Abstract
Tongue squamous cell carcinoma (TSCC) ranks as one of the most common cancers worldwide and has a poor prognosis. Myocyte-specific enhancer factor 2 (MEF2D) has recently been considered as a novel factor involved in cancer development. In the present study, the function and underlying mechanism of MEF2D in TSCC were investigated. The levels of MEF2D mRNA and protein were determined in human TSCC samples by RT-qPCR and western blot, respectively. The interaction between MEF2D expression and clinicopathologic features was evaluated by IHC and analysis of clinical information. MEF2D-mediated effects on proliferation, migration, and invasion were explored in TSCC cells after transfection with MEF2D-siRNA. The results showed higher expression of MEF2D at both the mRNA and protein levels in TSCC carcinoma tissues than in paracarcinoma tissues. Furthermore, high expression of MEF2D was positively correlated with tumor differentiation and lymphatic metastasis. MEF2D knocked down TSCCA cells also had reduced proliferative, migratory, and invasive abilities compared to those of control cells. Together, these data confirmed that knockdown of MEF2D might suppress the growth and metastasis of TSCC, which further indicated that MEF2D might serve as a therapeutic target for TSCC treatment.
Keywords: Tongue squamous cell carcinoma (TSCC), myocyte-specific enhancer factor 2 (MEF2D), tumorigenesis, proliferation
Introduction
Tongue squamous cell carcinoma (TSCC) is one of the most common cancers of the oral cavity and demonstrates a much higher rate of proliferation and nodal metastasis than other oral cancers [1,2]. The etiologic factors of TSCC have been found to be related to alcohol consumption, tobacco smoking, and betel quid chewing [3,4]. Epidemiological surveys have reported that an estimated 10,990 new cases of TSCC develop each year around the world, accounting for approximately 30% of all pharynx and oral cavity cancers [5]. Despite a variety of advances in treatments, including surgery, chemotherapy, and radiotherapy, the 5-year survival rate of TSCC patients has not significantly improved and has remained at less than 50% over the past several decades [6,7]. Local or regional relapse and cervical lymph node metastasis are the most prevalent causes of death in these patients [8]. Hence, many studies have been performed to identify suitable biomarkers for monitoring cancer recurrence or screening high-risk TSCC populations, to investigate the effects of various biologic factors on the aggressive potential of TSCC, and to further unravel the underlying molecular carcinogenesis of TSCC [9,10]. However, these processes are still not fully defined. Accordingly, understanding the above information associated with TSCC progression is pivotal for the development of effective therapeutic strategies for TSCC patients.
Myocyte-specific enhancer factor 2 (MEF2D), a myogenic transcription factor, was initially confirmed to play a critical role in skeletal muscle development and differentiation by regulating sarcomeric-related genes, such as M-line and Z-line proteins [11,12]. Moreover, accumulating previous studies have shown that mice lacking MEF2D are deficient in several skeletal muscle disorders, e.g., muscular dystrophy and microgravity-induced atrophy, and ultimately die around embryonic day 10 [13,14]. Additionally, extensive studies have also shown that overexpression of MEF2D can initiate cardiomyogenesis in embryonic carcinoma cells [15]. Nevertheless, in addition to this function of MEF2D, MEF2D also possesses much broader biological roles in processes such as bone development and neuronal development and melanocyte and craniofacial development [16]. Furthermore, in recent years, it has been verified that MEF2D might participate in cancer development and tumorigenicity [17,18]. For example, MEF2D promotes gefitinib resistance in hepatic cancer cells by regulating mitogen-inducible gene 6 (MIG6) transcription [19]. In this study, we aimed to explore the role of MEF2D in tumorigenesis and progression of TSCC.
Materials and methods
Patients and tissue samples
Samples of TSCC patients and their corresponding adjacent noncancerous tissues were collected during a routine checkup by medical professionals. These patients underwent primary surgical treatment at the Department of Otorhinolaryngology-Head and Neck Surgery, Qingdao Municipal Hospital between January 2012 and October 2018, were not subjected to radiotherapy or chemotherapy preoperatively, and had no history of other systemic diseases (e.g., diabetes, hypertension, etc.). The clinical characteristics of all these patients are summarized in Table 1. Formalin-fixed, paraffin-embedded sections of surgical specimens were subjected to immunohistochemical (IHC) analysis, and the remaining portions were flash-frozen in liquid nitrogen and stored at -80°C in our laboratory for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting (WB).
Table 1.
Association between MEF2D expression and clinicopathologic features in TSCC patients
| Clinicopathologic feature | No. of cases | MEF2D expression (N) | P value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| - | + | ++ | +++ | |||
| Age, years | 0.4344 | |||||
| ≥50 | 18 | 6 | 5 | 4 | 3 | |
| <50 | 22 | 6 | 8 | 4 | 4 | |
| Gender | 0.1372 | |||||
| Male | 19 | 6 | 6 | 3 | 4 | |
| Female | 21 | 6 | 7 | 5 | 3 | |
| Differentiation | 0.002 | |||||
| Well | 20 | 8 | 9 | 2 | 1 | |
| Moderately | 20 | 4 | 4 | 6 | 6 | |
| Lymphatic metastasis | 0.003 | |||||
| Yes | 19 | 3 | 5 | 6 | 5 | |
| No | 21 | 9 | 8 | 2 | 2 | |
Written informed consent was obtained from all patients before the study, and protocols were approved by the Institutional Ethics Review Board of the Ethics Committee of Qingdao Municipal Hospital. All procedures for clinical tissues were performed in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Helsinki Declaration and its later amendments.
RT-qPCR for gene examination
Total RNA from tissue samples was extracted using TRIpure Reagent (BioTeke, China) in accordance with the manufacturer’s protocols. Then, 1 µg of total RNA from each sample was reverse-transcribed into complementary DNA (cDNA) using random hexamer primers and SuperScript II reverse transcriptase (Invitrogen, USA) following the manufacturer’s guidelines. Next, quantitative PCR was performed using the 7500 Fast Real-Time PCR system in 96-well plates, which included 100 nM forward and reverse primers, 4 mM MgCl2, LightCycler SYBR-Green I and 2 µl cDNA template. The PCR amplification conditions were as follows: 20 s at 95°C followed by 40 cycles of 3 s of denaturation at 95°C and 30 s of annealing at 60°C. The relative expression level of MEF2D was normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous reference gene. The primers were designed and obtained from Shanghai Genechem Co., Ltd. (Shanghai, China), and the relative quantification values were calculated via the 2-∆∆CT method. The sequences of the primers used in this study are as follows: MEF2D forward, TCGAGATACCCACAACACAG and MEF2D reverse, AATTCGTTCCGGTGATCCTCT; GAPDH forward, ATCATCCCTGCCTCTACTGG and GAPDH reverse, CCCTCCGACGCCTGCTTCAC.
Western blotting (WB) for protein detection
Tissue specimens were lysed in ice-cold immunoprecipitation assay (IPA) buffer (Beyotime, China) containing phenylmethanesulfonyl fluoride (PMSF; Roche, Germany) and phosphatase inhibitors (Roche, Germany). Subsequently, the cell lysate was centrifuged at 12000 rpm for 10 min at 4°C to remove cell debris, and the protein concentration was further measured using the BCA Assay kit (Biosharp, China) with the manual. Proteins (20 µg per lane) that were denatured by heating at 95°C for 8 min were electrophoresed on a 10%~12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA) by electroblotting. Subsequent to blocking with 5% skim milk for 1 hour at room temperature, membranes were incubated with primary mouse monoclonal antibodies against human MEF2D (1:2000 dilution; Abcam, USA) and GAPDH (as an internal control, 1:500 dilution; bioPM, China) on a shaker at 4°C overnight. After 3 washes with TBST, the membranes were further incubated with horseradish peroxidase (HRP)-conjugated secondary antibody at 37°C for 1 hour. Eventually, target proteins were visualized using ECL reagent, and the relative expression of proteins was quantified by densitometry using ImageJ software (National Institutes of Health, USA).
IHC analysis
Four-micrometer (4-μm) thick sections were serially cut from each specimen block for IHC analysis. After being deparaffinized in xylene and dehydrated with an alcohol gradient, the tissue sections were processed with antigen retrieval by conventional microwave heating (750 W) in sodium citrate buffer (10 mmol/L, pH 6.0) for 10 min, followed by immersion in 0.3% hydrogen peroxide (H2O2) in methanol for 20 min to quench endogenous peroxidase activity. Then, sections were blocked using 5% goat serum for 30 min to eliminate nonspecific immunoglobulin-binding sites and were further incubated with the primary antibody overnight at 4°C: rabbit anti-MEF2D (1:200 dilution; Abcam, USA). After washing with PBS, the sections were incubated with secondary goat anti-rabbit antibody for 15 min at 37°C. After a further PBS wash, diaminobenzidine-HCl (DAB; China) was used as a substrate for visualization, and hematoxylin was further applied to counterstain the nuclei. The sections were mounted with a cover glass and observed under a microscope.
Cell culture and construction of MEF2D-siRNA interference in TSCC cells
The human TSCC cell line TSCCA, purchased from Zhongqiaoxinzhou (Shanghai, People’s Republic of China), was maintained in a mixture of Dulbecco’s modified Eagle’s medium and Ham’s F-12 (DMEM/F12) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 U/ml streptomycin in a humidified incubator (37°C, 5% CO2).
MEF2D-specific siRNA and negative control (NC) siRNA were synthesized and purchased from GenePharma (Suzhou, China). Transfection of siRNA plasmids was mediated by Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) into TSCC cells according to the manufacturer’s instructions to construct MEF2D-silenced TSCC cells and NC-TSCC cells. After 48 h of transfection, the knockdown efficiencies of MEF2D siRNA were determined by RT-qPCR.
In vitro cell proliferation assay
Cell proliferation rates were determined by the Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Japan) reagent according to the manufacturer’s instructions. Briefly, TSCCA or TSCCA-NC or TSCCA-MEF2D siRNA cells (6×103 cells per well) were plated in 96-well plates and incubated in culture medium at 37°C for 0, 1, 2, 3 or 4 days. At the indicated time, culture medium was removed, and fresh culture medium containing 10 μL CCK-8 solution was added to each well. After additional incubation for 2 h at 37°C with 5% CO2, viable cells were quantified by measuring the optical density (OD) values of the absorbance at 450 nm using a microplate reader.
Wound-healing assay
TSCCA, TSCCA-NC or TSCCA-MEF2D siRNA cells were seeded in each well of a 6-well plate at a density of 3×105 cells per well. When the cells reached 90~100% confluence, a wound scratch was made by a sterile 20 μL pipette tip in the central area of the confluent culture, followed by careful washing to remove detached cells. Next, the cells were sequentially cultured in a 37°C incubator with 5% CO2. Images of the wounded areas were captured using a phase-contrast microscope at 0, 6, 24 and 48 h.
Cell invasion assay
Transwell inserts with 8 µm pores (Corning, USA) coated with 40 µL of prediluted Matrigel (BD Biosciences, USA) were used to evaluate the cell invasive ability. TSCCA or TSCCA-NC or TSCCA-MEF2D siRNA cells suspended in serum-free DMEM/F12 medium were seeded onto the Transwell inserts at 6×104 cells per well, while DMEM/F12 medium containing 10% FBS was added to the bottom of the wells as a chemoattractant. After 24 hours of incubation at 37°C, the filters were removed, and cotton swabs were used to gently remove the noninvaded cells in the filters. Then, cells on the lower surface of the filters were fixed with 4% paraformaldehyde for 15 min and stained with crystal violet for 5 min. The invasive capacities were counted in five random fields for each insert under a phase-contrast microscope at 200× magnification.
Statistical analysis
All the quantitative parameters repeatedly obtained in the above assays are given as the mean ± standard deviation (SD) of three or more independent experiments. Differences in data between each condition and control were analyzed using a two-tailed Student’s t-test, while comparisons between more than two groups were evaluated by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls (S-N-K) post hoc test. P values less than 0.05 (two-sided) were considered significant. All statistical analyses were carried out with SPSS Version 20.0 or GraphPad Prism 6.
Results
MEF2D was upregulated in TSCC tissues
We first assayed the mRNA and protein expression patterns of MEF2D in 10 pairs of human TSCC tissues and relevant adjacent normal tongue tissues using RT-qPCR and WB methods. As shown in Figure 1, the expression of MEF2D was significantly upregulated in TSCC tissues compared with adjacent normal tongue tissues at both the mRNA and protein levels. Hence, these data suggested that aberrant MEF2D expression might be involved in the progression of TSCC.
Figure 1.

MEF2D is overexpressed in TSCC patient tissues. A. Expression of MEF2D mRNA in ten, randomly selected, paired TSCC samples assessed by RT-qPCR. B. Expressions of MEF2D protein in ten, randomly selected, paired TSCC samples analyzed by WB.
The relationship between MEF2D expression and the clinical characteristics of TSCC patients
To investigate the association between MEF2D expression and the clinical outcome of TSCC patients, MEF2D expression and tumor differentiation were first examined by IHC experiments. Immunoreactivity was semiquantitatively evaluated based on staining intensity and distribution using the immunoreactive score (IRS) method, which was calculated as the product of the intensity score and the proportion score as we reported previously. The intensity score was defined as follows: 0, negative; 1, weak, light yellow; 2, moderate, tan; and 3, strong, dark brown. The proportion score was defined as follows: 0, <5%; 1, 5%~25%; 2, 26%~50%; 3, 51%~75%; and 4, >75% positive cells [20,21]. Therefore, the total score ranged from 0 to 12. Accordingly, IHC results with an IRS of 0~1 were considered negative (-), 2~4 as weak positive (+), 5~8 as moderate positive (++) and 9~12 as strong positive (+++). After rigorous statistical analysis, it was observed (Figure 2A) that among 40 cases of carcinoma, 12 cases presented as negative (MEF2D expression was -), 13 cases presented as weak positive (MEF2D expression was +), 8 cases presented as moderate positive (MEF2D expression was ++), and 7 cases presented as strong positive (MEF2D expression was +++). However, among 40 paracarcinoma tissues, 21 were negative (MEF2D expression was -), 10 were weakly positive (MEF2D expression was +), 9 were moderately positive (MEF2D expression was ++), and 0 were strongly positive (MEF2D expression was +++). Representative images are shown in Figure 2B. Together, these results indicated that MEF2D expression might be closely related to the degree of tumor differentiation.
Figure 2.
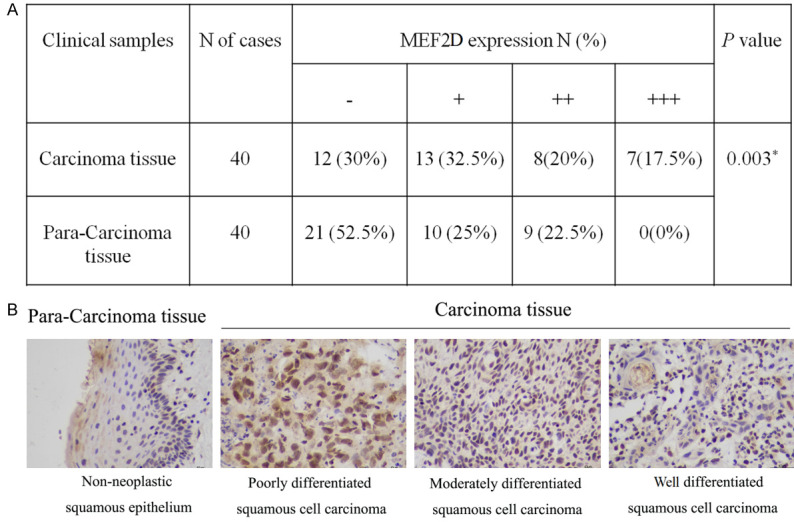
Expression of MEF2D in TSCC patients. A. Statistical analysis of MEF2D expression and the degree of tumor differentiation. B. Typical IHC staining for MEF2D expression in consecutive paraffin sections (bar = 50 μm).
Additionally, the association between MEF2D expression and clinicopathological features was further analyzed. As demonstrated in Table 1, there were significant differences among MEF2D expression and differentiation and lymphatic metastasis. Nevertheless, no significant correlation was noted between MEF2D expression and patient age, sex, or tumor size. Thus, these results further implied that MEF2D expression might be a prognostic factor for TSCC patient differentiation and lymphatic metastasis.
Establishment of MEF2D-silenced TSCC cells and suppression of MEF2D inhibited TSCC cell growth
To explore the role of MEF2D in the development of TSCC, we constructed a MEF2D-silenced TSCC cell model by transfecting the MEF2D-siRNA plasmid into TSCCA cells. The transfection efficiency was verified through RT-qPCR, which revealed that MEF2D expression was notably decreased in the MEF2D-siRNA group compared to the NC group (Figure 3A). Hence, these data suggest that a MEF2D-silenced TSCCA cell model could be established. Then, CCK-8 assays were applied to evaluate cell growth. As illustrated in Figure 3B, although the signal for the three experimental groups gradually increased with time, the rate of increase in the TSCCA-MEF2D siRNA group was remarkably lower than that in the TSCCA group or TSCCA-NC group. Therefore, these findings uncovered that the suppression of MEF2D might inhibit TSCC cell growth.
Figure 3.
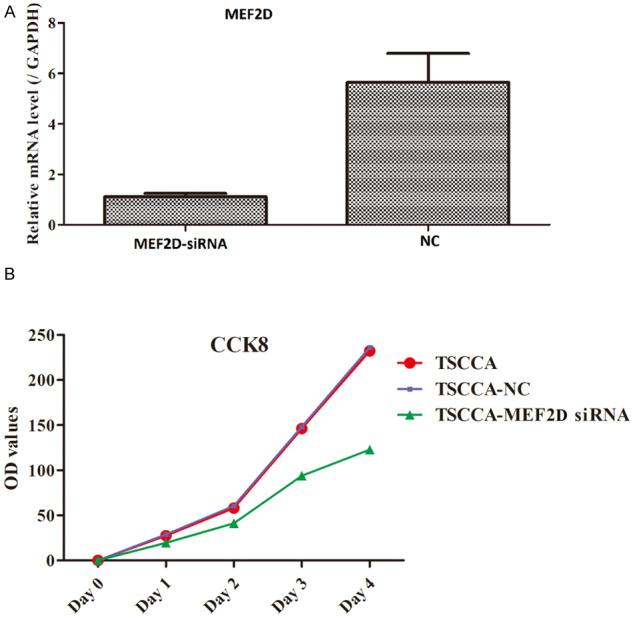
Proliferation of MEF2D-silenced TTSCCA cells was determined by the CCK-8 assay.
Silencing of MEF2D hindered cell migration in TSCC cells
A wound healing assay was utilized to assess the migratory ability of TSCC cells. At 0, 6, 24 and 48 h after creating a wound scratch, the migratory capacity was observed in the three groups. There were no marked differences at 0 h and 6 h among these three groups (Figure 4). However, the cells sharply converged at 24 h and 48 h in the TSCCA group and TSCCA-NC group, whereas the cells slightly, not fully, converged at 24 h and 48 h in the TSCCA-MEF2D siRNA group. Therefore, these results indicated that silencing MEF2D might hinder cell migration in TSCC cells.
Figure 4.
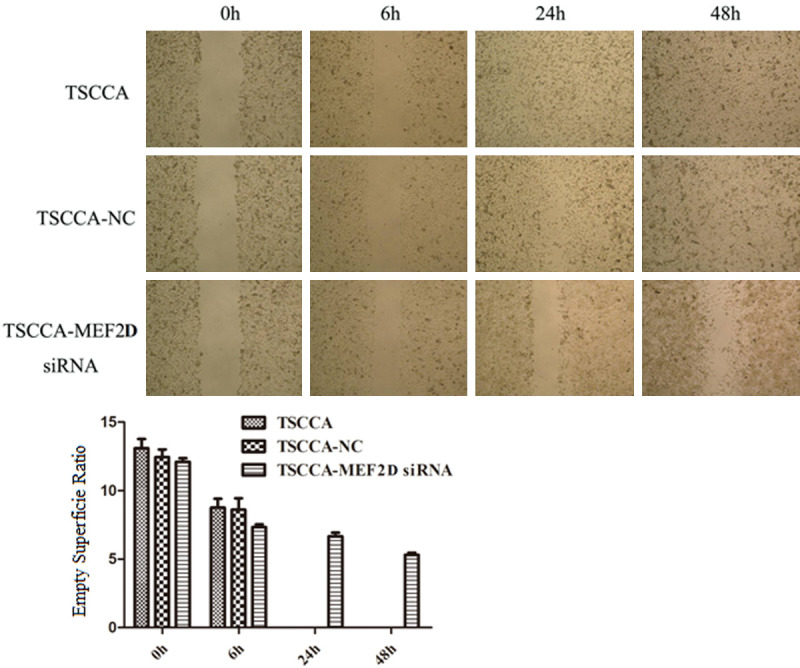
Wound-healing assay at different time courses. Data shown are from triplicate experiments.
Knockdown of MEF2D suppressed cell invasion in TSCC cells
Matrigel-based Transwell assays were used to analyze the invasiveness of TSCC cells. As depicted in Figure 5, TSCCA cells transfected with MEF2D-siRNA had a dramatically lower invasive ability, while TSCCA cells or TSCCA cells transfected with NC had an evidently higher invasive ability. Thus, these data indicated that knockdown of MEF2D might suppress cell invasion in TSCC cells.
Figure 5.

Suppression of MEF2D resulted in a decrease in the invasive ability of TTSCCA cells as measured by Transwell assay. *P<0.05.
Discussion
TSCC, accounting for 95% of oral cavity carcinomas, is a significantly aggressive form with a propensity for rapid local invasion and spread and a high recurrence rate [5,22]. Moreover, it has a 5-year survival rate of only approximately 50%, and the most important prognostic factors for patients with TSCC are intimately associated with lymph node metastases [8,23]. Surgical resection is the current mainstay strategy for TSCC patients during the disease’s early stage, but there are still no satisfactory treatments for patients with advanced TSCC [24,25]. In recent years, despite enormous improvements in modern medicine, the mortality rate of TSCC patients has not been effectively deceased [26]. Thus, TSCC remains a major health problem around the world. MEF2D belongs to the family of MADS box transcription regulators, which are mainly involved in skeletal and cardiac smooth muscle differentiation and neuronal survival [27]. However, MEF2D has also been implicated in the progression of various cancers, such as leukemia, colon cancer, pancreatic cancer and hepatocellular cancer [28,29]. Furthermore, MEF2D is also considered to have an oncogenic role in these cancers. Thus, in this study, we aimed to investigate whether MEF2D also presented aberrant expression in TSCC. First, the mRNA and protein expression levels of MEF2D were examined by RT-qPCR and WB. The results showed that MEF2D expression levels were significantly increased in TSCC carcinoma tissues compared to their paracarcinoma tissues, which suggested that MEF2D overexpression might participate in TSCC carcinogenesis. The abnormal expression of MEF2D was further evaluated in the context of the clinicopathologic features of these patients. The analysis of clinicopathologic features primarily included patient age, patient sex, tumor differentiation, lymphatic metastasis and tumor size. The results showed that MEF2D expression was correlated with tumor differentiation and lymphatic metastasis; therefore, MEF2D might act as a prognostic factor in the development of TSCC.
To further explore the detailed functions of MEF2D, we constructed a MEF2D-knockdown TSCCA cell model by transfecting MEF2D-siRNA into TSCCA cells. The RT-qPCR data showed that stable cell lines with silenced MEF2D were successfully established. Then, the proliferative ability was assessed by CCK-8 assay. Knockdown of MEF2D inhibited cell proliferation. Previous studies have demonstrated that dysregulation of cellular proliferation contributes to the occurrence and development of many cancers [30,31]. Thus, the changes in MEF2D might directly influence cell proliferation in TSCC. Additionally, metastasis is the key hallmark of cancer and the main cause of death in patients with advanced TSCC [32,33]. However, tumor metastasis is made up of a series of steps, including cell invasion, migration, and extravasation [34]. The migratory and invasive capacities were further detected by wound healing assay and Transwell assay, respectively. The results illustrated that decreased MEF2D expression impaired the cell migration and invasion of TSCCA cells, which implied that MEF2D might be involved in the regulation of TSCC metastasis.
Conclusion
Taken together, the findings of the current study uncovered that MEF2D expression presented an increasing trend, which was related to tumor differentiation and lymphatic metastasis. Moreover, the silencing of MEF2D hindered cell proliferation, migration and invasion in TSCCA cells. Collectively, these results suggested that MEF2D might serve as a tumor promoter in TSCC development. MEF2D might have critical implication for future therapy of TSCC.
Acknowledgements
All of the patients who provided samples were well-informed about the use of samples and informed consents were signed. The present study was approved by the Research Ethics Committee of the Qingdao University (No. 2011EA438 on October 30th, 2011) in accordance with the ethical guidelines of the Declaration of Helsinki.
This work was financially supported by Fund of CSCO-Qilu Oncology Research (No. Y-Q201802-044) and Qingdao Livelihood Science and Technology Program (No. 18-6-1-92nsh).
Disclosure of conflict of interest
None.
Abbreviations
- TSCC
Tongue squamous cell carcinoma
- MEF2D
Myocyte-specific enhancer factor 2
- MIG6
mitogen-inducible gene 6
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- WB
western blotting
- IHC
immunohistochemistry
- cDNA
complementary DNA
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IPA
immunoprecipitation assay
- SDS-PAGE
sulfate-polyacrylamide gel
- PVDF
transferred onto polyvinylidene difluoride
- ANOVA
analysis of variance
- IRS
immunoreactive score
References
- 1.Hassona Y, Sawair F, Matarweh D, Abdalhamid A, Thweib D, Scully C. Oral cancer early detection: what do patients need to know? J Cancer Educ. 2018;33:865–869. doi: 10.1007/s13187-017-1191-x. [DOI] [PubMed] [Google Scholar]
- 2.Silverman S Jr. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BR, Netterville JL, Sinard RJ, Mannion K, Rohde SL, Langerman A, Kim YJ, Lewis JS Jr, Lang Kuhs KA. Early onset oral tongue cancer in the United States: a literature review. Oral Oncol. 2018;87:1–7. doi: 10.1016/j.oraloncology.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan H, Paiboonrungruan C, Zhang X, Prigge JR, Schmidt EE, Sun Z, Chen X. Nrf2 regulates cellular behaviors and notch signaling in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2017;493:833–839. doi: 10.1016/j.bbrc.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 6.Gamez ME, Kraus R, Hinni ML, Moore EJ, Ma DJ, Ko SJ, Rwigema JCM, McGee LA, Halyard MY, Buras MR, Foote RL, Patel SH. Treatment outcomes of squamous cell carcinoma of the oral cavity in young adults. Oral Oncol. 2018;87:43–48. doi: 10.1016/j.oraloncology.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Siu A, Chang J, Lee C, Lee S, Lee C, Ramos DM. Expression of EMMPRIN modulates mediators of tumor invasion in oral squamous cell carcinoma. J Calif Dent Assoc. 2013;41:831–838. [PubMed] [Google Scholar]
- 8.Agarwal SK, Arora SK, Kumar G, Sarin D. Isolated perifacial lymph node metastasis in oral squamous cell carcinoma with clinically node-negative neck. Laryngoscope. 2016;126:2252–6. doi: 10.1002/lary.25954. [DOI] [PubMed] [Google Scholar]
- 9.Fang Z, Chen S, Zhao JF, Sun Q, Qiu F, Li XM. Effect of human trophoblast cell-surface antigen 2 gene expression by RNA interference on proliferation and apoptosis of tongue squamous cell carcinoma and its mechanism. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018;53:640–644. doi: 10.3760/cma.j.issn.1002-0098.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Cao J, Guo T, Dong Q, Zhang J, Li Y. miR-26b is downregulated in human tongue squamous cell carcinoma and regulates cell proliferation and metastasis through a COX-2-dependent mechanism. Oncol Rep. 2015;33:974–80. doi: 10.3892/or.2014.3648. [DOI] [PubMed] [Google Scholar]
- 11.Potthoff MJ, Arnold MA, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by MEF2D. Mol Cell Biol. 2007;27:8143–51. doi: 10.1128/MCB.01187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shum AM, Mahendradatta T, Taylor RJ, Painter AB, Moore MM, Tsoli M, Tan TC, Clarke SJ, Robertson GR, Polly P. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting. Aging (Albany NY) 2012;4:133–43. doi: 10.18632/aging.100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuma K, Nakao R, Inashima S, Hirata M, Kubo T, Yasuhara M. Marked reduction of focal adhesion kinase, serum response factor and myocyte enhancer factor 2C, but increase in RhoA and myostatin in the hindlimb dy mouse muscles. Acta Neuropathol. 2004;108:241–9. doi: 10.1007/s00401-004-0884-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamakuchi M, Higuchi I, Masuda S, Ohira Y, Kubo T, Kato Y, Maruyama I, Kitajima I. Type I muscle atrophy caused by microgravity-induced decrease of myocyte enhancer factor 2C (MEF2D) protein expression. FEBS Lett. 2000;477:135–40. doi: 10.1016/s0014-5793(00)01715-4. [DOI] [PubMed] [Google Scholar]
- 15.Skerjanc IS, Petropoulos H, Ridgeway AG, Wilton S. Myocyte enhancer factor 2C and Nkx2-5 up-regulate each other’s expression and initiate cardiomyogenesis in P19 cells. J Biol Chem. 1998;273:34904–10. doi: 10.1074/jbc.273.52.34904. [DOI] [PubMed] [Google Scholar]
- 16.Cante-Barrett K, Pieters R, Meijerink JP. Myocyte enhancer factor 2C in hematopoiesis and leukemia. Oncogene. 2014;33:403–10. doi: 10.1038/onc.2013.56. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhu B, Davie J. Alternative splicing of MEF2D pre-mRNA controls its activity in normal myogenesis and promotes tumorigenicity in rhabdomyosarcoma cells. J Biol Chem. 2015;290:310–24. doi: 10.1074/jbc.M114.606277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laszlo GS, Alonzo TA, Gudgeon CJ, Harrington KH, Kentsis A, Gerbing RB, Wang YC, Ries RE, Raimondi SC, Hirsch BA, Gamis AS, Meshinchi S, Walter RB. High expression of myocyte enhancer factor 2C (MEF2D) is associated with adverse-risk features and poor outcome in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. J Hematol Oncol. 2015;8:115. doi: 10.1186/s13045-015-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Liu W, Wang Z, Meng L, Wang Y, Yan H, Li L. MEF2C promotes gefitinib resistance in hepatic cancer cells through regulating MIG6 transcription. Tumori. 2018;104:221–231. doi: 10.1177/0300891618765555. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Qiao B, Zhao T, Hu F, Lam AK, Tao Q. Sox2 promotes tumor aggressiveness and epithelialmesenchymal transition in tongue squamous cell carcinoma. Int J Mol Med. 2018;42:1418–1426. doi: 10.3892/ijmm.2018.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu H, Jia B, Qiu X, Pan J, Sun X, Wang Z, Zhao J. Investigation of proliferation and migration of tongue squamous cell carcinoma promoted by three chemokines, MIP-3alpha, MIP-1beta, and IP-10. Onco Targets Ther. 2017;10:4193–4203. doi: 10.2147/OTT.S132855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Z, Xiao T, Huang J, Yuan Y, Ye Q, Xuan M, Xie H, Wang X. Elective neck dissection versus observation in squamous cell carcinoma of oral cavity with clinically N0 neck: a systematic review and meta-analysis of prospective studies. J Oral Maxillofac Surg. 2019;77:184–194. doi: 10.1016/j.joms.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Ong W, Zhao R, Lui B, Tan W, Ebrahimi A, Clark JR, Soo KC, Tan NC, Tan HK, Iyer NG. Prognostic significance of lymph node density in squamous cell carcinoma of the tongue. Head Neck. 2016;38(Suppl 1):E859–66. doi: 10.1002/hed.24113. [DOI] [PubMed] [Google Scholar]
- 24.Chen YH, Chien CY, Fang FM, Huang TL, Su YY, Luo SD, Huang CC, Lin WC, Li SH. Nox4 overexpression as a poor prognostic factor in patients with oral tongue squamous cell carcinoma receiving surgical resection. J Clin Med. 2018;7 doi: 10.3390/jcm7120497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao KN, Jagade M, Kale VD, Rengaraja D, Hekare A. Margin status and duration of surgery in resection of tongue carcinoma with ultrasound coagulation device: a comparative study. Indian J Surg Oncol. 2018;9:501–504. doi: 10.1007/s13193-018-0785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52:228–240. doi: 10.1016/j.semcancer.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Naya FJ, Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11:683–8. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 28.Bai X, Wu L, Liang T, Liu Z, Li J, Li D, Xie H, Yin S, Yu J, Lin Q, Zheng S. Overexpression of myocyte enhancer factor 2 and histone hyperacetylation in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134:83–91. doi: 10.1007/s00432-007-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai XL, Zhang Q, Ye LY, Liang F, Sun X, Chen Y, Hu QD, Fu QH, Su W, Chen Z, Zhuang ZP, Liang TB. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/beta-catenin signaling. Oncogene. 2015;34:4089–97. doi: 10.1038/onc.2014.337. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O, Majumder S, He C, Huang S. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606. e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Yang T, Shang D, Sun Z. miR-1254 promotes lung cancer cell proliferation by targeting SFRP1. Biomed Pharmacother. 2017;92:913–918. doi: 10.1016/j.biopha.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Lu Z, Deng Y, Wang W, He Q, Yan W, Wang A. Up-regulation of INSR/IGF1R by C-myc promotes TSCC tumorigenesis and metastasis through the NF-kappaB pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1873–1882. doi: 10.1016/j.bbadis.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Jiao J, Zhao X, Liang Y, Tang D, Pan C. FGF1-FGFR1 axis promotes tongue squamous cell carcinoma (TSCC) metastasis through epithelial-mesenchymal transition (EMT) Biochem Biophys Res Commun. 2015;466:327–32. doi: 10.1016/j.bbrc.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]


