ABSTRACT
BACKGROUND:
Cochlear morphology and cochlear duct length (CDL) play important roles in the selection of appropriate electrodes. Cochlear parameters such as diameter (A value) and width (B value) are used as inputs for calculating the CDL. Current measurements of these parameters are inefficient and time consuming. Recently developed otological planning software (OTOPLAN) allows surgeons to directly measure these parameters and then automatically calculate the CDL.
OBJECTIVES:
The primary objective was to validate this new software for measuring the cochlear parameters and CDL. The secondary aim was to investigate the correlation between each cochlear parameter with the calculated CDL.
DESIGN:
Retrospective.
SETTINGS:
Ear specialist hospital.
PATIENTS AND METHODS:
The measurement of cochlear diameter (A value) was chosen as the validation parameter. To do this, the A value was measured by a neurotologist on the new OTOPLAN planning software and was validated to the one measured on the currently used DICOM viewer. Upon the validation of the OTOPLAN software, the other two cochlear parameters, namely width (B value) and height (H value) were measured, and CDL was automatically calculated. Finally, the correlation of all parameters with the CDL was statistically analyzed.
MAIN OUTCOME MEASURES:
Validation of OTOPLAN and CDL estimation.
SAMPLE SIZE:
88 ears.
RESULTS:
There was no significant difference between the A-value measured on the DICOM viewing software and that on the new planning software by the two independent neurotologists (P=.27). Both A-and B-values showed a high positive correlation to the CDL. However, the B-value showed a stronger correlation to the CDL than the A-value (r=0.63 for A, and r=0.96 for B).
CONCLUSION:
The direct measurement of cochlea parameters and automatic calculation of the CDL could improve the efficiency of clinical workflow and make otology surgeons more independent. Moreover, the cochlear width (B) has a strong correlation to the CDL. Thus, we suggest using the combination of A and B to accurately estimate the CDL rather than using only one.
LIMITATIONS:
Single center and small sample size.
CONFLICT OF INTEREST:
None. No relationship with manufacturers.
INTRODUCTION
Cochlear implantation (CI) is the main treatment modality for sensorineural hearing loss where regular hearing aids do not provide any benefit. In patients who have undergone CI, the intracochlear electrode transmits electrical stimulation to the auditory nerve through spiral ganglion cells in the cochlea. However, a thorough understanding of the variability in anatomical dimensions and the intracochlear compartment is essential to minimize any possible trauma during electrode insertion.1 Since the cochlear structure dimensions can vary by as much as 30% to 40% between the shortest and longest cochleas,2-6 interindividual variability in the normal cochlear morphology and other parameters should be considered preoperatively by the surgical team to select the most suitable type of electrode that can (i) prevent damage to the cochlear structure and degeneration of cochlear cells, which will be reflected in the performance of speech understanding, and (ii) preserve the postoperative residual hearing.7-9 The electrode array of the implant should be sufficiently stimulate a wide frequency range.10 Thus, numerous electrode arrays with different lengths, sizes, designs, and number of channels are available. The first step toward selecting the most suitable electrode for the patient is to measure the cochlear duct length (CDL). Although several studies have attempted to determine the ideal approach in measuring the (CDL), their findings have yielded multiple approaches, including histological and radiological methods.10-14 Wurfel et al4 used 3D planar reconstruction in cone-beam computed tomography (CT) to measure the CDL by determining the length of a curved line drawn from the midpoint of a round window of the cochlea to the terminal point of the apical turn along the outer lateral wall of the cochlea. This method is, however, time consuming and requires a high-resolution CT image.
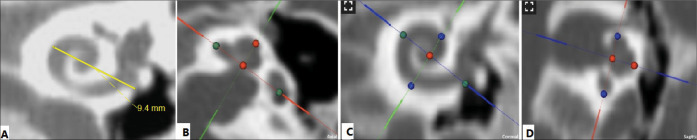
Currently the most clinically viable methods rely on cochlear basal turn parameters of diameter (Figure 1A) and width (Figure 1B), which are visible in routine clinical CT resolutions.6,15 In the current clinical practice workflow, these parameters are measured by the radiological DICOM viewing software from the CT scanner and sent to the surgeon. The surgeon then puts these parameters in one of the available formulas to calculate the CDL and select the suitable electrode. This workflow has the disadvantage of extra work and also requires the surgeon has to rely on the measurements performed by the radiologist.
Figure 1.
(A) DICOM viewing software from the CT scanner, The A-value is the length of the cochlear base, indicated by the yellow line. B, C and D: Cochlear view in the OTOPLAN: The A-value, indicated by green dots. The B-value is the width of the cochlear base, indicated by blue dots. The H-value is the height of the cochlea, indicated by red dots.
Recently developed systems allow the surgeon to obtain these measurements themselves. One such system, the OTOPLAN, is a new otological planning software program developed by CAScination AG (Bern, Switzerland) in collaboration with MED-EL (Innsbruck, Austria) that enables the surgeon to import and analyze patient images (Figure 1).
The primary aim of the current study was to compare the A-value measurements obtained using the OTOPLAN and DICOM viewing software from the CT scanner (PHILIPS ICT 256) by two independent neurotologists to validate the new tool, the OTOPLAN, to determine if it can provide equivalent measurements. The secondary aim was to examine the relationship between all measured cochlear parameters and CDL. There is disagreement in previous studies where Escude2 assumes a linear dependency of A and B and Schurzig15 argues that the ratio of A and B is not consistent but may vary quite substantially from one cochlea to another.. In this work we wanted to evaluate these correlations.
PATIENTS AND METHODS
This retrospective study was approved by the ethics committee of King Saud University, College of Medicine, Saudi Arabia, (Ref. No. 19/0338/IRB). Of 127 ears assessed, only 88 met the inclusion criteria; the selected ears belonged to children aged <7 years with prelingual deafness and normal cochlear morphology (2.5 turns) who underwent CI either unilaterally or bilaterally between 2013 and 2014 at King Abdullah Ear Specialist Center. Confirmation of normal cochlear morphology was performed using the 3D segmentation of the inner ear (3D Slicer) software (https://www.slicer.org/) (Figure 2). Additionally, we reviewed the findings from the high-resolution CT that was performed routinely before the surgery and used OTOPLAN to measure the cochlear parameters.
Figure 2.
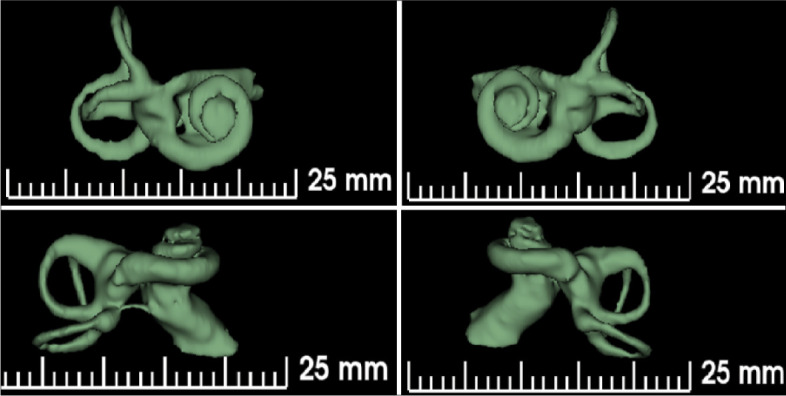
3D SLICER software segmentation for one of our sample shows normal inner ear morphology including full 2.5 turns cochlea.
Normal cochlear morphology confirmation
The images for each ear were uploaded into the 3D Slicer software to confirm whether the inner ear morphology was normal or malformed through a 3D reconstruction of the inner ear and internal auditory canal (Figure 2). Ears with abnormal morphology were excluded, 39 ears of 127 ears, including those showing abnormalities in the internal auditory canal, cochlea, vestibule, and semicircular canals.
Cochlear parameter measurements
For each sample, the neurotologist performed HRCT to measure the cochlear diameter (A-value), which was defined as the diameter of the cochlear base. The measurement was performed in the DICOM viewing software from the CT scanner (PHILIPS ICT 256) and determined by measuring the length of a straight line starting from the midpoint of the round window passing through the mid-modiolus axis and ending at the contralateral wall. Then, another independent neurotolo-gist with the same level of experience and performance in cochlear implants performed the same measurement using the OTOPLAN software. Both software consist of multiplanar reconstruction (MPR) viewers. Moreover, two other cochlear parameters, namely, (i) width of the cochlear base (B-value) measured as a straight line perpendicular to the A-value line at the mid-modiolus and (ii) height of the cochlea (H-value) as a straight line starting from the lowest point at the base to the end at the apex, were measured using the OTOPLAN software (Figure 1). Then, the A-values measured using the PHILIPS ICT 256 and OTOPLAN were compared. The ratios of the diameter to width and height of the cochlea were calculated in each case to determine if there were any correlations among the A-, B-, and H-values.
Cochlear length measurements
The A- and B-values were used to estimate the length of the basal turn of the cochlea at the level of the lateral wall BTL (LW) as shown in equation 1 using the ECA formulas:
In this study, it is assumed that the organ of Corti is located 0.5 mm from the lateral wall (Figure 3), as reported by Alexiades.6 Therefore, to calculate the BTLOC, 1 mm was subtracted from the A- and B-values to accommodate this distance (equation 2). Then, the full CDL (OC) was calculated by applying the BTL (OC) in the Alexiades formula, as shown in eq. 3. The 1.58 mm was added to the calculated CDL to accommodate for the organ of Corti in the hook region:
Figure 3.
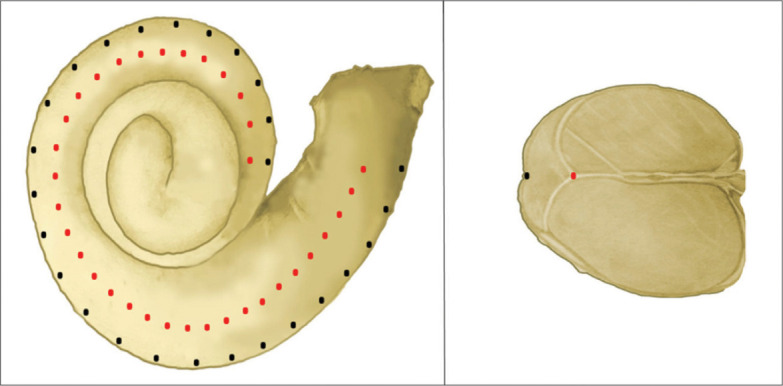
Cross-sectional of the cochlea shows the level of the organ of Corti (OC) as green dots and at level of lateral wall (LW) as blue dots. The organ of Corti (OC) is located 0.5 mm away from the lateral wall (LW).
Statistical analysis
The statistical analysis was performed using IBM SPSS (Armonk, NY: IBM Corp) version 24. The Kolmogorov test was performed to determine the normality of quantitative variables. Parametric variables were compared between paired data using the paired t test, while non-parametric variables were compared between paired data using Wilcoxon signed-rank test. Parametric variables were compared between the two groups using an independent sample t test, while the Mann-Whitney test was used to compare two groups of nonparametric variables. The correlation between parametric variables was assessed using Pearson correlation analysis, while the correlation between nonparametric variables was assessed using Spearman rank correlation. A P value <.05 indicated statistical significance.
RESULTS
Of 127 ears, 88 met the inclusion criteria. The age of the patients ranged from 1 to 7 years and the mean [SD] was 2.9 [1.3] years) (Table 1).
Table 1.
Distribution of sex and ear side in the study sample.
| N | % | |
|---|---|---|
| Sex | ||
| Females | 46 | 52.3 |
| Males | 42 | 47.7 |
| Ears | ||
| Left | 37 | 42 |
| Right | 51 | 58 |
A-value
The A-values obtained using the PHILIPS ICT 256 ranged from 7.50 to 9.40 mm (mean [SD] 8.43 [0.39] mm). The corresponding values obtained using the OTOPLAN ranged from 7.60 to 9.30 mm (mean [SD] 8.44 [0.39] mm). The statistical analysis showed no significant difference between the A-values obtained by the two independent neurotologists using both tools, namely, PHILIPS ICT 256 and OTOPLAN (P=.27), and the two sets of readings showed a significant positive linear correlation (r=0.989, P<.001). The mean A-value obtained using the OTOPLAN was 8.54 mm in males and 8.34 mm in females, and the values for males and females were significantly different (P=.016). However, the difference between the A-values of the right and left sides in all included patients was not significantly different (8.45 mm vs. 8.42 mm, P=.704).
B-value
The B-values obtained using the OTOPLAN ranged from 5.40 to 7.80 mm (mean [SD] 6.53 [0.40] mm), and the values in males and females were significantly different (6.62 mm vs. 6.45 mm, P=.044). No significant difference was noted between the values for the right and left ears (6.52 mm vs. 6.55 mm, P=.780).
H-value
The H-values obtained using the OTOPLAN ranged from 2.00 to 3.40 mm (mean [SD] 2.71 [0.31] mm), and no significant difference (P=.350) was observed between the mean values in males 2.74 mm and females 2.68 mm. No significant difference was noted between the values for the right and left ears (2.75 mm vs. 2.64 mm, P=.095). Additionally, the H-values showed a weak positive association to the previous values.
Correlation between A- and B-values
The A- and B-values obtained using the OTOPLAN showed a moderate positive correlation (r=0.398 and P<.01).
B/A ratio
The B/A ratio showed a weak negative correlation to the A-value obtained using the OTOPLAN (r=0.351 and P<.01). However, it showed a significant strong positive correlation with the B-value (r=718 and P<.01) and a very weak negative correlation with the H-value (r=0.087) obtained using the OTOPLAN. Moreover, the B/A ratio showed moderate positive correlations with CDL (oc) (r=0.498, P<.01). The ratio showed no signifi-cant difference between the right and left ears and between males and females.
H/A ratio
The H/A ratio showed an extremely weak negative correlation to all parameters, including the CDL (oc). This ratio also showed no significant difference between the right and left ears and between males and females.
CDL(oc)
Table 2 shows the mean values and range in each sex, showing a clear significant difference. In contrast, no significant difference was observed between the mean values for the right and left ears.
Table 2.
Cochlear duct length (CDL) at the level of the organ of corti(OC) and the first turn of the cochlea at the organ of corti(OC) and lateral wall(LW).
| Range (mm) | Mean (mm) | SD | N | Mean | SD | SE | P value | Significance | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CDL(OC) | 28.1–37.8 | 32.91 | 1.78 | Male | 42 | 33.39 | 1.52 | 0.23 | .014 | Significant |
| Female | 46 | 32.47 | 1.90 | 0.28 | ||||||
| 1.0 Turn(OC) | 15.39–21.08 | 18.22 | 1.05 | Male | 42 | 18.50 | 0.89 | 0.14 | .015 | Significant |
| Female | 46 | 17.96 | 1.12 | 0.16 | ||||||
| 1.0 Turn(LW) | 18.39–24.10 | 21.23 | 1.05 | Male | 42 | 21.51 | 0.89 | 0.14 | .015 | Significant |
| Female | 46 | 20.97 | 1.12 | 0.17 |
Cochlear duct length (CDL), organ of Corti (OC), lateral wall (LW).
First turn(oc) and first turn(LW)
These values were significantly different between males and females (Table 2). However, they did not significantly differ between the right and left ears.
Correlation of CDL and first turn to other parameters
Table 3 shows the positive correlation of all CDL(OC), first turn(OC), and first turn(LW) values to other parameters, except H/A ratio. There was an extremely strong significant positive correlation between CDL and B-value and a moderate positive correlation between CDL and A-value. Moreover, the CDL showed an extremely weak and negligible association with the H-value.
Table 3.
Correlation of cochlear duct length CDL(OC), 1.0 turn(OC) and 1.0 turn(LW) to other parameters, n=88.
| A-value OTOPLAN | B-value OTOPLAN | H-value OTOPLAN | B/A | H/A | CDL(OC) | 1.0 Turn(OC) | 1.0 Turn(LW) | ||
|---|---|---|---|---|---|---|---|---|---|
| CDL(OC) | Pearson correlation | .636** | .961** | 0.189 | .498** | -0.068 | 1 | 1.000** | 1.000** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.078 | 0.000 | 0.531 | 0.000 | 0.000 | ||
| 1.0 Turn(OC) | Pearson correlation | .636** | .961** | 0.189 | .498** | -0.068 | 1.000** | 1 | 1.000** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.078 | 0.000 | 0.531 | 0.000 | 0.000 | ||
| 1.0 Turn(LW) | Pearson correlation | .634** | .962** | 0.188 | .501** | -0.067 | 1.000** | 1.000** | 1 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.079 | 0.000 | 0.534 | 0.000 | 0.000 |
Cochlear duct length (CDL), organ of Corti (OC), lateral wall (LW).
DISCUSSION
Variations in cochlear structure dimensions, which can be as high as 40%, and interindividual anatomical variations in the normal cochlea affect the size and length of the cochlea itself. Thus, selection of an optimal electrode requires the use of a clear and valid method or tool that can help the surgeon assess the inner ear morphology and measure cochlear dimensions. Subtle cochlear variations are less likely to be detected by routine CT with single-plane assessments in the coronal and axial views.5,14,16
In the current clinical setting, if the surgeon is interested in selecting the right electrode, he/she would ask the radiologist for cochlear parameters measurements. The surgeon would then take these parameters and manually (using calculator or Excel) place them in one of the known CDL formulas to measure the cochlear length. After that, the surgeon uses the Greenwood function to calculate the frequency mapping of that specific cochlea.13 Only then he/she can see what length of electrode would suit the patient best. With the new planning software's electrode visualization feature, the calculation of CDL and frequency mapping is done automatically upon the measurement of A and B. In the first part of the study, measurements of the new planning software were validated. To do this equivalency in the A-value measured by the neurotologist using the DICOM viewing software from the CT scanner (PHILIPS ICT 256) and measured by another independent neurotologist using the OTOPLAN.
The A-values measured through the PHILIPS ICT 256 and OTOPLAN showed no statistically significant difference (P=.27). Thus, surgeons can utilize this new tool to independently measure the cochlear parameters.
The second aim of the study was to investigate the correlation of the cochlear parameters and CDL. Both A- and B-values showed a positive correlation, which was consistent with the findings of previous studies. An extremely weak positive correlation was shown between the H-value and both A- and B-values.
In the present study, the mean A-value obtained using the OTOPLAN was 8.44 mm, while the mean B-value was 6.53 mm (Table 4), indicating that our results were comparable to those reported in previous studies that used a similar radiological method.2,10,16-21 The mean A-value in our study was short but within the previously reported range, and the mean B-value was consistent with the average B-value in previous studies. However, participants' ethnic backgrounds may influence these measurements. Our study participants were from the Saudi population, which generally shows a shorter CDL than other populations. Thus, the lower values obtained in our study are consistent with the findings reported by Alanazi et al.22 Wurfel et al4 measured the CDL ranged from 30.8 to 43.2 mm. Similarly, Meng et al21 noted significant variations in the anatomy of 310 normal cochlea and suggested a CDL range of 30.7–42.2 mm for personalized cochlear implants. Sato et al23 reported a CDL range of 32.7–43.2 mm.
Table 4.
The different reported means of cochlear parameters values in the various studies that are done based on the radiological method in measurement including our current study.
| Author | Year | N | A (mm) | B (mm) | H (mm) |
|---|---|---|---|---|---|
| Escudé et al2 | 2006 | 84 | 9.23 | 6.9 | N/A |
| Martinez-Monedero et al16 | 2011 | 124 | 8.39 | 6.98 | N/A |
| Van der Marel et al17 | 2014 | 671 | 8.85 | 6.58 | N/A |
| Avci et al10 | 2014 | 16 | 9.2 | 7.0 | 4.4 |
| Pelliccia et al18 | 2014 | 482 | 9 | 6.8 | N/A |
| Meng J et al21 | 2016 | 178 men | 9.12 | 6.41 | N/A |
| 132 women | 8.93 | 6.22 | |||
| Liu et al19 | 2017 | 204 | 8.84 ± 0.29 | 6.30 ± 0.38 | 3.59 ± 0.12 |
| Rivas et al20 | 2017 | 309 | 9.22 ± 0.44 | N/A | |
| This study | 2019 | 88 | DICOM viewer 8.43 | N/A | N/A |
| OTOPLAN 8.44 | 6.53 | 2.71 |
The mean H-value in this study was 2.71 mm (range, 2.00–3.40 mm), which was significantly shorter than those reported in few available studies that measured the height of the cochlea, such as those by Avci et a l (H-value, 4.4 mm) and Liu et al (H-value, 3.59 [0.12] mm).10,21
In this study, we did not include age-related variables since this study aimed to evaluate the differences related to the tools we utilized and to identify the cochlear parameters that could allow more accurate estimations of CDL. With respect to sex, we found that males had slightly higher cochlear parameters than females. Alanazi et al conducted a study in a population with almost similar characteristics as our study population, and their results were consistent with our findings.22 While this finding is potentially debatable, other studies have also reported longer diameters in males than in females.4,17,23,24 However, no statistically significant difference was observed between the right and left cochlea, which indicated that there was no particular difference in morphology. Moreover, post-experimental studies support our findings.4,17,25,26
There were several limitations in our study. These included the small sample size and the fact that the subject's physical measurements, such as height and head circumference, were not included. In conclusion, in addition to HRCT, 3D-segmentation reconstruction can help surgeons analyze cochlear morphology. No significant differences were observed between the assessments performed by the two independent neurotologists. Therefore, the new OTOPLAN software can be validated as a tool for surgeons to independently perform preoperative planning, including electrode visualization. This will help toward optimizing the pre-operative clinical workflow. The study also showed that the cochlear width (B) had a higher correlation to the CDL and the combination of A- and B-values to estimate the CDL was more accurate than A- or B-value alone. More research is needed to further improve CDL estimation.
Funding Statement
None.
REFERENCES
- 1.Braun K, Böhnke F, Stark T.. Three-dimensional representation of the human cochlea using micro-computed tomography data: presenting an anatomical model for further numerical calculations. Acta Otolaryngol. 2012;132:603–13. [DOI] [PubMed] [Google Scholar]
- 2.Escudé B, James C, Deguine O, Cochard N, Eter E, Fraysse B.. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurotol. 2006;11 Suppl 1:27–33. [DOI] [PubMed] [Google Scholar]
- 3.Hardy M. The length of the organ of Corti in man. Am J Anat. 1938;62:291–311. [Google Scholar]
- 4.Würfel W, Lanfermann H, Lenarz T, Majdani O.. Cochlear length determination using cone beam computed tomography in a clinical setting. Hear Res. 2014;316:65–72. [DOI] [PubMed] [Google Scholar]
- 5.Verbist BM, Ferrarini L, Briaire JJ, Zarowski A, Admiraal-Behloul F, Olofsen H, et al. Anatomic considerations of cochlear morphology and its implications for insertion trauma in cochlear implant surgery. Otol Neurotol. 2009;30:471–77. [DOI] [PubMed] [Google Scholar]
- 6.Alexiades G, Dhanasingh A, Jolly C.. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36:904–7 [DOI] [PubMed] [Google Scholar]
- 7.O'Connell BP, Cakir A, Hunter JB, Francis DO, Noble JH, Labadie RF, et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37:1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landsberger DM, Mertens G, Punte AK, Van De Heyning P.. Perceptual changes in place of stimulation with long cochlear implant electrode arrays. J Acoust Soc Am. 2014;135:EL75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gstoettner W, Plenk H, Franz P, Hamzavi J, Baumgartner W, Czerny C, et al. Cochlear implant deep electrode insertion: extent of insertional trauma. Acta Otolaryngol. 1997;117:274–7. [DOI] [PubMed] [Google Scholar]
- 10.Avci E, Nauwelaers T, Lenarz T, Hamacher V, Kral A.. Variations in microanatomy of the human cochlea. J Comp Neurol. 2014;522:3245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawano A, Seldon HL, Clark GM.. Computer-aided three-dimensional reconstruction in human cochlear maps: measurement of the lengths of organ of Corti, outer wall, inner wall, and Rosenthal's canal. Ann Otol Rhinol Laryngol. 1996;105:701–9. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos P, Muren C.. Anatomic variations of the cochlea and relations to other temporal bone structures. Acta Radiol. 1990;31:439–44. [PubMed] [Google Scholar]
- 13.Koch RW, Ladak HM, Elfarnawany M, Agrawal SK.. Measuring cochlear duct length – a historical analysis of methods and results. J Otolaryngol Head Neck Surg. 2017;46:19. doi: 10.1186/s40463-017-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erixon E, Högstorp H, Wadin K, Rask-Andersen H.. Variational anatomy of the human cochlea. Otol Neurotol. 2009;30:14–22. [DOI] [PubMed] [Google Scholar]
- 15.Schurzig D, Timm ME, Batsoulis C, Salcher R, Sieber D, Jolly C, et al. A novel method for clinical cochlear duct length estimation toward patient-specific cochlear implant selection. OTO Open. 2018;2:2473974X18800238. doi: 10.1177/2473974X18800238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Monedero R, Niparko JK, Aygun N.. Cochlear coiling pattern and orientation differences in cochlear implant candidates. Otol Neurotol. 2011;32:1086–93. [DOI] [PubMed] [Google Scholar]
- 17.Van der Marel KS, Briaire JJ, Wolterbeek R, Snel-Bongers J, Verbist BM, Frijns JH.. Diversity in cochlear morphology and its influence on cochlear implant electrode position. Ear Hear. 2014;35:e9–20. doi: 10.1097/01.aud.0000436256.06395.63 [DOI] [PubMed] [Google Scholar]
- 18.Pelliccia P, Venail F, Bonafé A, Makeieff M, Iannetti G, Bartolomeo M, et al. Cochlea size variability and implications in clinical practice. Acta Otorhinolaryngol Ital. 2014;34:42. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YK, Qi CL, Tang J, Jiang ML, Du L, Li ZH, et al. The diagnostic value of measurement of cochlear length and height in temporal bone CT multiplanar reconstruction of inner ear malformation. Acta Otolaryngol. 2017;137:119–26. [DOI] [PubMed] [Google Scholar]
- 20.Rivas A, Cakir A, Hunter JB, Labadie RF, Zuniga MG, Wanna GB, et al. Automatic cochlear duct length estimation for selection of cochlear implant electrode arrays. Otol Neurotol. 2017;38:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng J, Li S, Zhang F, Li QY, Qin Z.. Cochlear size and shape variability and implications in cochlear implantation surgery. Otol Neurotol. 2016;37:1307–13. [DOI] [PubMed] [Google Scholar]
- 22.Alanazi A, Alzhrani F.. Comparison of cochlear duct length between the Saudi and non-Saudi populations. Ann Saudi Med. 2018;38:125–9. doi: 10.5144/0256-4947.2018.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato H, Sando I, Takahashi H.. Sexual di-morphism and development of the human cochlea. Computer 3-D measurement. Acta Otolaryngol. 1991;111:1037–40. [DOI] [PubMed] [Google Scholar]
- 24.Miller JD. Sex differences in the length of the organ of Corti in humans. J Acoust Soc Am. 2007;121:EL151–5. [DOI] [PubMed] [Google Scholar]
- 25.Hochmair I, Arnold W, Nopp P, Jolly C, Müller J, Roland P.. Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryngol Stockh. 2003;123(5):612–7. [PubMed] [Google Scholar]
- 26.Lenarz T, Stöver T, Buechner A, Paasche G, Briggs R, Risi F, et al. Temporal bone results and hearing preservation with a new straight electrode. Audiol Neurotol. 2006;11:34–41. [DOI] [PubMed] [Google Scholar]