedullo P., Howard L.S., Jaïs X. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT- 1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet. Respir. Med. 2017;5(10) doi: 10.1016/S2213-2600(17)30305-3. [DOI] [PubMed] [Google Scholar]
- 29.Ghofrani H.A., Wiedemann R., Rose F., Olschewski H., Schermuly R.T., Weissmann N. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann. Intern. Med. 2002;136(7):515–522. doi: 10.7326/0003-4819-136-7-200204020-00008. [DOI] [PubMed] [Google Scholar]
- 30.Voswinckel R., Reichenberger F., Enke B., Kreckel A., Krick S., Gall H. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm. Pharmacol. Ther. 2008;21(5):824–832. doi: 10.1016/j.pupt.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Swisher J.W., Elliott D. Combination therapy with riociguat and inhaled treprostinil in inoperable and progressive chronic thromboembolic pulmonary hypertension. Respir. Med. Case Rep. 2017 doi: 10.1016/j.rmcr.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.