ABSTRACT
BACKGROUND:
Mesenteric traction syndrome (MTS), which is characterized by arterial hypotension and tachycardia following mesenteric traction (MT), frequently occurs during abdominal surgery. Dexmedetomidine, commonly used in general anesthesia during major surgery, has a sympatholytic effect and attenuates the compensatory response to hypotension.
OBJECTIVE:
Assess the effect of dexmedetomidine on hypotension following mesenteric traction.
DESIGN:
Prospective, randomized, controlled clinical trial.
SETTING:
Department of Anesthesiology, Zhenjiang First People's Hospital in China.
PATIENTS AND METHODS:
Patients were randomly divided into three groups. Dexmedetomidine, 0.5 or 1.0 µg/kg, was intravenously administered over 15 minutes before skin incision followed by a maintenance rate of 0.5 µg/kg/h in groups D1 and D2, respectively; saline was administered in group C.
MAIN OUTCOME MEASURE(S):
The duration of hypotension, heart rate and plasma norepinephrine level in patients with MTS were recorded within 60 minutes following MT.
SAMPLE SIZE:
75 patients.
RESULTS:
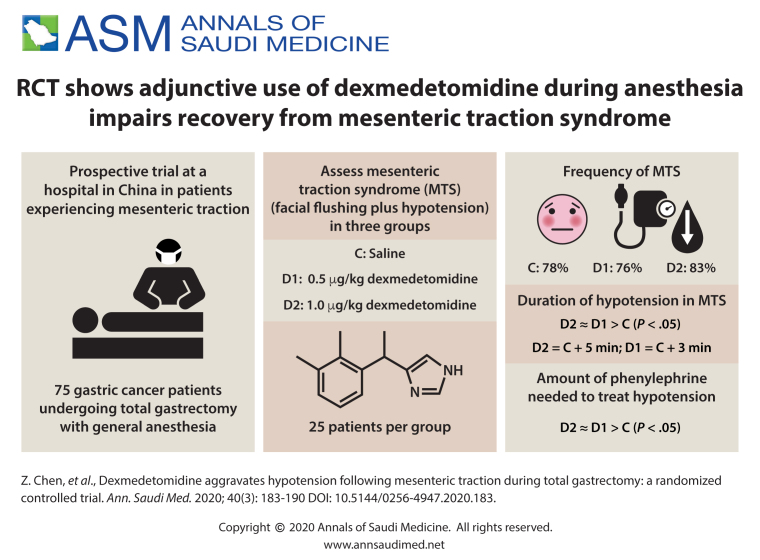
The duration of hypotension in the MTS patients in group D1 and D2 was significantly longer than that in groups C (D1 vs. C, P<.05; D2 vs. C, P<.01). Significantly more phenylephrine was required to treat hypotension in group D1 and D2 than was required for patients in group C (P<.05). The increase in heart rate during the first 15 minutes of MT in group D2 was significantly attenuated compared to that in group C (P<.0083). The increases in norepinephrine levels during the first 15 minutes of MT in group C were significantly higher than those in groups D1 and D2 (P<.0167).
CONCLUSION:
Adjunctive dexmedetomidine in general anesthesia aggravates hypotension during MTS in open total gastrectomy.
LIMITATIONS:
Postoperative complications were not evaluated.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Traction of the mesentery in major abdominal surgery leads to systemic hypotension, tachycardia and facial flushing, which is defined as mesenteric traction syndrome (MTS).1 Mesenteric traction (MT) stimulates endothelial cells of the mesenteric vessels and activates cyclooxygenase-1 (COX-1), which induces the production of prostacyclin I2 (PGI2). The release of PGI2 causes systemic vasodilation and hypotension.2-4 The stress response system is activated to attenuate hypotension and stabilized hemodynamics when MTS occurs. Brinkmann et al reported that hypo-tension following MT was accompanied by enhanced release of the counterregulatory vasopressor hormone, such as catecholamine and arginine vasopressin.5
Dexmedetomidine, a commonly used adjuvant drug in general anesthesia, can reduce the perioperative usage of anesthetics. Dexmedetomidine is a highly selective α2-adrenergic agonist that inhibits sympathetic nervous system reactions to surgical stimuli, which can stabilize intraoperative hemodynamics.6 However, its sympatholytic effect inhibits protective stress responses to blood pressure decreases, and increases the incidence of hypotension.7,8 Therefore, we postulated that dexmedetomidine may increase the severity of MTS because of its inhibitory counterregulatory mechanisms. The primary aim of this study was to investigate the effects of dexmedetomidine on hypotension and heart rate. The secondary aim was to measure the plasma concentration of norepinephrine following MT during open total gastrectomy.
PATIENTS AND METHODS
The prospective, randomized controlled clinical trial was approved by the medical ethics committee of Zhenjiang First People's Hospital, Zhenjiang, Jiangsu Province, (K-20170030-W) and is registered at www.chictr.org (ChiCTR1800014506). The study was implemented between January 2018 and May 2018. The study was conducted in accordance with the Declaration of Helsinki. The CONSORT guidelines were followed in reporting the study.9
Eligibility
Gastric cancer patients confirmed by gastroscope and pathology and scheduled for elective open total gastrectomy with general anesthesia were recruited. Other inclusion criteria were American Society of Anesthesiologists (ASA) physical status classification I-II and age 40-65 years old. The exclusion criteria included body mass index ≥28 kg/m2, nonsteroidal anti-inflammatory drug treatment, poor preoperative control of hypertension, hepatic failure, renal dysfunction, and cardiac diseases, including bradycardia and atrioventricular block. Written informed consent was obtained from all participants. Patients were randomly allocated into three groups: the control group (group C), dexmedetomidine 0.5 µg/kg group (group D1) and dexmedetomidine 1.0 µg/kg group (group D2); there were 25 patients in each group.
Anesthesia procdeures
A 16-G central venous catheter was inserted into the right internal jugular vein, and 8 mL/kg of lactated Ringer's solution was infused before anesthesia induction. Invasive radial artery blood pressure and bispectral index (BIS) were monitored. Propofol and reminifendine were infused by effect-site infusion for anesthesia induction via a commercially available TCI pump (Veryark Concert-I; Veryark Medical Technology Co., Ltd., Guangxi, China). The initial concentration of propofol was set at 3.0 μg/mL and was gradually increased until BIS values reached 40-60. Then, remifentanil was infused, and the concentration was set at 3.0 ng/mL. When the effect-site concentration of remifentanil reached the target value, rocuronium was administered to facilitate endotracheal intubation.
After completion of anesthesia induction, the infusion of remifentanil was discontinued temporarily. Dexmedetomidine (Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China) was intravenously administered at 0.5 or 1.0 µg/kg over 15 minutes before skin incision followed by the same maintenance rate of 0.5 µg/kg/h in groups D1 and D2, respectively; volume-matched normal saline was administered in group C. The effect-site concentration of propofol was adjusted to maintain BIS values at 40-60. Remifentanil infusion was restored 3 minutes before skin incision, and the initial concentration was set at 3.0 ng/mL. The effect-site concentration of remifentanil was adjusted according to blood pressure. The lungs were ventilated with a tidal volume of 6 mL/kg and 60% oxygen/40% air mixture. The respiratory frequency was adjusted to maintain an end-tidal carbon dioxide partial pressure between 30 and 40 mm Hg. Cisatracurium was intermittently injected to maintain muscle relaxation. Lactated Ringer's solution was continuously infused at 8 mL/kg/h.
Dexmedetomidine was discontinued before peritoneal closure. After propofol and remifentanil infusions were stopped before skin anastomosis, 3 μg/kg fentanyl was administered and patient-controlled intravenous analgesia was used to maintain a visual analog scale (VAS) score ≤3. Patients were routinely transferred to a postoperative anesthesia care unit and extubated after consciousness and muscular relaxation recovery.
After the incision of the peritoneum, the surgeon routinely explored the abdominal cavity, and assessments continued until 60 minutes post MT. Facial flushing after abdominal opening was judged every 2 minutes by two nurse anesthetists. If the systolic blood pressure (SBP) was below 70% of the baseline value or below 90 mm Hg, which was defined as hypotension, the 3 ng/mL concentration of remifentanil was maintained, and phenylephrine 1.0 µg/kg was administered to elevate blood pressure and repeated as necessary. If the SBP was more than 130% of baseline values or above 160 mm Hg, which was defined as hypertension, the concentration of remifentanil was increased. If the heart rate (HR) was below 45 bpm or if a second-degree atrioventricular block appeared, atropine 0.5 mg was administered. In our study, MTS was defined as facial flushing accompanied by hypotension within 30 minutes after MT. The duration of hypotension was defined as the interval from the onset of hypotension to the time of last phenylephrine administration, adding 3 minutes of vasopressor action of phenylephrine. If hypotension was corrected by one dose of phenylephrine, we took 3 minutes as the duration of hypotension.
Blood samples were collected before anesthesia induction and at 15 minutes and 60 minutes following MT. The blood samples were centrifuged at 2300 g for 15 minutesat 4°C, and the separated plasma was stored at -70°C. The plasma was thawed for the determination of the plasma concentrations of 6-keto-prostaglandin-1α (6-keto-PGF1α) and norepinephrine by ELISA at the end of the study.
The outcome measures were the onset and duration of facial flushing and concomitant hypotension, the incidence of MTS, and the usage of phenylephrine during MTS. The SBP, HR, and remifentanil concentration were monitored at the moment of skin incision, the moment of MT (T0), and 15 minutes (T15), 30 minutes (T30), 45 minutes (T45), and 60 minutes (T60) following MT in patients with MTS.
Sample size and randomization
The primary end-point was defined as the duration of hypotension. In a pilot study, the duration of hypotension of MTS was a mean (standard deviation) of 10 (5) minutes under the same protocol as the present study in pilot investigation. We speculated that dexmedetomidine, 0.5 µg/kg and 1.0 µg/kg, would prolong hypo-tension by 3 and 6 min, respectively.
A sample size of 25 subjects per group was required for a two-tailed α of 0.05 and β of 0.10, assuming a 10% loss to follow-up and an 80% incidence of MTS.10 We thus planned to enroll 75 subjects in this study.
Randomization was achieved with a random number generator in Excel to yield 75 random numbers to equally assign patients into three groups. The grouping scheme was enclosed in opaque envelopes, which were encoded by consecutive numbers. Subjects were enrolled in light of inclusion and exclusion criteria. One anesthetist applied dexmedetomidine or placebo according to the grouping after opening the corresponding numbered envelope and adjusted the propofol concentration under BIS guidance. Another anesthetist, who was blinded to patient grouping and propofol concentration, administered intraoperative anesthesia according to the study protocol.
Statistical analysis
Statistical analysis was performed with SPSS version 22.0 (Armonk, NY), and P<.05 was considered statistically significant unless otherwise mentioned. Data are expressed as the mean (SD) or median (with inter-quartile range, IQR), and number when appropriate. Categorical data were compared using the chi-square test. Numerical variables were compared using parametric the one-way ANOVA with post hoc Student-Newman-Keuls test or the nonparametric Kruskal-Wallis analysis with the post hoc Nemenyi test for between-group comparisons after testing for normal distribution and homogeneity variance.
For the heart rate (HR) data, the Wilcoxon signed rank test was used to analyze two comparisons of time points (T15 vs T0 and T60 vs T0) within each group. For SBP, remifentanil concentration, 6-keto-PGF1α and norepinephrine levels, the Wilcoxon signed rank test was performed to analyze two time points (T15 vs baseline and T60 vs baseline) within each group. Because multiple tests were performed, a Bonferroni correction was employed, and P<.05/6 was considered statistically significant. The changes in HR from T0 to T15 (ΔT0-15) and T0 to T60 (ΔT0-60) were analyzed using Wilcoxon rank sum test with Bonferroni's correction between groups (P<.05/6). Intergroup comparisons of increases in 6-keto-PGF1α and norepinephrine levels during the first 15 minutes after MT were analyzed by the Wilcoxon rank sum test with Bonferroni's correction (P<.05/3). The relationship of the plasma concentrations of 6-keto-PGF1α with the duration of hypotension in all MTS patients was analyzed by Spearman's rank correlation.
RESULTS
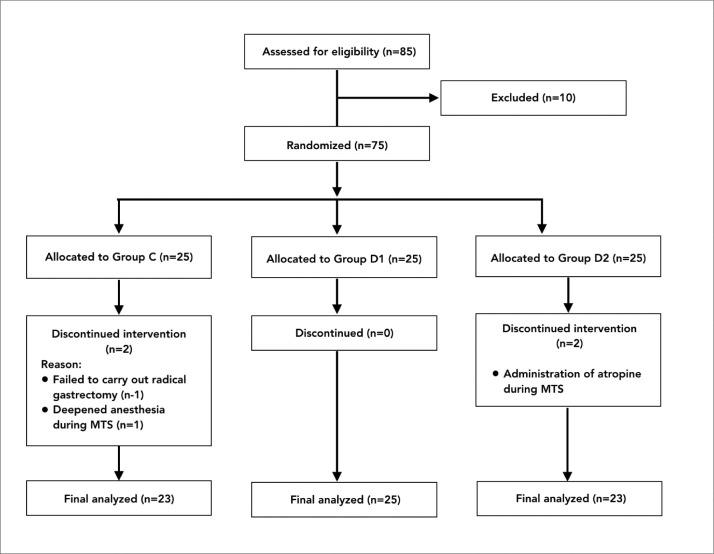
One patient from group C was excluded from the study because a tumor invaded the surrounding tissue, and the surgeon failed to carry out a radical gastrectomy. In one patient from group C (also excluded), facial per-spiration appeared during MTS and gradually vanished after deepening anesthesia by increasing the remifentanil concentration to 5 ng/mL. Two patients with MTS in group D2 were excluded from the study because of the administration of atropine to treat severe bradycardia at 17 and 19 minutes following MT, respectively. Thus, the data of 23, 25 and 23 patients, respectively, in groups C, D1 and D2 were finally included in the statistical analysis (Figure 1). There were no differences in gender, age, body mass index or preoperative complications among the groups (Table 1).
Figure 1.
Study flow diagram.
Table 1.
Characteristics of patients allocated to treatment arm (n=71).
| Group C (n=23) | Group D1 (n=25) | Group D2 (n=23) | |
|---|---|---|---|
| Sex (M/F) | 20/4 | 17/8 | 16/7 |
| Age (y) | 61 (4) | 59 (6) | 60 (5) |
| Body mass index (kg/m2) | 23.1 (1.7) | 22.9 (2.8) | 22.8 (1.8) |
| Hypertension | 9 | 6 | 8 |
| Diabetes mellitus | 5 | 2 | 3 |
Data are presented as the mean (SD), or number of patients.
MTS was observed in 56 patients: 18 of 23 patients (78%) in group C, in 19 of 25 patients (76%) in group D1 and in 19 of 23 patients (83%) in group D2. There were no significant differences in the incidence of MTS among the groups. In cases of MTS, there were no significant differences in the onset times of facial flushing or hypotension or in the duration of facial flushing among the three groups. The duration of hypotension in the MTS patients in group D1 and D2 were significantly longer than that in groups C (D1 vs. C, P<.05; D2 vs. C, P<.01). There were no significant differences in the duration of hypotension between groups D1 and D2 (P>.05). Significantly more phenylephrine was required to treat hypotension in the MTS patients in group D1 and D2 than was required in group C (P<.05). There were no significant differences in phenylephrine requirements between groups D1 and D2 (P>.05) (Table 2).
Table 2.
Comparisons of the intraoperative data in cases of mesenteric traction syndrome (n=56).
| Group C (n=18) | Group D1 (n=19) | Group D2 (n=19) | |
|---|---|---|---|
| The onset of facial flushing (min) | 7.4 (1.8) | 7.3 (1.5) | 7.4 (1.8) |
| The duration of facial flushing (min) | 24 (9) | 25 (9) | 26 (8) |
| The onset of hypotension (min) | 8.7 (1.3) | 8.6 (1.3) | 8.7 (1.9) |
| The duration of hypotension (min) | 9.4 (5.1) | 12.8 (5.6)a | 14.8 (3.9)b |
| Usage of phenylephrine (μg/kg) | 3 (2-8) | 7 (6-13)a | 10 (8-14)a |
Data are presented as the mean (SD) or the median (interquartile range in the case of phenylephrine usage).
P<.05,
P<.01 compared with group C.
There was a significant correlation between the increases in 6-keto-PGF1α from T0 to T15 and the duration of hypotension in all MTS patients (P<.05). At T15 HR increased significantly compared to T0 in patients with MTS within all three groups (P<.0083). There were no significant changes at T60 compared with T0 within all three groups. The increase in HR during the first 15 minutes of MT in group D2 was significantly attenuated compared to that in group C (ΔT0-15 minutes 6 [4-10] vs. 14 [10-19] bpm, P=.001). There were no differences in HR changes between groups D1 and C (ΔT0-15 minutes 11 [1-16] vs. 14 [10-19] bpm, P=.124) or D1 and D2 (ΔT0-15 minutes 11 [1-16] vs. 6 [4-10] bpm, P=.209). There were no significant differences in ΔT0-60 minutes HR among the groups (Figure 2).
Figure 2.
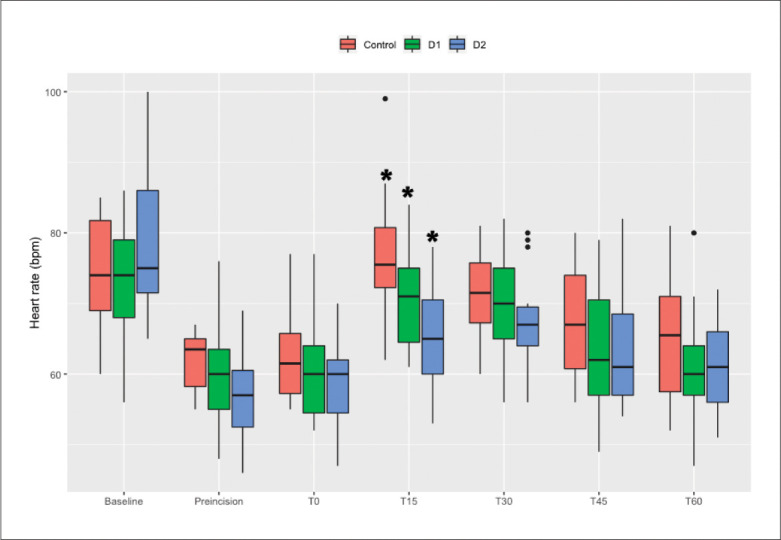
Trend for changes in heart rate. T0 is the time point at the moment of mesenteric traction. T15, T30, T45, and T60 are at 15, 30, 45, and 60 minutes after mesenteric traction. * P<.0083 compared with T0.
SBP decreased significantly at T15 compared to the baseline value within all three groups, notwithstanding administration of a vasopressor (P<.0083). There were no significant differences between the baseline values and T60 within all three groups. The remifentanil concentrations were 3 ng/mL in all patients with MTS at T15 and were significantly more than the initially determined value at T60 within all three groups (P<.0083) (Figures 3 and 4).
Figure 3.
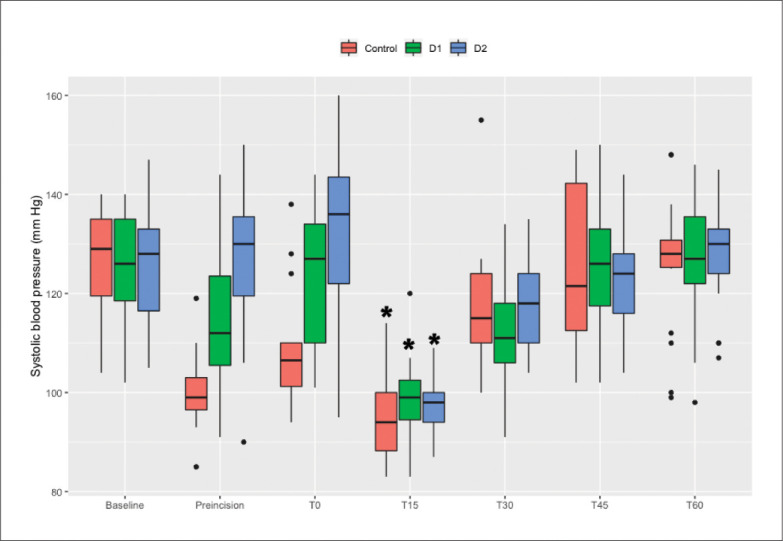
Trend for changes in SBP. T0 is the time point at the moment of mesenteric traction. T15, T30, T45, and T60 are at 15, 30, 45, and 60 minutes after mesenteric traction. * P<.0083 compared with baseline.
Figure 4.
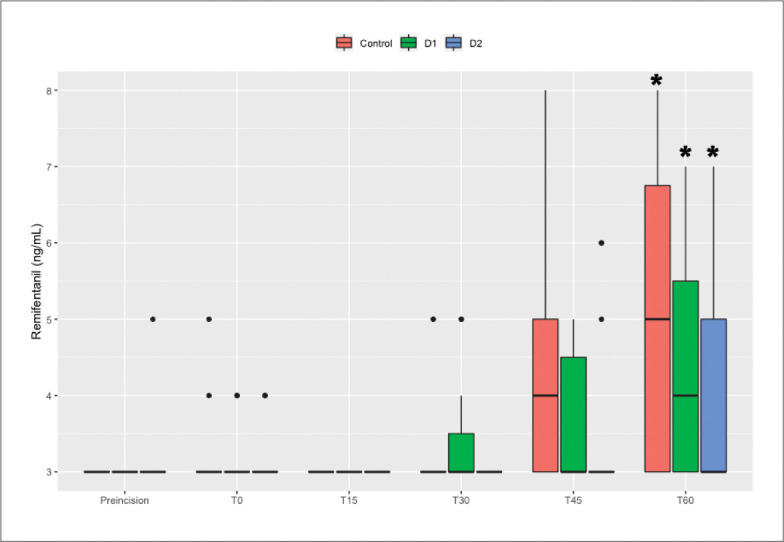
Trend for changes in remifentanil concentration. T0 is the time point at the moment of mesenteric traction. T15, T30, T45, and T60 are at 15, 30, 45, and 60 minutes after mesenteric traction. *P<.0083 compared with baseline.
The norepinephrine and 6-keto-PGF1α levels at T15 and T60 were significantly higher than the baseline values within all three groups (P<.0083). The increases in norepinephrine levels during the first 15 minutes of MT in group C were significantly higher than those in groups D1 and D2 (C vs. D1, P=.012; C vs. D2, P=.005), and there were no differences between groups D1 and D2 (P=.231). There were no differences in the increases in 6-keto-PGF1α levels during the first 15 minutes of MT between groups (Figures 5 and 6).
Figure 5.
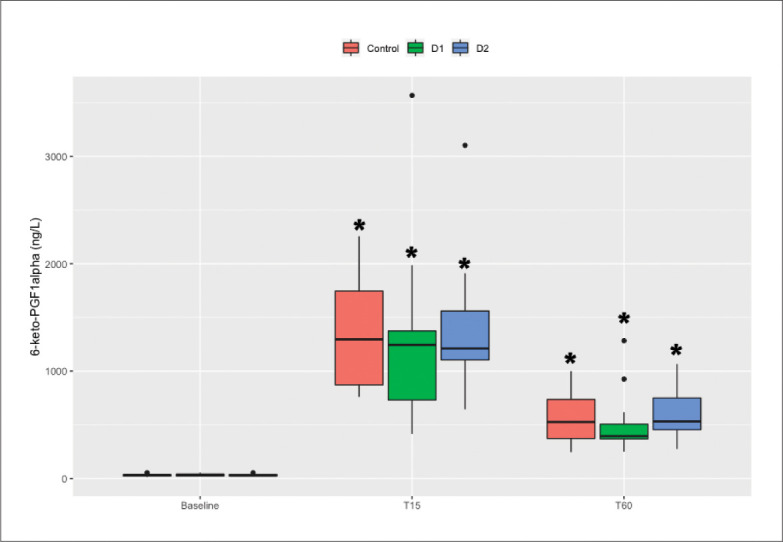
The plasma concentrations of 6-keto-PGF1α and norepinephrine were determined before anesthesia induction and at 15 minutes and 60 minutes following MT. * P<.0083 compared with baseline.
Figure 6.
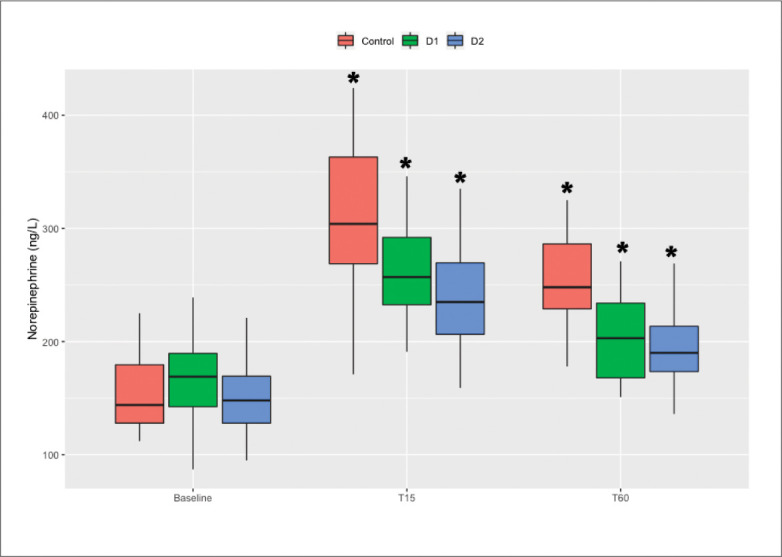
The plasma concentrations of 6-keto-PGF1α and norepinephrine were determined before anesthesia induction and at 15 minutes and 60 minutes following MT. * P<.0083 compared with baseline.
DISCUSSION
We demonstrated that a common adjunctive dose of dexmedetomidine in general anesthesia extends the duration of hypotension and requires an increase in the dosage of phenylephrine in patients with MTS undergoing open total gastrectomy. In previous studies, MTS was diagnosed by a single sign of facial flushing following MT and was classified by scope and degree of flushing.10-12 Dark-skinned and significantly anemic individuals are difficult to evaluate by facial flushing, and the simple observation of facial flushing has no practical significance in the treatment of MTS. Therefore, facial flushing and accompanied hypotension were regarded as diagnostic conditions in the present study. Adjunctive dexmedetomidine in general anesthesia had no effect on the incidence of MTS, and the total incidence of MTS was 76%, which was compatible with that in a previous study.2,10 However, the requirements of phenylephrine were increased, and the duration of hypotension was prolonged by 3 and 5 minutes when administering a loading of dexmedetomidine 0.5 and 1.0 µg/kg with a maintenance rate of 0.5 µg/kg/h in patients with MTS, respectively.
The release of PGF1α following MT results in systemic vasodilation and a decrease in blood pressure. Fujimoto et al showed that the extent of increased PGF1α was associated with a decrease in blood pressure following MT.10 Our study also showed that the increase of PGF1α concentration was correlated with the duration of hypotension in MTS patients. A number of factors, including the depth of anesthesia, surgical stress and blood volume status, can affect intraoperative blood pressure. To date, no studies have focused on the duration of hypotension following MT. To avoid the influence of the depth of anesthesia on blood pressure, BIS was maintained in the range of 40-60, and the lowest concentration of remifentanil was similarly maintained. An equal volume of fluid was infused before induction and during surgery, and the same type of surgery was performed, which attenuated the effects of blood volume and surgical stress on hemodynamics. Under our study protocol, hypotension continued for approximately 10 minutes during MTS.
Systemic vasodilation following MT, which activates counterregulatory systems, such as the sympathetic nervous system, contributes to hemodynamic stability.5 Therefore, the duration of blood pressure decrease was shorter than the change in PGF1α following MT. In the present study, blood pressure gradually stabilized and was not maintained by vasopressor 15-30 minutes following MT. With the disappearance of vasodilation action of PGF1α at 45 minutes after MT, hypertension occurred, and the concentration of remifentanil was increased to maintain blood pressure at the baseline level.
Dexmedetomidine cannot affect the activation of tissue COX-1 or the production of PGI2. The concentration of PGF1α was not changed following administration of dexmedetomidine. However, dexmedetomidine weakened the activation of the sympathetic nervous system following MT-induced hypotension, and catecholamine (e.g., norepinephrine) release was decreased. Therefore, MTS can be promoted by the sympatholytic effect of dexmedetomidine, and the duration of hypotension was prolonged by adjunctive dexmedetomidine before MT, especially in the case of the application of a loading of 1.0 µg/kg with a maintenance rate of 0.5 µg/kg/h. Compensatory increases in HR accompanied by hypotension were a clinical manifestation of the start-up of a redemptive mechanism, which led to an increase in cardiac output and blood pressure. The reduction in HR elevation indicated that dexmedetomidine can attenuate the compensatory response to hypotension and worsen hypotension.13,14
In the course of MTS, the HR decreased below 45 bpm in two patients who were administered a loading of dexmedetomidine 1.0 µg/kg with a maintenance rate of 0.5 µg/kg/h. The HR was raised by atropine administration with simultaneous blood pressure increase and subsequent vasopressor discontinuing, which also indicated that the inhibition effect of dexmedetomidine on HR raise can aggravate hypotension.
Severe MTS increases postoperative complications in major abdominal surgery.15,16 We did not evaluate postoperative complications, which was a limitation of our study. However, hypotension was corrected in a timely manner by monitoring continuous invasive blood pressure, and hypotension had no impact on postoperative recovery. Previous studies have shown that intravenous flurbiprofen axetil, an injectable nonsteroidal anti-inflammatory drug (NSAID), reduced the incidence of MTS and attenuated hypotension following MTS during abdominal surgery.10,17 Therefore, a prophylactic administration of flurbiprofen axetil before surgery could stabilize the hemodynamics instability due to MTS, especially in patients with decreased circulatory compensative capacity. However, NSAIDs have contra-indications and many clinical anesthetists cannot obtain the drug. In conclusion, the inhibitory effects of dexmedetomidine on the compensatory response to MTS aggravate hypotension and increase the required vasopressor dose. Dexmedetomidine should be avoided before MT in abdominal surgery, especially relatively large doses.
Funding Statement
Science and Technology Planning Social Development Project of Zhenjiang City SH2014087. ChiCTR registry: ChiCTR1800014506
REFERENCES
- 1.Krohn PS, Ambrus R, Zaar M, Secher NH, Svendsen LB.. Mesenteric traction syndrome. Ugeskr Laeger. 2014;176:V09130546. [PubMed] [Google Scholar]
- 2.Ring LL, Strandby RB, Henriksen A, Ambrus R, Sørensen H, Gøtze JP, et al. . Laser speckle contrast imaging for quantitative assessment of facial flushing during mesenteric traction syndrome in upper gastrointestinal surgery. J Clin Monit Comput. 2019;33:903-10. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi H, Shida D, Tagawa K, Suzuki T.. Hemodynamics of mesenteric traction syndrome measured by FloTrac sensor. J Clin Anesth. 2016;30:46-50. [DOI] [PubMed] [Google Scholar]
- 4.Bucher M, Kees FK, Messmann B, Lunz D, Rath S, Zelenka M, et al. . Prostaglandin I2 release following mesenteric traction during abdominal surgery is mediated by cyclooxygenase-1. Eur J Pharmacol. 2006;536:296-300. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann A, Seeling W, Wolf CF, Kneitinger E, Schönberger C, Vogt N, et al. . Vasopressor hormone response following mesenteric traction during major abdominal surgery. Acta Anaesthesiol Scand. 1998;42:948-56. [DOI] [PubMed] [Google Scholar]
- 6.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P.. Clinical Pharmacokinetics and Pharmaco-dynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56:893-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinger RY, White WD, Hale B, Habib AS, Bennett-Guerrero E.. Hemodynamic impact of dexmedetomidine administration in 15,656 noncardiac surgical cases. J Clin Anesth. 2012;24:212–20. [DOI] [PubMed] [Google Scholar]
- 8.Malone B, Firstenberg MS.. The importance of identifying patients at risk of dexmedetomidine-associated hypotension. Int J Crit Illn Inj Sci. 2016;6:165-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz KF, Altman DG, Moher D.. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ [Internet]. 2010. March 24 [cited 2020 May 7];340 Available from: https://www.bmj.com/content/340/bmj.c332 [DOI] [PMC free article] [PubMed]
- 10.Fujimoto Y, Nomura Y, Hirakawa K, Hotta A, Nakamoto A, Yoshikawa N, et al. . Flurbiprofen axetil provides a prophylactic benefit against mesenteric traction syndrome associated with remifentanil infusion during laparotomy. J Anesth. 2012;26:490-5. [DOI] [PubMed] [Google Scholar]
- 11.Nomura Y, Funai Y, Fujimoto Y, Hori N, Hirakawa K, Hotta A, et al. . Remifentanil increases the incidence of mesenteric traction syndrome: preliminary randomized controlled trial. J Anesth. 2010;24:669-74. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Shida D, Tagawa K, Iwamoto R, Arita M, Arai H, et al. . Therapeutic effects of flurbiprofen axetil on mesenteric traction syndrome: randomized clinical trial. BMC Surg. 2017;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong M, Man Y, Fu Q.. Incidence of bradycardia in pediatric patients receiving dexmedetomidine anesthesia: a meta-analysis. Int J Clin Pharm. 2017;39:139-47. [DOI] [PubMed] [Google Scholar]
- 14.Piao G, Wu J.. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci. 2014;10:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrus R, Svendsen LB, Secher NH, Goetze JP, Rünitz K, Achiam MP.. Severe Postoperative Complications may be Related to Mesenteric Traction Syndrome during Open Esophagectomy. Scand J Surg. 2017;106:241-48. [DOI] [PubMed] [Google Scholar]
- 16.Olsen AA, Strandby RB, Nerup N, Ambrus R, Gøtze JP, Svendsen LB, et al. . Development of a severe mesenteric traction syndrome during major abdominal surgery is associated with increased postoperative morbidity: Secondary data analysis on prospective cohorts. Langenbecks Arch Surg. 2020;405:81-90. [DOI] [PubMed] [Google Scholar]
- 17.Takada M, Taruishi C, Sudani T, Suzuki A, Iida H.. Intravenous flurbiprofen axetil can stabilize the hemodynamic instability due to mesenteric traction syndrome–evaluation with continuous measurement of the systemic vascular resistance index using a FloTrac® sensor. J Cardiothorac Vasc Anesth. 2013;27:696-702. [DOI] [PubMed] [Google Scholar]