Abstract
Herein reported is the unique case of a small hepatocellular carcinoma (HCC) with several foci of a minor (10% in area) component of “malignant ductular reactions”. The patient was 51-year-old man who was a drinker. HBV/HCV were negative. The tumor was small (12×10×11 mm), solid, expansile and reddish-brown, and contained fibrous septa. The background was cirrhotic without alcoholic features. Histologically, the tumor was well differentiated HCC, and, besides the HCC, it contained several small foci consisting of the following four biliary epithelial elements: clusters of small cells (CSC), ductules (D), ductular hepatocytes (DH), and bile ducts (BD). The proportion of area was as follows: HCC 90%, CSC 3%, D 3%, DH 2%, and BD 2%. These non-HCC elements were intimately admixed and formed several foci that were characteristically located in the fibrous septa (FS), except for CSC which were situated among HCC cells close to FS. There were gradual merges between HCC and CSC, CSC and D, D and DH, and D and BD, respectively. Cells of CSC and D resembled rat oval cells. Cells of these four elements had atypical features regarded as malignant. Immunohistochemically (IHC), HCC were positive for arginase, HepPar1, and less frequently CK7. CSC were positive for CK7. D were positive for arginase, HepPar1, CK7, CK19, EMA, and EpCAM. DH were positive for arginase, HepPar1, and CK7. BD were positive for CK7, CK19, EMA, EpCAM and mucin. Although such tumors as this have been termed stem cell-related cancers, our case lacked definite evidence for stem cell origin in histology as well as in the IHC that showed negativity for KIT, CD34, and OCT3/4. The above findings suggest that CSC, D, DH and BD are analogous to the ductular reaction seen in hepatic inflammation. Therefore, we termed the phenomenon “malignant ductular reaction”. It is suggested in the present tumor that at first only HCC developed, and then HCC cells in the interface with FS transformed to CSC, like a fetal ductal plate. Then, the CSC gave rise to D, which in turn led to DH and BD in FS, all findings of which are most likely sequential considering embryonic biliary development. The idea that the present tumor was at first D carcinoma and then D developed on one hand into CSC and HCC, and on the other into DH and BD seems possible, but its probability appears low because the vast majority of the present tumor had the phenotype of HCC.
Keywords: HCC, ductular reaction, liver stem cells, case report
Introduction
The proximal biliary system is composed of complex biliary structures containing bile canaliculi, Herring ducts, ductules, and interlobular ducts [1]. Herring ducts are located in the periphery of the liver parenchyma, and are composed of hepatocytes and/or ductules. The ductules are situated in the peripheral portal tracts and consist of small cuboidal cholangiocytes that are not accompanied by hepatic arteries, in contrast to interlobular ducts which are always situated next to hepatic arteries. Ductules are categorized as typical and atypical; the former has lumens while the latter do not. Hepatic progenitor cells or stem cells are thought by some to reside in Herring ducts and ductules [1,2]. The proliferated ductules, both typical and atypical, are called the ductular reaction [1,3]. The ductular reaction occurs in any type of portal inflammations, but is most prominent in extrahepatic obstruction and primary biliary cirrhosis. Interlobular ducts have obvious round lumens, are composed of columnar biliary epithelium, and are thought not to have proliferative activity whereas Herring canals and ductules can proliferate. Interlobular bile ducts drain into common bile duct by septal (having connective tissue walls), area, segmental, and hepatic ducts, along with peribiliary glands. Cholangiocarcinomas arise from these particular cell types and show each particular histologic phenotype [4].
The majority of malignant liver tumors are hepatocellular carcinoma (HCC) and cholangiocarcinoma; the former shows hepatocellular phenotypes or is derived from hepatocytes and the latter cholangiocytic phenotypes or arises from cholangiocytes. Cholangiolocellular carcinoma was first described by Steiner and Higginson [5] for the liver carcinoma displaying structures resembling cholangioles or ductules. Recently, there have been some reports and studies of unusual liver carcinomas which have shared features of HCC, cholangiocarcinoma, and/or cholangiolocellular carcinoma. Such cases have been reported as various terms such as cholangiolocellular carcinoma, combined hepato-cholangiocarcinoma, stem cell malignancy, intermediate carcinoma, and hepato-cholangiocarcinoma with stem cell features [5-9]. We herein report a case of HCC with a minor component of cholangiocytic structures resembling a ductular reaction.
Case report
A 51-year-old man had been followed up because of cirrhosis of probable alcoholic origin. HBV and HCV were negative, and the patient was an alcoholic drinker. During the follow-up, he was found to have a small nodule in the liver segment 8 by CT/angio-CT, and its resection was carried out under the clinical diagnosis of HCC. Grossly, the tumor was small, solid and expansile, and measured 12×10×11 mm (Figure 1). Fibrous septa were present.
Figure 1.
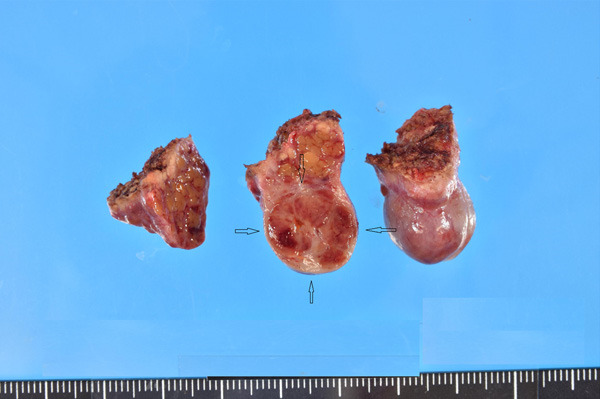
Gross features of the cut surface of the resected liver tumor (arrows). The tumor measures 12×10×11 mm, is solid, expansile, and is well-defined. Fibrous septa are seen. The background shows cirrhosis.
Microscopically, the nodule was composed largely of HCC (percentage of area: 90%), and the remaining of biliary elements consisting of clusters of small cells (3%), ductules (3%), ductular hepatocytes (2%) [10], and bile ducts (2%). The HCC was well to moderately differentiated, corresponding to Grade II of Edmondson and Steiner [11] (Figure 2A). The structural pattern was compact and less frequently trabecular. No portal tracts were present in the HCC. Fibrous septa were noted in places. Tumor vessels were also present. No rims of macroregenerative nodule [12], atypical adenomatous hyperplasia [13], or dysplastic nodule were present. The tumor was not focal nodular hyperplasia or hepatocellular adenoma because of the presence of atypical features and background cirrhosis. The non-HCC elements were intimately mixed up and formed several foci. They were characteristically located in the fibrous septa except for the clusters of small cells which were situated among HCC cells close to fibrous septa.
Figure 2.
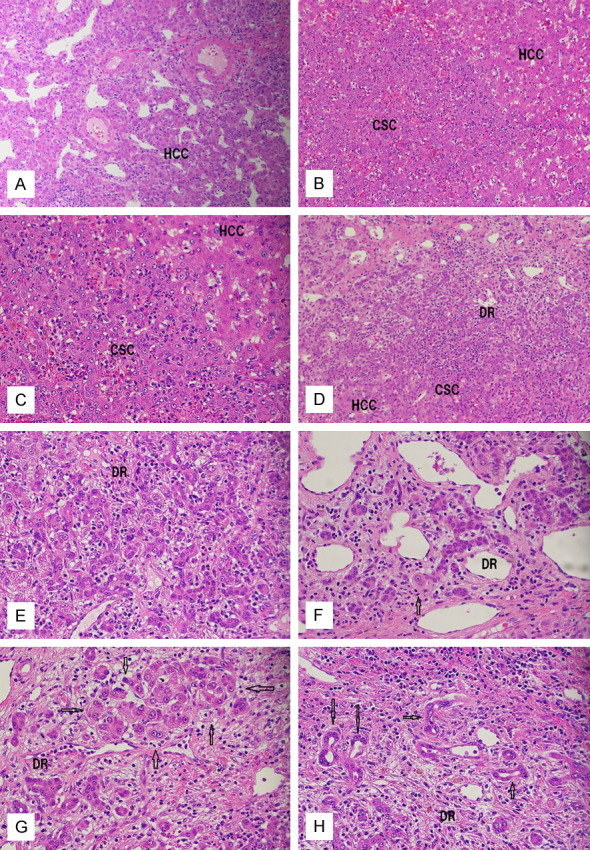
Histopathologic features of the liver tumor. (A) Hepatocellular carcinoma (HCC) area showing typical compact and trabecular patterns. Tumor vessels are present. H&E, ×40. (B, C) The area of cluster of small cells (CSC) shows an aggregate of small oval cell-like cells with scant cytoplasm, increased nucleo-cytoplasmic ratio, nucleoli, and neutrophilic infiltrations. (B) ×40. (C) ×100. (D, E) The areas of fibrous septa of HCC showing malignant ductular reaction (DR), clusters of small cells (CSC), and HCC cells. There are gradual transitions between HCC cells and CSC cells and also between CSC cells and ductular cells. (D, E) ×100. (F, G) The areas of ductular hepatocytes (arrows) in fibrous septa of HCC showing a mixture of ductular cells and ductular hepatocytes (arrows) that are present singly (F) or in clusters (G). There is a gradual transition between the ductular cells and ductular hepatocyte (F, arrow). (F, G) ×100. (H) The area of fibrous septa of the HCC showing bile duct differentiation (arrows) and ductules (DR). (H) ×100.
The clusters of small cells were present in the HCC parenchyma next to the fibrous septa (Figure 2B and 2C), and they were frequently merged with ductules within the fibrous septa or in the interfaces between HCC cells and the septa. They formed discrete nodules and consisted of small cells with scant ovoid cytoplasm, increased nucleo-cytoplasmic ratio, oval cells-like features, and nucleoli. They were characteristically accompanied by heavy neutrophilic infiltrations. The ductules were complex-tubular or cord-like structures consisting of cuboidal or oval cholangiocytes, thus resembling bile ductules in the ductular reaction (Figure 2D and 2E). These ductular structures were characteristically infiltrated by neutrophils (Figure 2D), and these cells were similar cytologically to those of the clusters of small cells (Figure 2E). The ductular hepatocytes were found in the fibrous septa, mixed with or very close to the ductular areas, and were present singly (Figure 2F) or in clusters (Figure 2G). There were obvious transitions between ductules and ductular hepatocytes (Figure 2F). No direct merges between ductular hepatocytes and HCC cells were seen. The ductular hepatocytes had abundant acidophilic cytoplasms similar to normal hepatocytes or HCC cells. The bile ducts were also located in the fibrous septa, and were continuous with ductules (Figure 2H). The bile ducts had apparent lumens that were larger than those of ductules. Direct transitions between the bile ducts and HCC cells were not noted. Thus, the clusters of small cells were similar to rat oval cells, the ductules to normal ductules, the ductular hepatocytes to hepatocytes or HCC cells, and the bile ducts to interlobular bile ducts. However, all of these unusual elements in the present tumor had significant cellular and structural atypia to be diagnosed malignant. The background liver was micronodular cirrhosis (a2, f4) without specific changes such as fatty changes, Mallory-Denk bodies, perivenular fibrosis, or ballooning.
An immunohistochemical study was carried out by the Dako Polymer method using an automatic immunohistochemical stainer (DAKO). The results are shown in Table 1. KIT, CD34 and OCT3/4 are markers of stem cells and other primitive or tumour cells. Cytokeratin (CK) 7 and CK19 are expressed in cholangiocytes of the adult and fetal livers. HepPar1 is expressed in hepatocytes and other cell types. Arginase is a lineage biomarker of hepatocytes. AFP is an oncofetal and expressed in HCC cells in the liver. EMA is a differentiated antigen expressed in hepatocytes and primitive cholangiocytes in the liver. The expression of EpCAM is seen in primitive cholangiocytes. Mucins are present in bile duct cells but not in hepatocytes.
Table 1.
Expression of various antigens in each subset of the hepatocellular carcinoma
| HCC | CSC | Ductules | Ductular hepatocytes | Bile ducts | |
|---|---|---|---|---|---|
| Arginase | ++ | - | + | + | - |
| HepPar1 | +++ | - | + | ++ | - |
| AFP | - | - | - | - | - |
| CK7 | + | + | +++ | + | ++ |
| CK19 | - | - | + | - | + |
| EMA | - | - | + | - | + |
| CEA | - | - | - | - | - |
| KIT | - | - | - | - | - |
| CD34 | - | - | - | - | - |
| Oct3/4 | - | - | - | - | - |
| EpCAM | - | - | + | - | + |
| AB-PAS | - | - | - | - | + |
CK: cytokeratin. AB-PAS: alcian blue/PAS double staining for mucins. HCC: hepatocellular carcinoma. CSC: clusters of small cells.
The HCC areas were positive for arginase, HepPar1, and less frequently CK. The clusters of small cells are positive for CK7. Ductules were positive for arginase, HepPar1, CK7, CK19, epithelial membrane antigen (EMA), and epithelial cell adhesion molecules (EpCAM). The ductular hepatocytes were positive for arginase, HepPAR1, and CK7. Bile ducts were positive for CK7, CK19, EMA, EpCAM, and mucins. KIT and CD34 labeled only mast cells and endothelial cells, respectively (Figure 3).
Figure 3.

Immunohistochemical features of the liver tumor. A: Arginase is expressed in hepatocellular carcinoma (HCC) areas and to a lesser extent in the ductules (small arrows) and ductular hepatocytes (wide arrows). Immunostain for arginase, ×40. B: HepPar1 is expressed in the ductules (DR) and ductular hepatocytes (arrows). Immunostain using HepPar1, ×60. C: CK7 expression. The ductules (DR) are strongly positive for CK7 and relatively weakly in the HCC cells. Immunostain for CK7, ×80. D: CK19 expression. CK19 is expressed in the ductules (DR). Immunostain for CK19, ×80. E: EMA is positive in the ductulular cells (DR) and bile ducts. Immunostain for EMA, ×80. F: EpCAM expression is seen in the ductular cells (DR) and bile ducts. Immunostain for EpCAM, ×80.
Discussion
We herein report a unique case HCC with several foci of atypical biliary or hepatocellular elements (clusters of small cells, ductules, ductular hepatocytes, and bile ducts) which were intimately admixed and located in fibrous septa. The vast majority of the present tumor was HCC, and characteristically only 10% of the tumor was composed of non-HCC epithelial elements. Theise et al. [2] and others [3-9] have suggested the presence of stem cells or progenitor cells in the ductules and Herring ducts. The stem cells have been thought to differentiate both into hepatocytes and cholangiocytes. Such liver tumors as the present tumor have been reported by some pathologists as stem cell-related ones such as hepato-cholangiocarcinoma with stem cell features [3-9], and such tumors have been thought by some to be associated with stem cells. We could not identify, however, the stem cells in the present liver tumor. We could not investigate this case by electron microscopy, and employed only immunohistochemical features. However, liver stem cells were difficult to identify by electron microscopy; in the present case, liver stem cells were best identified by KIT expression. The most reliable stem cell antigens were KIT and CD34, which were totally negative in the present case. The less specific stem cell biomarker was OCT3/4, which was also negative in this case. EpCAM and EMA, which are less specific markers for stem cells, were positive in only ductules in the present case. Therefore, we could not find any evidence of stem cells in the current case; thus, we classified our tumor as HCC with a minor component of ductular reactions. The stem cell is defined as a specialized cell type which after cell division gives rise to another specific cell type and to one that remains a “stem cell”. Thus, the stem cell hypothesis is quite conceptual, and the identification of stem cells is very difficult. Therefore, we think that the concept of stem cells cannot be overused.
The non-HCC epithelial elements of the present case are very similar to the ductular reaction that is seen in various inflammatory hepatobiliary diseases [3]. The non-HCC biliary epithelial elements of the present case consisted of clusters of small cells, ductules, ductular hepatocytes, and bile ducts. It is of interest that these four elements are closely located, admixed, and formed a focus of cell clusters, suggesting that these represent a unit of a cellular system. It is also of interest that these units resemble the ductular reaction seen in non-neoplastic livers with inflammation. There was significant atypia in the non-HCC elements of the present case, and we think that the cell unit is the malignant counterpart of the ductular reaction, i.e. “malignant ductular reaction”. It is of interest that the malignant ductular reaction that was characteristically accompanied by severe neutrophilic infiltration; this phenomenon is also seen in the usual non-malignant ductular reaction. This suggests the validity of our hypothesis. We also speculate the presence of the cell renewal downstream from clusters of small cells to ductules, and then from ductules to ductular hepatocytes or to bile ducts. That is, we postulate in this case that cells of the clusters of small cells gave rise to ductules, which in turn led to ductular hepatocytes and bile ducts. This hypothesis is similar to that of the ductular reaction in chronic liver diseases. Therefore, we think that the present case was an HCC with a malignant ductular reaction. The upstream of the cell renewal from clusters of small cells to HCC remains to be investigated.
The similarity between the ductular reaction and the ductal plate has been advocated [3]. In fact, the ductal plate, which is seen in embryonic and fetal livers, resembles morphologically the ductular reaction [14-17]. The ductal plate is a double-layered cylindrical structure which leads to the development of ductules, bile ducts, periportal hepatocytes, peribiliary glands, and pancreatic acinar cells [18-23]. The developmental process involves various molecular changes, such as CK profile, apoptosis, and cell proliferation, peribiliary capillary plexus, glycosylation, and epithelial-mesenchymal interactions [14-26]. Our recent study showed there are stem cell-like cells expressing C-erbB2, CD56, chromogranin, synaptophysin, bcl2, NSE, NCAM, KIT, and PDGFRA in the human fetal ductal plate, suggesting the presence of stem cells in the human ductal plate [26]. Thus, the ductular reactions resemble the fetal ductal plate in many functions as well as in histologic features. It is interesting that the malignant ductular reaction resembles the fetal ductal plate from the viewpoint that the carcinogenesis/progression of tumours recapitulates the ontogeny/embryology. It helps to indicate that, apart from the ductal plate, the human fetal liver has other kinds of embryonic or fetal stem cells in the hilar and large portal tracts [28-30]. These obvious stem cells are located particularly near the nerve fibers [30]. This association between stem cells and neurons is interesting in that stem cells are positive for neuroendocrine antigens such as chromogranin A, synaptophysin, NCAM and NSE, and these molecules are biomarkers for stem cells [31].
Most of the previously reported cases of these cholangiolocellular or hepato-cholangiocarcinomas with stem cell features have been concerned with large liver cancers and with liver cancers in which the “stem cell features” were predominant and HCC/cholangiocarcinoma elements are very scant. In contrast, the present tumor was largely composed of HCC (90%), with only a minor component of scattered foci of malignant ductular reaction (10%). We previously demonstrated CK19-poisitve ductal and ductular structures in atypical adenomatous hyperplasia and small HCC [32]. We also reported a case combined HCC-cholangiocarcinoma arising in an atypical adenomatous hyperplasia, now called dysplastic nodules [33]. These cases had suggested that combined liver tumors might have emerged early in HCC development, i.e. in atypical adenomatous hyperplasia and small HCC. The present case is a small HCC (12 mm); thus it supports the above statements. In addition, we speculate in the current case that the malignant ductular reaction emerged in an already-developed HCC because the vast majority of the tumor was conventional HCC. We speculate in the current case that clusters of small cells emerged in small HCC and then they yielded the ductules which in turn gave rise to ductular hepatocytes and bile ducts. The hepatoblasts in human fetus transform to the ductal plate, a mimic of ductular reaction. Therefore, it is conceivable that the clusters of small cells might have been derived from the ordinary HCC cells.
Ductular hepatocytes have been studied under inflammatory conditions. They are seen in the furan-treated rats [10], and they were seen to develop from cholangiocytes in mouse models, in which hepatocytes could be differentiated from cholangiocytes by integrin scaffolds under liver injury [34]. In the present case, we suspect that the ductules transform into the ductular hepatocytes. This phenomenon of ductular hepatocyte is the first report in HCC, to the best of our knowledge, and indicates that so-called cholangiocellular carcinoma can transform in part to HCC cells. We also think that the bile duct elements are derived from the ductules.
It is important that epithelial and mesenchymal interactions play important roles in the human liver embryogenesis [35,36] as well as in the ductular reaction [3]. In the present case, the malignant ductular reaction was located in the fibrous septa next to HCC parenchyma. As is well known, the ductal plate develops and matures in the interface between hepatoblasts and portal mesenchyme in embryonic livers. Therefore, it is very likely that the ductular reaction is in close association with the functions of the portal tract connective tissue and fibrous septa in HCC. We previously reported that fibrosis within HCC is causally related to activated stellate cells in Disse spaces of HCC, also known as fat-storing cells [37], suggesting indirectly that the malignant ductular reactions may be associated with activated stellate cells.
Immunohistochemically, the HCC element was positive for hepatocyte lineage antigens, arginase and HepPar1 and CK7; the latter CK7 finding is relevant and indicates that the HCC component shows cholangiocytic features. The clusters of small cells was positive for CK7 but negative for arginase and HepPar1, indicating that they show cholangiocytic phenotype but not hepatocellular lineage. The ductules were positive for arginase and HepPar1 as well as CK7, CK19, EMA and EpCAM, implying that they have hepatocellular features in addition to cholangiocytic characters. The ductular hepatocytes showed positive reactions for arginase, HepPar1, and CK7, indicating that they harbor cell lineage of both hepatocytes and cholangiocytes. The bile ducts were mucin-positive.
Conclusions
We present a unique case of small HCC with the following four minor (10%) components of atypical biliary epithelium: clusters of small cells resembling oval cells in rodent chemical hepatocarcinogenesis, ductules, ductular hepatocytes, and bile ducts. We tentatively termed these malignant ductular reactions because they resembled the ductular reaction in hepatobiliary inflammations. They were located in the fibrous septa, indicating that epithelial mesenchymal interactions played important roles in their development, similar to the human ductal plate ontogeny. The putative transformation of the ductules into ductular hepatocytes and bile ducts is noteworthy, and suggests that malignant ductules can give rise to malignant hepatocytes and cholangiocytes. We could not identify a stem cell nature in this tumour. We speculate in this tumor that at first an HCC emerged and then these four biliary elements transformed from the HCC. We postulate the nature of these biliary components in the context of human liver ontogeny.
Acknowledgements
The authors are very grateful to the staff of Department of Pathology, Shizuoka General Hospital, for their excellent technical assistance.
Informed consent was obtained from the patient.
Disclosure of conflict of interest
None.
References
- 1.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 2.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 3.Desmet VJ. Ductal plates in hepatic ductular reactions. Hypothesis and implications. III. Implications for liver pathology. Virchows Arch. 2011;458:271–279. doi: 10.1007/s00428-011-1050-9. [DOI] [PubMed] [Google Scholar]
- 4.Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 5.Steiner PE, Higginson J. Cholangiolocellular carcinoma of the liver. Cancer. 1959;12:753–759. doi: 10.1002/1097-0142(195907/08)12:4<753::aid-cncr2820120420>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 9.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–22. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 10.Sirica AE, Gainey TW, Mumaw VR. Ductular hepatocytes. Evidence for a bile ductular cell origin in furan-treated rats. Am J Pathol. 1994;145:375–383. [PMC free article] [PubMed] [Google Scholar]
- 11.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Terada T, Nakanuma Y. Survey of iron-accumulative macroregenerative nodules in cirrhotic livers. Hepatology. 1989;10:851–854. doi: 10.1002/hep.1840100517. [DOI] [PubMed] [Google Scholar]
- 13.Terada T, Nakanuma Y. Cell proliferative activity in adenomatous hyperplasia of the liver and small hepatocellular carcinoma. An immunohistochemical study demonstrating proliferating cell nuclear antigen. Cancer. 1992;70:591–598. doi: 10.1002/1097-0142(19920801)70:3<591::aid-cncr2820700309>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 15.Desmet VJ, Van Eyken P, Sciot R. Cytokeratins for probing cell lineage relationships in developing liver. Hepatology. 1990;12:1249–1251. doi: 10.1002/hep.1840120530. [DOI] [PubMed] [Google Scholar]
- 16.Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development. A possible role in biliary cell migration. Am J Pathol. 1995;147:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 17.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995;146:67–74. [PMC free article] [PubMed] [Google Scholar]
- 18.Terada T. Differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling ductal plate in human fetuses. Hepatology. 2012;56:2004–2005. doi: 10.1002/hep.25750. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Nakanuma Y. Expression of pancreatic enzymes (alpha-amylase, trypsinogen, and lipase) during human liver development and maturation. Gastroenterology. 1995;108:1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers. Heterotopic pancreas in the liver. Gastroenterology. 1990;98:1333–1337. doi: 10.1016/s0016-5085(12)90353-4. [DOI] [PubMed] [Google Scholar]
- 21.Terada T. Human fetal ductal plate revisited. I. ductal plate expresses NCAM, KIT, MET, PDGFRA, and neuroendocrine antigens (NSE, chromogranin, synaptophysin, and CD56) Microsc Res Tech. 2014;77:814–824. doi: 10.1002/jemt.22404. [DOI] [PubMed] [Google Scholar]
- 22.Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenbaum LE. The ductal plate: a source of progenitors and hepatocytes in the adult liver. Gastroenterology. 2011;141:1152–1155. doi: 10.1053/j.gastro.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Terada T, Nakanuma Y. Development of human intrahepatic peribiliary glands. Histological, keratin immunohistochemical, and mucus histochemical analyses. Lab Invest. 1993;68:261–269. [PubMed] [Google Scholar]
- 25.Terada T, Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology. 1994;20:388–397. [PubMed] [Google Scholar]
- 26.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: a lectin-histochemical and immunohistochemical study. Hepatology. 1993;18:529–536. [PubMed] [Google Scholar]
- 27.Terada T. Human ductal plate and its derivatives express antigens of cholangiocellular, hepatocellular, hepatic stellate/progenitor cell, stem cell, and neuroendocrine lineages, and proliferative antigens. Exp Biol Med (Maywood) 2017;242:907–917. doi: 10.1177/1535370216644684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terada T. Development of extrahepatic bile duct excluding gall bladder in human fetuses: histological, histochemical, and immunohistochemical analysis. Microsc Res Tech. 2014;77:832–840. doi: 10.1002/jemt.22406. [DOI] [PubMed] [Google Scholar]
- 29.Terada T. Huge clusters of embryonic stem cells in human embryos: a morphologic study. Microsc Res Tech. 2014;77:825–831. doi: 10.1002/jemt.22405. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. Ontogenic development of nerve fibers in human fetal livers: an immunohistochemical study using neural cell adhesion molecule (NCAM) and neuron-specific enolase (NSE) Histochem Cell Biol. 2015;143:421–429. doi: 10.1007/s00418-014-1286-y. [DOI] [PubMed] [Google Scholar]
- 31.Van Haele M, Roskams T. Hepatic progenitor cells: an update. Gastroenterol Clin North Am. 2017;46:409–420. doi: 10.1016/j.gtc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Hoso M, Nakanuma Y. Distribution of cytokeratin 19-positive biliary cells in cirrhotic nodules, hepatic borderline nodules (atypical adenomatous hyperplasia), and small hepatocellular carcinomas. Mod Pathol. 1995;8:371–379. [PubMed] [Google Scholar]
- 33.Harada K, Terada T, Nakanuma Y, Furukawa Y, Kurumaya H. A case of small combined hepatocellular and cholangiocellular carcinoma arising in a nodule of atypical adenomatous hyperplasia of the liver. Am J Gastroenterol. 1993;88:1968–1969. [PubMed] [Google Scholar]
- 34.Raven A, Lu WY, Man TY, Ferreira-Gonzalez S, O’Duibhir E, Dwyer BJ, Thomson JP, Meehan RR, Bogorad R, Koteliansky V, Kotelevtsev Y, Ffrench-Constant C, Boulter L, Forbes SJ. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of epithelial-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development: a possible role in bile duct morphogenesis. J Hepatol. 1998;28:263–269. doi: 10.1016/0168-8278(88)80013-8. [DOI] [PubMed] [Google Scholar]
- 36.Terada T, Ohta T, Nakanuma Y. Expression of transforming growth factor-alpha and its receptor during human liver development and maturation. Virchows Arch. 1994;424:669–675. doi: 10.1007/BF00195783. [DOI] [PubMed] [Google Scholar]
- 37.Terada T, Makimoto K, Terayama N, Suzuki Y, Nakanuma Y. Alpha-smooth muscle actin-positive stromal cells in cholangiocarcinomas, hepatocellular carcinomas and metastatic liver carcinomas. J Hepatol. 1996;24:706–712. doi: 10.1016/s0168-8278(96)80267-4. [DOI] [PubMed] [Google Scholar]


