Abstract
Despite progress in developing cell therapies, such as T cell or stem cell therapies to treat diseases, immunoincompatibility remains a major barrier to clinical application. Given the fact that a host's immune system may reject allogeneic transplanted cells, methods have been developed to genetically modify patients' primary cells. To advance beyond this time-consuming and costly approach, recent research efforts focus on generating universal pluripotent stem cells to benefit a broader spectrum of patients. In this review, we first summarize current achievements to harness immunosuppressive mechanisms in cells to reduce immunogenicity. Then, we discuss several recent studies demonstrating the feasibility of genetically modifying pluripotent stem cells to escape immune attack and summarize the methods to evaluate hypoimmunogenicity. Although challenges remain, progress to develop genetically engineered universal pluripotent stem cells holds the promise of expediting their use in future gene and cell therapeutics and regenerative medicine.
Subject Areas: Biological Sciences, Genetic Engineering, Cell Biology, Stem Cells Research
Biological Sciences; Genetic Engineering; Cell Biology; Stem Cells Research
Introduction
Given their potential for unlimited proliferation and differentiation to various types of tissues, human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSC) represent promising tools for the treatment of cancer as well as neuronal, cardiovascular, and liver diseases (Rami et al., 2017). In fact, hESC-derived cells have entered clinical trials to treat macular degeneration, spinal cord injury, type 1 diabetes, Parkinson disease, and cancer (Liu et al., 2017, Trounson and DeWitt, 2016). Pluripotent stem cell-differentiated chimeric antigen receptor (CAR)-T cells as well as natural killer (NK) cells show great potency in pre-clinical studies, and particularly, iPSC-derived CAR-NK cells have recently entered cancer clinical trials for safety evaluation (Hodgins et al., 2019). CAR-T cell therapy has also achieved great success in treating patients with refractory and relapsed hematologic diseases such as B cell acute lymphocytic leukemia, chronic lymphocytic leukemia, and non-Hodgkin's lymphoma (McCreedy et al., 2018).
Despite such progress, immuno-incompatibility remains a major barrier to clinical application of stem cell therapy. Human leukocyte antigen (HLA) is the main cause of immuno-incompatibility. HLA genes encode the major histocompatibility complex (MHC) membrane-bound glycoproteins in humans. HLA complex is composed of a series of genes that can be divided into three categories: Class I, Class II, and Class III. Allogeneic cells can be eliminated by a host's cytotoxic CD8+ T cells and CD4+ T helper cells through exposure of foreign HLA Class I and Class II molecules, whereas Class III molecules are not involved in immunization activities. In recent years, the field has attempted to reduce immunogenicity, or the rejection of allogeneic transplanted cells by the host's immune system, via immunosuppressive drugs, HLA matching, and gene editing. Transplantation of autologous cells eliminates the possibility of immune rejection; however, manufacturing autologous cells in an individual patient basis makes it a costly therapeutic product. It is estimated that the median costs of the autologous hematopoietic cell transplantation (HCT) are $109,000 (range $26,000–490,000) (Khera et al., 2013). As such, some researchers attempted to establish HLA-matched iPSC libraries to cover the majority of certain populations (Solomon et al., 2015, Turner et al., 2013), yet this requires a large number of samples—more than 4 million in the United States alone—to be screened to derive enough HLA homozygous cell lines. Current registry and iPSC banking efforts around the world would not be able to provide matches for the majority of people in their respective countries (Ilic and Ogilvie, 2017, Nakatsuji et al., 2008, Pappas et al., 2015, Riolobos et al., 2013, Taylor et al., 2012). Given these challenges, and the fact that immunosuppressive drugs such as azathioprine cause myelosuppression, hepatotoxicity, alopecia, and gastrointestinal adverse effects (Rossi et al., 1993), the field is turning to a new approach, genetic editing. Modifying the genome encoding the immunogenicity elements of the transplanted cell products, to enhance hypoimmunogenicity, makes large-scale manufacturing of these “off-the-shelf” products possible. In this review, we first summarize current achievements to harness immunosuppressive mechanisms in genetically engineered cells to reduce immunogenicity. Then, we discuss several recent studies demonstrating the feasibility of genetically modifying pluripotent stem cells to escape immune attack and summarize the methods to evaluate hypoimmunogenicity. Although challenges remain, such as safety problems, progress to develop genetically engineered pluripotent stem cells holds the promise of expediting the translation of universal cell therapies for use in the clinic.
Approaches to Reduce Immunogenicity
Harnessing the Immunosuppressive Machinery
Recent strategies to harness immunosuppressive machinery in genetically engineered cells derive from observations of the placenta or cancer cell activities. Previous studies have shown that the syncytiotrophoblast cells from placental origin, a barrier between fetal blood and allogeneic maternal blood, have low MHC I and MHC II expression levels, but high expression of CD47 and other features that protect the fetal cells from maternal immune attack (Deuse et al., 2019, Makrigiannakis et al., 2008). To protect themselves from immune attack, cancer cells express immunosuppressive molecules, including cytotoxic T lymphocyte antigen 4 (CTLA4), programmed death ligand-1 (PD-L1), CD47, CD24, or the β2-microglobulin subunit of the HLA-I (Bradley, 2019, Brightwell et al., 2016, Li et al., 2013, Xu et al., 2019), that send signals through corresponding immune cell receptors. CTLA4 and PD-L1 maintain peripheral tolerance by restraining T cell activity (Lanza et al., 2019), and PD-L1 expression may also protect engrafted cells from attack by PD-1+ NK cells (Beldi-Ferchiou et al., 2016, Della Chiesa et al., 2016) and PD-1+ macrophages (Gordon et al., 2017).
Such findings prompted studies to explore whether overexpressing CTLA4-immunoglobulin fusion protein (CTLA4-Ig) or PD-L1 in allogenic cells prevents clearance by the immune system. For instance, Rong et al. knocked CTLA4-Ig or PD-L1 into the HPRT1 locus of hESCs and found that the modified allogeneic hESCs and their differentiated progenies effectively avoid immune surveillance by inhibiting T cell activity, preventing T cell infiltration, and increasing the number of Treg cells (Fife and Bluestone, 2008, Rong et al., 2014). Importantly, the cells gain immune protection only when both molecules are present. Hence, these findings demonstrate that up-regulating immunosuppressive molecule expression in engrafted cells protects engineered ESC-derived cells from attack by the allogenic host. However, this approach does not modify HLA molecules and does not prevent hyperacute rejection of transplanted cells by anti-HLA antibody; such rejection occurs due to pre-formed antibodies present in the recipient's serum that react to donor antigens expressed on engrafted cells (Afzali et al., 2007, Masson et al., 2007).
Leveraging the interactions between other cancer cell immunosuppressive molecules and other types of immune cells such as NK cells and macrophages may also hold promise. CD47, an immunoglobulin-like protein expressed on the surface of red blood cells, all human solid tumor cells, and syncytiotrophoblast cells, interacts with SIRPα on macrophages to decrease phagocytosis (Willingham et al., 2012). CD47 has been utilized in genetically engineered iPSCs to confer immune tolerance to innate immune cells (Chhabra et al., 2016, Han et al., 2019, Jaiswal et al., 2009). In addition, CD47 plays an inhibitory role in NK cell-mediated cytotoxicity against cancer cells, mainly through CD47 ligand thrombospondin-1 (Nath et al., 2019). Deuse et al., found that CD47-overexpressing cells significantly reduce interferon-γ release from activated NK cells in vitro, thus indicating a reduced immune response. They also found that CD47 transgene-expressing mouse iPSC-induced endothelial cells (miECs) evade NK cells' attack in vivo, whereas wild-type miECs do not. They were able to achieve the same results with engineered human cells (Deuse et al., 2019). Of note, CD24, a sialoglycoprotein expressed on mature granulocytes and B cells, and overexpressed in cancers including breast (TNBC) and ovarian, prevents macrophagic phagocytosis through interaction with Siglec-G/10 (Bradley, 2019, Chan et al., 2019, Chen et al., 2014). However, CD24 has not yet been utilized in genetically engineering iPSCs. Together, these findings demonstrate that natural immunosuppressive machineries can be engineered into pluripotent stem cells to confer hypoimmunogenicity, and the growing list of genes in these machineries could be tested for further development of hypoimmunogenic cells. These types of approaches rely on the immunosuppressive receptor-ligand pairs, and on whether they are present on engrafted cells and immune cells, and thus introduction of only these genes may not necessarily confer full hypoimmunogenecity. These strategies do not involve genetic depletion of endogenous genes and may have less off-target effects and toxicity to the engineered cells, but any side effects of ectopic expression still need to be characterized.
Editing HLA Class I Genes to Escape from Allogeneic T Cells
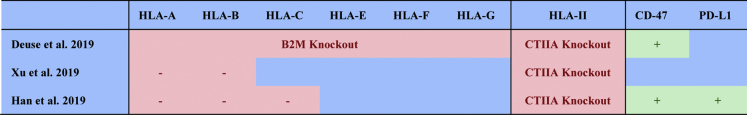
HLA Class I genes, which are expressed on almost all nucleated cells and platelets, present intracellularly processed peptides to CD8+ cytotoxic T cells and enable elimination of antigen-expressing or virus-infected cells. The HLA Class I molecule is composed of highly polymorphic heavy chain alpha and β2-microglobulin, which is encoded by the B2M gene. In the HLA Class I-mediated antigen presentation pathway, transporters associated with antigen processing (TAP)1 and TAP2 are responsible for peptide delivery from the cytosol to the lumen of the endoplasmic reticulum (Abele and Tampe, 2004). Even though mutations in TAP1 or TAP2 result in structural changes that can cause severe reduction in the cell surface expression of HLA Class I molecules and alter antigen recognition and presentation (Koch et al., 2004, Yang et al., 2003), individuals with TAP1 or TAP2 mutations are relatively healthy (Zimmer et al., 2005). Given B2M knockout mice exhibit relative health except for the lack of CD4+ CD8+ T cells (Riolobos et al., 2013), knocking out B2M gene is the preferred method to reduce HLA I-mediated immunogenicity (Riolobos et al., 2013, Wang et al., 2015, Xu et al., 2019).
Such reductions in immunogenicity do not go unnoticed. NK cells, a group of cytotoxic innate lymphoid cells that play a significant role in the first human immune defense line against pathogenic invasion and malignant cell transformation (Arnon et al., 2004, Chaushu et al., 2012), detect the lack of HLA-I expression on target cells (Dressel et al., 2010, Kruse et al., 2015, Wang et al., 2015). Furthermore, inhibitory receptors such as the Killer Cell Immunoglobulin-like receptors (KIRs) and the NKG2A receptor are responsible for sensing the “missing self,” which identifies cells with lower HLA-I expression. Given many NK cell subsets exhibit heterogeneous receptor expression and different specificity toward HLA-A, HLA-B, HLA-C, and HLA-E (Ichise et al., 2017), complete elimination of HLA Class I in donor cells will activate host NK cells to attack the HLA Class I-deficient cells (Gornalusse et al., 2017, Torikai et al., 2016, Xu et al., 2019). Thus, researchers now seek to develop comprehensive strategies to cope with NK cell attack resulting from B2M knockout as described in the following paragraph.
As HLA-E is a major ligand for the NK inhibitory receptor CD94/NKG2A (Lee et al., 1998), overexpression of single-chain HLA-E could suppress NKG2A+ NK cells. Of note, HLA-E introduced and overexpressed in B2M knockout pluripotent stem cells lysed less compared with HLA-E-negative cells when co-cultured with NK cells in vitro (Gornalusse et al., 2017). However, this strategy cannot suppress KIR2D+ NK cell populations because KIR2D+ NK cells are suppressed by HLA-C (Ichise et al., 2017) or HLA-G (Zhao et al., 2014). Thus, another group selectively knocked out HLA-A/B/C and overexpressed HLA-G to evade the NK cells including the KIR2D+ population (Han et al., 2019). An alternative method is to selectively knock out HLA-A and HLA-B and retain HLA-C (Xu et al., 2019). Due to the heterogeneity of NK cell subtypes, with diverse HLA/NK receptor immunosuppressive pairs, the leaving question is which population can be suppressed by HLA-C/G/E, and whether all NK cell subtypes can be adequately suppressed.
Editing HLA Class II Genes to Further Escape from Allogeneic T Cells
HLA Class II proteins (HLA-DR, HLA-DP, HLA-DQ) present peptides to CD4+ helper T cells, thus the potential exists to manipulate HLA Class II genes similar to HLA Class I genes. Class II proteins are composed of polypeptide α and β chains. Individuals with HLA-II deficiency present with CD4+ T cell lymphopenia, because HLA-II-dependent thymic maturation of T cells is impaired (Farrokhi et al., 2018). There are four categories of HLA-II deficiency based on which of the following four genes exhibit defects: RFXANK, RFX5, RFXAP, and CIITA (Farrokhi et al., 2018). RFXANK, RFX5, and RFXAP encode transcription factors that bind to HLA-II promoters and recruit the transcription factor CIITA to activate HLA-II gene expression. Recent studies show knockout of CIITA using CRISPR-Cas9 technologies reduces HLA-II molecules (Deuse et al., 2019, Han et al., 2019, Xu et al., 2019). Deuse et al. reported that HLA-I and HLA-II double-knockout mouse iPSCs evaded immune attack more effectively with more teratomas formed (10 out of 10) compared with HLA-I single knockout (6 out of 10) (Deuse et al., 2019). Their work showed that HLA-II deficiency further confers hypoimmunogenicity of transplanted cells. One potential concern in a specific case of application is that if the final differentiation products are dendritic cells (DCs), knocking out HLA-II molecules will ablate their antigen presentation functions.
Comprehensive Strategies to Develop Hypoimmunogenicity in Recent Studies
To obtain hypoimmunogenic cells, researchers have sought to ablate HLA Class I and Class II molecules, while simultaneously preventing attack from cytotoxic CD8+ T and CD4+ helper T cells. Not long ago, CRISPR-Cas9 genome editing technologies were used to deplete HLA Class I expression in human pluripotent stem cells (hPSCs) and hematopoietic stem cells (HSCs) by knocking out B2M (Mandal et al., 2014, Mattapally et al., 2018, Riolobos et al., 2013) and to eliminate HLA Class II expression by targeting CIITA (Chen et al., 2015). However, knocking out B2M prevents the surface expression of the nonpolymorphic HLA Class Ib molecules HLA-E and HLA-G, which are required for immune tolerance of NK cell (Ferreira et al., 2017, Lee et al., 1998). Overexpression of CD47 to prevent cells from being engulfed by NK cells has been conducted to synergize with the immune evasion effect from deletion of B2M and CIITA (Deuse et al., 2019). Xu et al. attempted to selectively delete HLA-A/-B and CIITA genes but retain HLA-C gene, which has the ability to suppress NK cells (Xu et al., 2019). HLA-C can still present peptides to T cells, which means the edited cells could potentially be recognized by T cells. Combined with the above-mentioned efforts, Han et al. simultaneously knocked out HLA-A/-B/-C and CIITA molecules and expressed the immunomodulatory factors PD-L1, HLA-G, and CD47, to evade immune surveillance by T cells, NK cells, and macrophages, respectively (Han et al., 2019). All the experiments showed significantly lower level of activation of T cells and NK cells, indicating that engineered cells are hypoimmunogenic (Figures 1 and 2). Han et al.'s strategy is comprehensive, but they can still observe residual activation of T cells in vitro. They pointed out that it might be caused by additional antigens generated in cell culture or due to the genetic modifications.
Figure 1.
Comprehensive Strategies to Develop Hypoimmunogenic Pluripotent Stem Cells in Recent Studies
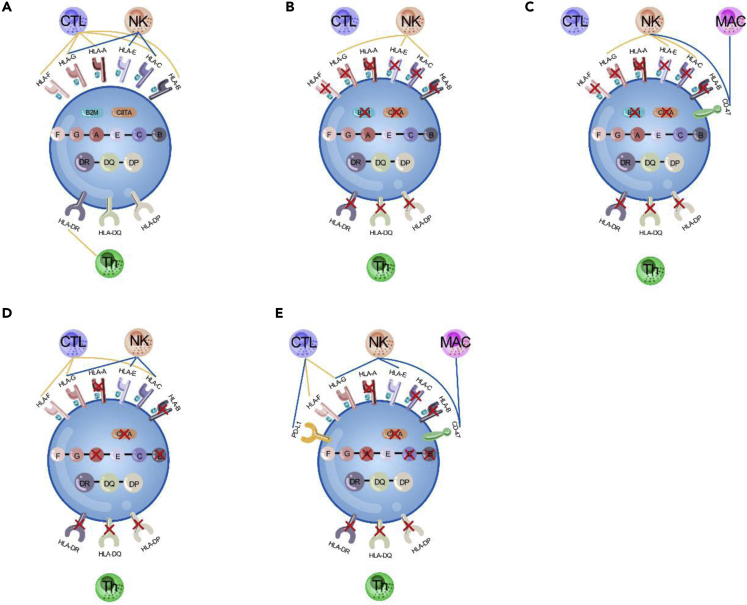
Figure 2.
Overview of Genetic Modification Strategies Employed to Generate Hypoimmunogenic Cells
Allogeneic cells can be rejected through innate immunity (NK cells and macrophages) as well as adaptive immunity (T cells).
(A) Allogeneic cells activate T cells by HLA-I and HLA-II molecules. HLA-I molecules suppress NK cells.
(B) Elimination of HLA-I by knocking out B2M and elimination of HLA-II by knocking out CIITA can evade T cell surveillance but trigger NK cell activity.
(C) Elimination of HLA-I and HLA-II to evade T cell attack combined with expression of the CD47 molecules to inactivate NK cells and macrophages (Deuse et al., 2019).
(D) Simultaneous deletion of HLA-A/B and HLA-II genes, and not HLA-C, allows HLA-C/G and HLA-E to suppress NK cells (Xu et al., 2019).
(E) Simultaneous knockout of HLA-A/B/C and HLA-II combined with expression of the immunomodulatory factors PD-L1, HLA-G, and CD47 (Han et al., 2019). These strategies combined can make the engineered cells to evade immune surveillance by T cells, NK cells, and macrophages.
Orange lines and blue lines depict pathway activation and suppression, respectively. NK, natural killer; Th, T helper; MAC, macrophage; CTL, cytotoxic lymphocyte.
Methods to Evaluate Hypoimmunogenicity of Genetically Engineered Pluripotent Stem Cells
Here, we summarize a set of in vitro and in vivo methods to evaluate the modified hypoimmunogenicity ordered by method stringency. Multiple in vitro assays can be employed to evaluate hypoimmunogenicity. Among these, the most straightforward but the least stringent methods to detect successful ablation of HLA gene expression are quantitative PCR and flow cytometry analyses (Han et al., 2019, Wang et al., 2015). Taking into account both adaptive immune and innate immune responses, it is recommended to quantify T cell and NK cell-mediated immune responses as well as macrophage engulfment in vitro (Han et al., 2019). For instance, to investigate whether removing polymorphic HLA molecules or introducing PD-L1 is sufficient to prevent T cell-mediated immune responses in vitro, T cell proliferation, activation, and killing assays were performed in a recent report (Han et al., 2019). Here, Han et al. found that all CD3/4/8+ T cell subpopulations' proliferation and activation markers were reduced in the presence of HLA-I knockout iECs. In addition, CD8+ cells, but not CD4+ T cells, were further suppressed by overexpression of PD-L1 in an HLA-null background. Consistent with results from proliferation assays, cytotoxicity of CD8+ T cell was suppressed by HLA-A/B/C knockout iECs, and even further by PD-L1 expression. Further in vitro assays indicated that when co-incubated with HLA-A/B/C knockout and PD-L1, CD47, and HLA-G overexpressed (KI-PHC) vascular smooth muscle cells (VSMCs) derived from KI-PHC hPSCs, NK cell activity is inhibited by HLA-G expression, manifested by a decline of NK cell marker CD107a. Analogously, co-culturing with pH-sensitive fluorescence dye (pHrodo-Red)-labeled VSMCs can indicate macrophage lysis activity because engulfed cells can be transported to low-pH lysosomes for digestion, and it was found that overexpression of CD47 in KI-PHC hPSC-derived VSMCs indeed reduced macrophage engulfment.
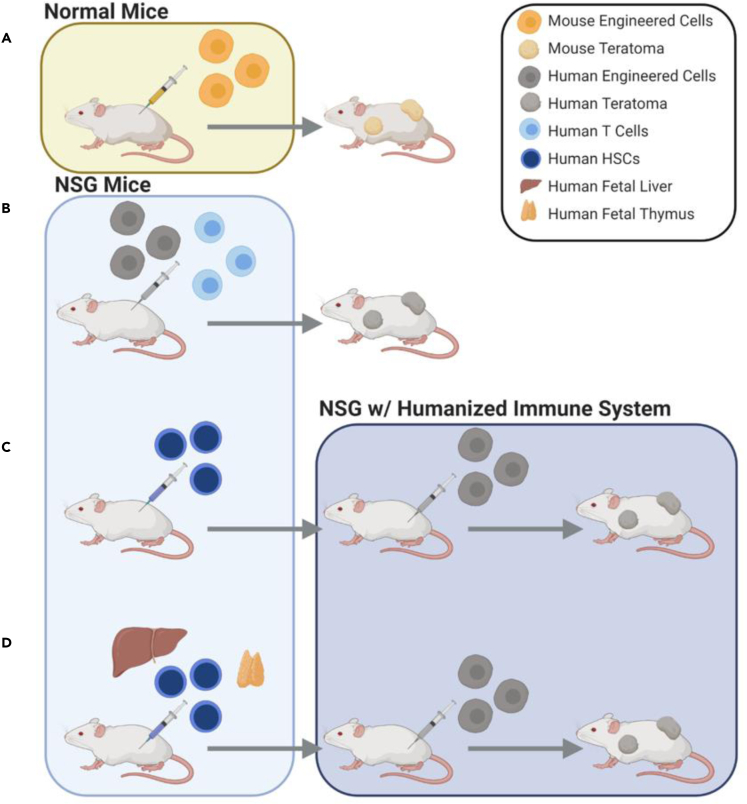
Given it is critical to evaluate hypoimmunogenicity in vivo, the field recently introduced a series of animal models that have evolved based on the origin of species for low immunogenicity test of the modified cells (Figure 3). For example, immunocompetent mice were used to test modified mouse hypoimmunogenic cells. Deuse et al. injected B2m−/−Ciita−/−CD47 and wild-type mouse iPSCs into the allogeneic mice and measured teratoma size (Deuse et al., 2019). Teratoma formed in immunocompetent allogeneic mice means that the modified iPSCs inside the mice are not eliminated by immune systems. Wang et al. first used anti-asialo GM1 to ablate the NK cells in immunocompetent mice and injected B2M−/−CIITA−/− human iPSCs, followed by teratoma size measurements (Wang et al., 2015).
Figure 3.
Evolution of In Vivo Models to Evaluate Hypoimmunogenicity of Genetically Engineered Pluripotent Stem Cells
(A) Size of teratomas measured following injection of engineered cells into immunocompetent mice to evaluate mouse cell engraftment.
(B) NSG mice injected with engineered cells plus T cells before measurement of teratoma size.
(C) To recapitulate the human immune system, human HSCs transplanted into NSG mice and injected with the engineered cells.
(D) To promote T cell reconstitution, maturation, and selection, human HSC cells, fetal liver, and fetal thymus tissue transplanted into NSG mice before injection with the engineered cells to evaluate immunogenicity.
However, due to the contrasting differences of human and mice immune systems, it is not completely informative to evaluate the immunogenicity of human cells with results from mouse cells. Some studies used severe combined immunodeficiency mice that lack T cells and B cells and used anti-asialo GM1 to deplete the NK cells (Wang et al., 2015). To completely eliminate mouse lymphoid cells, NSG (Han et al., 2019) or NRG (Xu et al., 2019) mice were used. For example, Han et al. injected both activated human T cells and KI-PHC human iPSCs into NSG mice and measured the growth of teratomas (Han et al., 2019).
Given immunodeficient mouse models cannot be used to test the interaction between engrafted cells and the human immune microenvironment, researchers have generated humanized mice to mimic the human immune system. Deuse et al. (Deuse et al., 2019) transplanted B2M−/−CIITA−/−CD47 hypoimmunogenic cells into allogeneic humanized CD34+ HSC-reconstituted NSG-SGM3 mice. To promote T cell reconstitution, maturation, and selection, BLT-humanized mice, which are NSG mice transplanted with human fetal liver, thymic tissue, and CD34+ HSCs, were used; four of five B2M−/−CIITA−/−CD47 engrafted iPSC lines survived, whereas all wild-type grafts underwent rapid rejection.
Challenges of Genetically Engineered Universal Pluripotent Stem Cells
Despite great progress to develop universal pluripotent stem cells, many challenges still exist. Although genetic modifications such as loss of MHC/HLA Class I and Class II do not lead to transformation per se (Hanna and Etzioni, 2014, Street et al., 2004), there are reports that human pluripotent stem cells exhibit mutations in the TP53 gene, which encodes tumor suppressor TP53, and that the mutation rate increases with the passage number (Avior et al., 2019, Merkle et al., 2017). Given that multiple rounds of genetic modifications may increase the risk of off-target events, it is essential for genetically engineered universal cells to be regularly screened for oncogenic mutations. Moreover, even though there is no evidence of human cancer cells transmitting from person to person, there are infectious cancer cells that evade allogeneic immune surveillance in other species, due to loss of MHC. Devil facial tumor disease is a transmittable tumor spread through biting and is the primary reason of death in the Tasmanian devil population, which is near extinction (Caldwell et al., 2018). Another contagious cancer, canine transmissible venereal tumor, can transmit through coitus, licking, biting, or sniffing tumor-affected areas in dogs (Welsh, 2011). Hence, it is essential to carefully examine mutations in the genome of the pluripotent stem cells and their differentiated progenies that are to be transplanted and to determine the infectiousness potential of hypoimmunogenic cells (Trounson, 2017).
One potential safety solution is to introduce a suicide gene into the modified iPSCs (Li and Xiang, 2013). For instance, herpes simplex virus thymidine kinase can be knocked in downstream of the OCT4 (POU5F1) promoter (Hara et al., 2008) or CDK1 gene (Harding et al., 2019) and selective elimination of undifferentiated embryonic stem cells could be achieved upon ganciclovir (GCV) treatment. Another suicide system is inducible Caspase-9. Caspase-9 was knocked in downstream of the miR302/367 promoter to enable selective ablation of the undifferentiated iPSCs (Ando et al., 2015, Villanueva et al., 2019).
One challenge that cannot be ignored is the loss of antigen presentation function of universal cells once MHC genes are depleted. For most cases of differentiated cells, this is not an issue. However, if the differentiated cell products are DCs, for instance, in the case of DC-based vaccines, it becomes more sophisticated to consider the effects of MHC depletion. The activation of naive antigen-specific T cells by professional antigen-presenting cells, such as DCs and macrophages, is the foundation to generate anti-tumor T cell immune response (Joffre et al., 2012). To date, many studies have shown that neoantigens presented by DCs are key to the success of immunotherapies and development of certain tumor vaccines (Desrichard et al., 2016, Gubin et al., 2014). Autologous iPSC may be a superior source of DCs without immunological rejection. However, the application of autologous iPSC is limited by inevitable time lag in the process of obtaining the iPSC and the economic factor also needs to be considered. The universal cells that can still present antigens may be the best source of DCs. Different from the role in other cell types, MHC molecules play an essential character in DCs' physiological function—antigen presentation (Alloatti et al., 2017). Therefore, semi-allogeneic DC differentiated from partially engineered iPSCs that have one or more MHC loci matched with recipients may be a practical strategy. For instance, if MHC Class I molecules are depleted and MHC Class II molecule are retained in one line of iPSC, this line can be applied to MHC Class II loci-matched population, without losing the capability of antigen presentation to stimulate CD4+ T cells. Another strategy is to leave MHC molecules intact, but fully unleash the potential of immunosuppressive machineries. Recent research shows that expression of eight immunomodulatory genes, including Pdl1, Fasl, Serpinb9, Cd47, Cd200, H2-M3, Ccl21, and Mfge8, allows this type of engineered cells to survive in immunocompetent, allogeneic recipients (Harding et al., 2019). Although this study did not demonstrate the contribution of each immunomodulatory gene, it provided an inspiring strategy to achieve hypoimmunogenicity without disturbing MHC molecules. More rigorous research needs to be performed to test whether the antigen presentation function is preserved, and whether these cells can be used as a source of DC-based tumor vaccines.
In summary, with increased understanding of the host immune surveillance system and the immunogenicity mechanisms of grafted cells, we can utilize genome engineering approaches to engineer hypoimmunogenic pluripotent stem cells, which will be useful to generate universal allogeneic transplantation cell products. Even though these strategies hold great promise, it is imperative to gather solid evidence demonstrating the robust hypoimmunogenicity and safety of such cells in vivo and bring them to clinical applications.
References
- Abele R., Tampe R. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology. 2004;19:216–224. doi: 10.1152/physiol.00002.2004. [DOI] [PubMed] [Google Scholar]
- Afzali B., Lechler R.I., Hernandez-Fuentes M.P. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69:545–556. doi: 10.1111/j.1399-0039.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- Alloatti A., Rookhuizen D.C., Joannas L., Carpier J.M., Iborra S., Magalhaes J.G., Yatim N., Kozik P., Sancho D., Albert M.L. Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity. J. Exp. Med. 2017;214:2231–2241. doi: 10.1084/jem.20170229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Nishimura T., Yamazaki S., Yamaguchi T., Kawana-Tachikawa A., Hayama T., Nakauchi Y., Ando J., Ota Y., Takahashi S. A safeguard system for induced pluripotent stem cell-derived rejuvenated T cell therapy. Stem Cell Rep. 2015;5:597–608. doi: 10.1016/j.stemcr.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon T.I., Achdout H., Lieberman N., Gazit R., Gonen-Gross T., Katz G., Bar-Ilan A., Bloushtain N., Lev M., Joseph A. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- Avior Y., Eggan K., Benvenisty N. Cancer-related mutations identified in primed and naive human pluripotent stem cells. Cell Stem Cell. 2019;25:456–461. doi: 10.1016/j.stem.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Beldi-Ferchiou A., Lambert M., Dogniaux S., Vely F., Vivier E., Olive D., Dupuy S., Levasseur F., Zucman D., Lebbe C. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget. 2016;7:72961–72977. doi: 10.18632/oncotarget.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C.A. CD24 - a novel 'don't eat me' signal. Nat. Rev. Drug Discov. 2019;18:747. doi: 10.1038/d41573-019-00146-0. [DOI] [PubMed] [Google Scholar]
- Brightwell R.M., Grzankowski K.S., Lele S., Eng K., Arshad M., Chen H., Odunsi K. The CD47 "don't eat me signal" is highly expressed in human ovarian cancer. Gynecol. Oncol. 2016;143:393–397. doi: 10.1016/j.ygyno.2016.08.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell A., Coleby R., Tovar C., Stammnitz M.R., Kwon Y.M., Owen R.S., Tringides M., Murchison E.P., Skjødt K., Thomas G.J. The newly-arisen Devil facial tumour disease 2 (DFT2) reveals a mechanism for the emergence of a contagious cancer. Elife. 2018;7:e35314. doi: 10.7554/eLife.35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.H., Tsai K.W., Chiu S.Y., Kuo W.H., Chen H.Y., Jiang S.S., Chang K.J., Hung W.C., Wang L.H. Identification of the novel role of CD24 as an oncogenesis regulator and therapeutic target for triple-negative breast cancer. Mol. Cancer Ther. 2019;18:147–161. doi: 10.1158/1535-7163.MCT-18-0292. [DOI] [PubMed] [Google Scholar]
- Chaushu S., Wilensky A., Gur C., Shapira L., Elboim M., Halftek G., Polak D., Achdout H., Bachrach G., Mandelboim O. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8:e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Brown N.K., Zheng P., Liu Y. Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology. 2014;24:800–806. doi: 10.1093/glycob/cwu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li Y., Lin X., Cui D., Cui C., Li H., Xiao L. Functional disruption of human leukocyte antigen II in human embryonic stem cell. Biol. Res. 2015;48:59. doi: 10.1186/s40659-015-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A., Ring A.M., Weiskopf K., Schnorr P.J., Gordon S., Le A.C., Kwon H.S., Ring N.G., Volkmer J., Ho P.Y. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci. Transl. Med. 2016;8:351ra105. doi: 10.1126/scitranslmed.aae0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Chiesa M., Pesce S., Muccio L., Carlomagno S., Sivori S., Moretta A., Marcenaro E. Features of memory-like and PD-1(+) human NK cell subsets. Front. Immunol. 2016;7:351. doi: 10.3389/fimmu.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrichard A., Snyder A., Chan T.A. Cancer neoantigens and applications for immunotherapy. Clin. Cancer Res. 2016;22:807–812. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- Deuse T., Hu X., Gravina A., Wang D., Tediashvili G., De C., Thayer W.O., Wahl A., Garcia J.V., Reichenspurner H. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019;37:252–258. doi: 10.1038/s41587-019-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R., Nolte J., Elsner L., Novota P., Guan K., Streckfuss-Bomeke K., Hasenfuss G., Jaenisch R., Engel W. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 2010;24:2164–2177. doi: 10.1096/fj.09-134957. [DOI] [PubMed] [Google Scholar]
- Farrokhi S., Shabani M., Aryan Z., Zoghi S., Krolo A., Boztug K., Rezaei N. MHC class II deficiency: report of a novel mutation and special review. Allergol. Immunopathol. 2018;46:263–275. doi: 10.1016/j.aller.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Ferreira L.M.R., Meissner T.B., Tilburgs T., Strominger J.L. HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. 2017;38:272–286. doi: 10.1016/j.it.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Gordon S.R., Maute R.L., Dulken B.W., Hutter G., George B.M., McCracken M.N., Gupta R., Tsai J.M., Sinha R., Corey D. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornalusse G.G., Hirata R.K., Funk S.E., Riolobos L., Lopes V.S., Manske G., Prunkard D., Colunga A.G., Hanafi L.A., Clegg D.O. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017;35:765–772. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Wang M., Duan S., Franco P.J., Kenty J.H., Hedrick P., Xia Y., Allen A., Ferreira L.M.R., Strominger J.L. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. U S A. 2019;116:10441–10446. doi: 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna S., Etzioni A. MHC class I and II deficiencies. J. Allergy Clin. Immunol. 2014;134:269–275. doi: 10.1016/j.jaci.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Hara A., Aoki H., Taguchi A., Niwa M., Yamada Y., Kunisada T., Mori H. Neuron-like differentiation and selective ablation of undifferentiated embryonic stem cells containing suicide gene with Oct-4 promoter. Stem Cells Dev. 2008;17:619–627. doi: 10.1089/scd.2007.0235. [DOI] [PubMed] [Google Scholar]
- Harding J., Vintersten-Nagy K., Shutova M., Yang H., Tang J.K., Massumi M., Izaidfar M., Izadifar Z., Zhang P., Li C. Induction of long-term allogeneic cell acceptance and formation of immune privileged tissue in immunocompetent hosts. bioRxiv. 2019:716571. [Google Scholar]
- Hodgins J.J., Khan S.T., Park M.M., Auer R.C., Ardolino M. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Invest. 2019;129:3499–3510. doi: 10.1172/JCI129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise H., Nagano S., Maeda T., Miyazaki M., Miyazaki Y., Kojima H., Yawata N., Yawata M., Tanaka H., Saji H. NK cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Rep. 2017;9:853–867. doi: 10.1016/j.stemcr.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D., Ogilvie C. Concise review: human embryonic stem cells-what have we done? what are we doing? where are we going? Stem Cells. 2017;35:17–25. doi: 10.1002/stem.2450. [DOI] [PubMed] [Google Scholar]
- Jaiswal S., Jamieson C.H., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O.P., Segura E., Savina A., Amigorena S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Khera N., Storer B., Sandmaier B.M., Chapko M.K., Lee S.J. Costs of second allogeneic hematopoietic cell transplantation. Transplantation. 2013;96:108–115. doi: 10.1097/TP.0b013e318294caf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J., Guntrum R., Heintke S., Kyritsis C., Tampe R. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP) J. Biol. Chem. 2004;279:10142–10147. doi: 10.1074/jbc.M312816200. [DOI] [PubMed] [Google Scholar]
- Kruse V., Hamann C., Monecke S., Cyganek L., Elsner L., Hubscher D., Walter L., Streckfuss-Bomeke K., Guan K., Dressel R. Human induced pluripotent stem cells are targets for allogeneic and autologous natural killer (NK) cells and killing is partly mediated by the activating NK receptor DNAM-1. PLoS One. 2015;10:e0125544. doi: 10.1371/journal.pone.0125544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza R., Russell D.W., Nagy A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019;19:723–733. doi: 10.1038/s41577-019-0200-1. [DOI] [PubMed] [Google Scholar]
- Lee N., Llano M., Carretero M., Ishitani A., Navarro F., Lopez-Botet M., Geraghty D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. U S A. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.L., Fu L., Uchtenhagen H., Achour A., Burshtyn D.N. Cis association of leukocyte Ig-like receptor 1 with MHC class I modulates accessibility to antibodies and HCMV UL18. Eur. J. Immunol. 2013;43:1042–1052. doi: 10.1002/eji.201242607. [DOI] [PubMed] [Google Scholar]
- Li W., Xiang A.P. Safeguarding clinical translation of pluripotent stem cells with suicide genes. Organogenesis. 2013;9:34–39. doi: 10.4161/org.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li W., Fu X., Xu Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front. Immunol. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiannakis A., Karamouti M., Drakakis P., Loutradis D., Antsaklis A. Fetomaternal immunotolerance. Am. J. Reprod. Immunol. 2008;60:482–496. doi: 10.1111/j.1600-0897.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Mandal P.K., Ferreira L.M.R., Collins R., Meissner T.B., Boutwell C.L., Friesen M., Vrbanac V., Garrison B.S., Stortchevoi A., Bryder D. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–652. doi: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson E., Stern M., Chabod J., Thevenin C., Gonin F., Rebibou J.M., Tiberghien P. Hyperacute rejection after lung transplantation caused by undetected low-titer anti-HLA antibodies. J. Heart Lung Transpl. 2007;26:642–645. doi: 10.1016/j.healun.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Mattapally S., Pawlik K.M., Fast V.G., Zumaquero E., Lund F.E., Randall T.D., Townes T.M., Zhang J. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 2018;7:e010239. doi: 10.1161/JAHA.118.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy B.J., Senyukov V.V., Nguyen K.T. Off the shelf T cell therapies for hematologic malignancies. Best Pract. Res. Clin. Haematol. 2018;31:166–175. doi: 10.1016/j.beha.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Merkle F.T., Ghosh S., Kamitaki N., Mitchell J., Avior Y., Mello C., Kashin S., Mekhoubad S., Ilic D., Charlton M. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545:229–233. doi: 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji N., Nakajima F., Tokunaga K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008;26:739–740. doi: 10.1038/nbt0708-739. [DOI] [PubMed] [Google Scholar]
- Nath P.R., Pal-Nath D., Mandal A., Cam M.C., Schwartz A.L., Roberts D.D. Natural killer cell recruitment and activation are regulated by CD47 expression in the tumor microenvironment. Cancer Immunol. Res. 2019;7:1547–1561. doi: 10.1158/2326-6066.CIR-18-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas D.J., Gourraud P.A., Le Gall C., Laurent J., Trounson A., DeWitt N., Talib S. Proceedings: human leukocyte antigen haplo-homozygous induced pluripotent stem cell haplobank modeled after the California population: evaluating matching in a multiethnic and admixed population. Stem Cells Transl. Med. 2015;4:413–418. doi: 10.5966/sctm.2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami F., Beni S.N., Kahnamooi M.M., Rahimmanesh I., Salehi A.R., Salehi R. Recent advances in therapeutic applications of induced pluripotent stem cells. Cell Reprogram. 2017;19:65–74. doi: 10.1089/cell.2016.0034. [DOI] [PubMed] [Google Scholar]
- Riolobos L., Hirata R.K., Turtle C.J., Wang P.R., Gornalusse G.G., Zavajlevski M., Riddell S.R., Russell D.W. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z., Wang M., Hu Z., Stradner M., Zhu S., Kong H., Yi H., Goldrath A., Yang Y.G., Xu Y. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell. 2014;14:121–130. doi: 10.1016/j.stem.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S.J., Schroeder T.J., Hariharan S., First M.R. Prevention and management of the adverse effects associated with immunosuppressive therapy. Drug Safety. 1993;9:104–131. doi: 10.2165/00002018-199309020-00004. [DOI] [PubMed] [Google Scholar]
- Solomon S., Pitossi F., Rao M.S. Banking on iPSC--is it doable and is it worthwhile. Stem Cell Rev. 2015;11:1–10. doi: 10.1007/s12015-014-9574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street S.E., Hayakawa Y., Zhan Y., Lew A.M., MacGregor D., Jamieson A.M., Diefenbach A., Yagita H., Godfrey D.I., Smyth M.J. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.J., Peacock S., Chaudhry A.N., Bradley J.A., Bolton E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–152. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Torikai H., Mi T., Gragert L., Maiers M., Najjar A., Ang S., Maiti S., Dai J., Switzer K.C., Huls H. Genetic editing of HLA expression in hematopoietic stem cells to broaden their human application. Sci. Rep. 2016;6:21757. doi: 10.1038/srep21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A. Potential pitfall of pluripotent stem cells. N. Engl. J. Med. 2017;377:490–491. doi: 10.1056/NEJMcibr1706906. [DOI] [PubMed] [Google Scholar]
- Trounson A., DeWitt N.D. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 2016;17:194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- Turner M., Leslie S., Martin N.G., Peschanski M., Rao M., Taylor C.J., Trounson A., Turner D., Yamanaka S., Wilmut I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Villanueva J., Nishimura T., Nakauchi H. Using the inducible caspase-9 suicide-safeguard system with iPSC and bioluminescent tracking. Methods Mol. Biol. 2019;2048:259–264. doi: 10.1007/978-1-4939-9728-2_20. [DOI] [PubMed] [Google Scholar]
- Wang D., Quan Y., Yan Q., Morales J.E., Wetsel R.A. Targeted disruption of the beta2-microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl. Med. 2015;4:1234–1245. doi: 10.5966/sctm.2015-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J.S. Contagious cancer. The Oncologist. 2011;16:1–14. doi: 10.1634/theoncologist.2010-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Wang B., Ono M., Kagita A., Fujii K., Sasakawa N., Ueda T., Gee P., Nishikawa M., Nomura M. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24:566–578.e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Yang T., McNally B.A., Ferrone S., Liu Y., Zheng P. A single-nucleotide deletion leads to rapid degradation of TAP-1 mRNA in a melanoma cell line. J. Biol. Chem. 2003;278:15291–15296. doi: 10.1074/jbc.M300954200. [DOI] [PubMed] [Google Scholar]
- Zhao L., Teklemariam T., Hantash B.M. Heterelogous expression of mutated HLA-G decreases immunogenicity of human embryonic stem cells and their epidermal derivatives. Stem Cell Res. 2014;13:342–354. doi: 10.1016/j.scr.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Zimmer J., Andres E., Donato L., Hanau D., Hentges F., de la Salle H. Clinical and immunological aspects of HLA class I deficiency. QJM. 2005;98:719–727. doi: 10.1093/qjmed/hci112. [DOI] [PubMed] [Google Scholar]