Abstract
Background: Nanog and CD133 are biomarkers of cancer stem cells (CSCs) that regulate cancer progression. The WW domain-containing oxidoreductase (WWOX) is a tumor suppressor protein that can inhibit tumor cell proliferation. The purpose of this study was to investigate the expression and clinical significance of Nanog, CD133, and WWOX in infiltrating breast cancer (IBC). Methods: Expressions of Nanog, CD133, and WWOX in 204 IBC specimens and their corresponding control specimens were detected by immunohistochemistry. Patients’ clinicopathologic and follow-up data were also collected. Results: The rates of positive expression of Nanog and CD133 were significantly higher in IBC specimens than in control specimens, and their expression was positively associated with tumor size, grade, and tumor stages, lymph node metastasis (LNM), and tumor-node-metastasis (TNM) stage. The rate of positive expression of WWOX was significantly lower in IBC specimens than in control specimens, and its expression was inversely associated with tumor size, grade, and tumor stages, LNM, and TNM stage. Patients whose specimens expressed Nanog, CD133, or HER2 had a reduced overall survival (OS) when compared with patients not expressing these proteins. However, patients whose specimens expressed WWOX, ER, or PR had an increased OS when compared with patients who did not show expression. Multivariate analysis demonstrated that expression of Nanog, CD133, WWOX, ER, and HER2, and the TNM stage were independent prognostic factors for IBC patients. Conclusions: Therefore, Nanog, CD133, and WWOX should be considered as promising prognostic factors and therapeutic targets in IBC.
Keywords: Infiltrating breast cancer, Nanog, CD133, WWOX, cancer stem cells, prognosis
Introduction
In 2018, there were an estimated 2.1 million new breast cancer cases worldwide, which accounted for approximately 1 in 4 cancer cases among women [1]. In China, there was an estimate of 270,000 new cases in 2015 [2]. Many breast cancer patients are diagnosed at advanced stages in China because the cancer does not show obvious symptoms during the early stages.
Tumor heterogeneity has emerged as a hallmark of the malignant state, leading to persistent growth, therapy resistance, and metastasis [3]. This heterogeneity may arise from a subpopulation of tumor cells called cancer stem cells (CSCs). CSCs are capable of self-renewal, multi-directional differentiation, and progression and are naturally resistant to chemo or radiotherapy.
Nanog is a homeodomain-containing transcription factor that plays a critical role in the self-renewal and maintenance of the embryonic stem cell (ESC) network [4,5]. The human Nanog gene is located at chromosome 12 and contains 4 exons and 3 introns. The protein consists of 305 amino acids and is divided into an N-terminal, homeobox domain, and C-terminal regions. Nanog is overexpressed in many cancers [6], and its knockdown or knockout can reduce cancer malignancy [7,8]. Moreover, Nanog overexpression has been often associated with poor prognosis in cancers such as head and neck squamous cell carcinoma, lung carcinoma, breast carcinoma, and colorectal carcinoma [5,9-12].
CD133, also known as prominin-1, is a common CSC biomarker. The protein is a member of the 5 transmembrane glycoprotein family and a cell surface marker of hematopoietic and progenitor cells. It has 97 kDa and contains an extracellular N-terminal domain, five transmembrane segments, two extracellular loops, and an intracellular C-terminal domain. CD133 overexpression often correlates with unfavorable overall survival (OS) and increased recurrence rates [13,14]. Cells expressing CD133 are resistant to chemo- and radiotherapy [15].
The inactivation of tumor suppressor genes is a hallmark of cancer [16] that leads to reduced OS. The WW domain-containing oxidoreductase (WWOX) was initially considered as a tumor suppressor gene in breast cancer [17]. Most cancer types inactivate WWOX, mostly by hemizygous but also by homozygous deletions [18]. WWOX is a 46 kDa protein that contains 2 N-terminal WW domains and a C-terminal domain [19]. Recent studies demonstrated that WWOX inactivation could cause tumorigenesis and promote tumor progression and angiogenesis [20], whereas increased WWOX expression inhibited tumor metastasis [21].
Nanog, CD133, and WWOX are associated with tumor progression and metastasis in different types of cancer. However, an association between these proteins and IBC has not been reported. This study aimed to assess the hypothesis that these proteins correlate with IBC progression and prognosis.
Materials and methods
Patients and specimens
We recruited 204 patients (median age: 50.8 years; range: 26-77 years) who were diagnosed with IBC from January 2011 to December 2012 by the Department of Pathology of our hospital and collected samples of cancer tissue and the corresponding normal mammary epithelial tissue form all patients. Patients who received any anti-cancer therapy were excluded. This study was approved by the ethical committee of Bengbu Medical University and carried out according to the guidelines issued in the Declaration of Helsinki. All patients provided written informed consents. Patients’ data included clinicopathologic characteristics, demography, and follow-up data. OS was calculated from the patients’ surgery date to their death date or to December 2017 (range: 11-82 months; mean OS: 56.0 months). The tumor-node-metastasis (TNM) stage was evaluated using the 8th edition of the guidelines issued by the American Joint Committee on Cancer (AJCC). See Table 1 for specific characteristics.
Table 1.
Patient characteristics
| Patient characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| < 50 | 134 | 65.7 |
| ≥ 50 | 70 | 34.3 |
| Location | ||
| Left | 103 | 50.5 |
| Right | 94 | 46.1 |
| Bilateral | 7 | 3.4 |
| Smoking | ||
| No | 170 | 83.3 |
| Yes | 34 | 16.7 |
| Size (cm) | ||
| ≤ 2.0 | 59 | 28.9 |
| 2.0 < S ≤ 5.0 | 122 | 59.8 |
| > 5.0 | 23 | 11.3 |
| Differentiation | ||
| G 1 | 50 | 24.5 |
| G 2 | 98 | 48.0 |
| G 3 | 56 | 27.5 |
| Tumor stage | ||
| T1 | 60 | 29.4 |
| T2 | 117 | 57.4 |
| T3 | 18 | 8.8 |
| T4a | 9 | 4.4 |
| Lymph node metastasis | ||
| N0 | 106 | 52.0 |
| N1 | 69 | 33.8 |
| N2 | 24 | 11.8 |
| N3 | 5 | 2.5 |
| TNM stagesII | ||
| I | 33 | 16.2 |
| II | 126 | 61.8 |
| III | 45 | 22.1 |
| ER expression | ||
| Negative | 94 | 46.1 |
| Positive | 110 | 53.9 |
| PR expression | ||
| Negative | 120 | 58.8 |
| Positive | 84 | 41.2 |
| HER2 expression | ||
| Negative | 145 | 71.1 |
| Positive | 59 | 28.9 |
Immunohistochemistry
The rabbit anti-human polyclonal antibodies against Nanog and WWOX, and the mouse anti-human monoclonal antibody against CD133 were purchased from Abcam, Co., Ltd (USA). The mouse anti-human monoclonal antibodies against HER2, ER, and PR and other reagents were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd (China). All the tissues were fixed in 10% formalin buffer solution, embedded in paraffin, and cut into 4-μm-thick sections. Immunohistochemistry was carried out following the ElivisionTM Plus method and the Kit instructions. A citrate buffer solution was used for antigen retrieval, and a solution of methanol containing 3% H2O2 was used to block endogenous peroxidase activity. Nanog, CD133, WWOX, ER, PR, and HER2 primary antibodies were added to all sections and incubated at 4°C overnight. Reagents A and B were added by turns. Finally, sections were developed in DAB substrate and re-dyed with hematoxylin.
Immunohistochemistry evaluation
We randomly selected ten high-power-fields (HPF) for each section to reduce potential intratumoral cell heterogeneity in biomarker expression. The intensity and extent of the immunohistochemistry were calculated as previously described [22]. Intensity: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. Extent: 1, < 11%; 2, 11%-50%; 3, 51%-75%; 4, > 75%. The final scores (range 0-12) were calculated by multiplying the intensity score by the extent score. We followed the 2013 ASCO/CAP guidelines to asses positive expression. HER2 expression in at least 10% and ER and PR expression in at least 1% of IBC cells was considered positive. A score above 2 indicated positive staining. For samples that were positive for Nanog, CD133, WWOX, ER, PR, and HER2, an average of the final score of each section was considered.
Statistical analysis
We used the Chi-square test to evaluate the expression of Nanog, CD133, and WWOX in the IBC and control tissues as well as the associations between their expression and the IBC clinicopathologic characteristics. The correlation analysis was performed using the Spearman correlation test. The univariate OS analysis was carried out using the Kaplan-Meier method with log-rank tests. The multivariate OS analysis was conducted using the Cox regression model test. P < 0.05 was considered significant. We acquired all data for statistical analysis using the SPSS 19.0 software (Chicago, IL).
Results
Expression of Nanog, CD133, and WWOX in IBC, and its association with clinicopathologic characteristics
The positive staining of Nanog was confined to the nuclei and cytoplasm of IBC cells, that of CD133 was confined to the cell membrane and cytoplasm, and that of WWOX was confined to the cell cytoplasm. Overall, 59.3% (121/204) of IBC cells and 2.0% (4/204) of normal breast epithelial cells were positive for Nanog expression (Figure 1A and 1B). The difference between the groups was significant (P < 0.001). Nanog expression positively correlated with tumor size, differentiation, and tumor stages, LNM, and TNM stages, but not with patient age, smoking status, and tumor location (Table 2).
Figure 1.
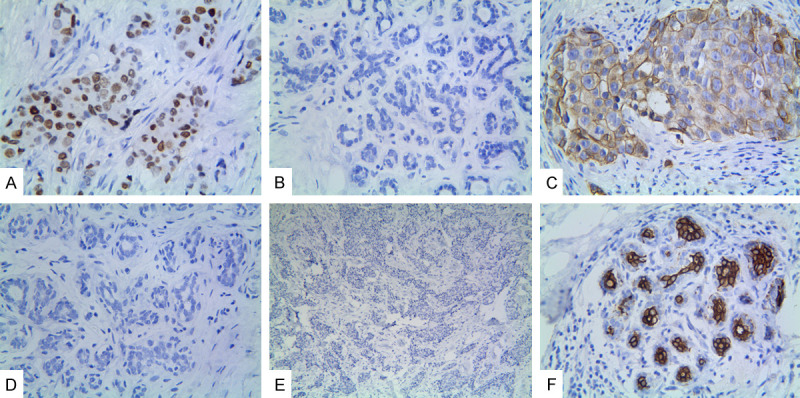
Expression of Nanog, CD133, and WWOX in invasive breast cancer. A. Positive Nanog expression in the cytoplasm and nucleus of cancer cells (×400 magnification). B. Negative Nanog expression in the “normal” breast epithelial cells (×100 magnification). C. Positive CD133 expression in the membrane and cytoplasm of cancer cells (×400 magnification). D. Negative CD133 expression in the “normal” breast epithelial cells (×100 magnification). E. Negative WWOX in the cancer cells (×40 magnification). F. Positive WWOX expression in the cytoplasm of “normal” breast epithelial cells (×100 magnification).
Table 2.
Associations between expression of Nanog, CD133, and WWOX and clinicopathologic characteristics of invasive breast carcinoma (IBC)
| Variable | Nanog | P | CD133 | P | WWOX | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| - | + | - | + | - | + | ||||
| Age (years) | 0.876 | 0.592 | 0.619 | ||||||
| < 50 | 54 | 80 | 53 | 81 | 70 | 64 | |||
| ≥ 50 | 29 | 41 | 25 | 45 | 34 | 36 | |||
| Location | 0.477 | 0.537 | 0.885 | ||||||
| Left | 46 | 57 | 40 | 63 | 52 | 51 | |||
| Right | 34 | 60 | 34 | 60 | 49 | 45 | |||
| Bilateral | 3 | 4 | 4 | 3 | 3 | 4 | |||
| Smoking | 0.483 | 0.122 | 0.168 | ||||||
| No | 71 | 99 | 69 | 101 | 83 | 87 | |||
| Yes | 12 | 22 | 9 | 25 | 21 | 13 | |||
| Size (cm) | < 0.001 | 0.014 | 0.002 | ||||||
| ≤ 2.0 | 38 | 21 | 31 | 28 | 23 | 36 | |||
| 2.0 < S ≤ 5.0 | 43 | 79 | 42 | 80 | 62 | 60 | |||
| > 5.0 | 2 | 21 | 5 | 18 | 19 | 4 | |||
| Differentiation | < 0.001 | < 0.001 | < 0.001 | ||||||
| G 1 | 39 | 11 | 34 | 16 | 13 | 37 | |||
| G 2 | 37 | 61 | 38 | 60 | 52 | 46 | |||
| G 3 | 7 | 49 | 6 | 50 | 39 | 17 | |||
| Tumor stage | < 0.001 | 0.046 | 0.005 | ||||||
| T1 | 39 | 21 | 31 | 29 | 22 | 38 | |||
| T2 | 40 | 77 | 41 | 76 | 61 | 56 | |||
| T3 | 2 | 16 | 4 | 14 | 14 | 4 | |||
| T4a | 2 | 7 | 2 | 7 | 7 | 2 | |||
| LNM | 0.001 | < 0.001 | < 0.001 | ||||||
| N0 | 57 | 49 | 55 | 51 | 34 | 72 | |||
| N1 | 20 | 49 | 21 | 48 | 45 | 24 | |||
| N2 | 6 | 18 | 1 | 23 | 20 | 4 | |||
| N3 | 0 | 5 | 1 | 4 | 5 | 0 | |||
| TNM stages | < 0.001 | < 0.001 | < 0.001 | ||||||
| I | 26 | 7 | 24 | 9 | 7 | 26 | |||
| II | 48 | 78 | 49 | 77 | 60 | 66 | |||
| III | 9 | 36 | 5 | 40 | 37 | 8 | |||
| ER expression | 0.074 | 0.255 | 0.011 | ||||||
| Negative | 32 | 62 | 32 | 62 | 57 | 37 | |||
| Positive | 51 | 59 | 46 | 64 | 47 | 63 | |||
| PR expression | 0.011 | 0.085 | 0.026 | ||||||
| Negative | 40 | 80 | 40 | 80 | 69 | 51 | |||
| Positive | 43 | 41 | 38 | 46 | 35 | 49 | |||
| HER2 expression | 0.002 | 0.002 | < 0.001 | ||||||
| Negative | 69 | 76 | 65 | 80 | 57 | 88 | |||
| Positive | 14 | 45 | 13 | 46 | 47 | 12 | |||
There was a significant difference (P < 0.001) in CD133 expression between the IBC (61.8%, 126/204) and control cells (12.3%, 25/204; Figure 1C and 1D). The expression of CD133 positively correlated with tumor size, differentiation, and tumor stages, LNM, and TNM stages, but not with patient age, smoking status, and tumor location (Table 2).
There were significantly fewer IBC cells (49.0%, 100/204) expressing WWOX than control cells (85.8%, 175/204; P < 0.001; Figure 1E and 1F). The positive expression of WWOX inversely correlated with tumor size, differentiation, tumor T stageLNM, and TNM stage, but not with patient age, smoking status, and tumor location (Table 2).
Univariate and multivariate analyzes
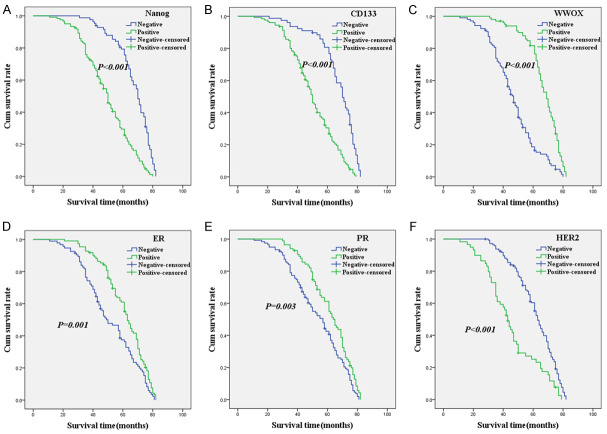
As shown in Figure 2A, the Kaplan-Meier survival analysis indicated that the OS time of IBC patients who expressed Nanog was significantly lower than that of patients who did not express the protein (log-rank = 54.217, P < 0.001). The OS time for CD133-positive patients was significantly lower than that of CD133-negative patients (log-rank = 53.793, P < 0.001; Figure 2B), showing similar results to those of Nanog expression. The relationship between WWOX expression and OS time was the opposite to that of Nanog and CD133, with patients who expressed WWOX surviving longer than those who did not express the protein (log-rank = 63.399, P < 0.001; Figure 2C). Moreover, OS was significantly associated with ER (log-rank = 10.061, P = 0.001; Figure 2D), PR (log-rank = 8.661, P = 0.003; Figure 2E), and HER2 (log-rank = 27.043, P < 0.001; Figure 2F) expression.
Figure 2.
Kaplan-Meier analysis of the survival rate of patients with IBC. The y-axis represents the percentage of patients; the x-axis represents their survival in months. (A) Overall survival of all patients in relation to Nanog (log-rank = 54.217, P < 0.001); (B) Overall survival of all patients in relation to CD133 expression (log-rank = 53.793, P < 0.001); (C) Overall survival of all patients in relation to WWOX expression (log-rank = 63.399, P < 0.001); (D) Overall survival of all patients in relation to ER expression (log-rank = 10.061, P = 0.001); (E) Overall survival of all patients in relation to PR expression (log-rank = 8.661, P = 0.001); (F) Overall survival of all patients in relation to HER2 expression (log-rank = 27.043, P < 0.001). In (A-F) analyses, the green line represents patients with positive expression of biomarkers and the blue line represents the negative expression of biomarkers.
The multivariate analysis suggested that the expression of Nanog, CD133, WWOX, ER, and HER2, as well as the TNM stage, were independent prognostic factors for IBC (Table 3).
Table 3.
Results of multivariate analyses of overall survival (OS) time
| Covariate | B | SE | P | HR | 95% CI |
|---|---|---|---|---|---|
| Nanog | 0.822 | 0.211 | < 0.001 | 2.276 | 1.505-3.443 |
| CD133 | 0.598 | 0.200 | 0.003 | 1.818 | 1.227-2.691 |
| WWOX | -0.598 | 0.182 | 0.001 | 0.550 | 0.385-0.786 |
| ER | -0.621 | 0.259 | 0.016 | 0.538 | 0.324-0.892 |
| HER2 | 0.462 | 0.189 | 0.014 | 1.588 | 1.096-2.299 |
| TNM stage | 0.534 | 0.268 | 0.046 | 1.706 | 1.008-2.886 |
Association between the expression of Nanog, CD133, WWOX, ER, PR, and HER2 and IBC
The Spearman correlation coefficient analysis showed a negative association between WWOX expression and Nanog (r = -0.485, P < 0.001), CD133 (r = -0.480, P < 0.001), or HER2 (r = -0.366, P < 0.001) expression and a positive relationship between WWOX expression and ER (r = 0.179, P = 0.011) or PR (r = 0.156, P = 0.026) expression. There was also a positive relationship between Nanog expression and CD133 (r = 0.416, P < 0.001) or HER2 (r = 0.220, P = 0.002) expression. The expression of Nanog negatively correlated with PR expression (r = -0.179, P = 0.010) and the expression of CD133 was positively associated with HER2 expression (r = 0.213, P = 0.002) (Table 4).
Table 4.
Correlation among expression of Nanog, CD133, and WWOX in IBC
| Variable | Nanog | r | P | CD133 | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | - | + | |||||
| Nanog | 0.416 | < 0.001* | ||||||
| - | 52 | 31 | ||||||
| + | 26 | 95 | ||||||
| WWOX | -0.485 | < 0.001@ | -0.480 | < 0.001@ | ||||
| - | 18 | 86 | 16 | 88 | ||||
| + | 65 | 35 | 62 | 38 | ||||
positive association;
negative association.
Discussion
IBC is the most common malignant tumor among women. Its heterogeneity makes it a threat to life and health. Therefore, it is urgent to find effective biomarkers that comprehensively predict the biologic behavior of this type of cancer.
Nanog is a biomarker of CSCs, whose overexpression can promote tumor cell proliferation, invasion, and resistance to therapy [23]. Inhibiting Nanog expression can induce apoptosis [23]. In this study, we investigated Nanog expression in IBC and the corresponding normal mammary (control) tissues and found that the IBC tissue expressed higher levels of the protein than control tissue. Moreover, Nanog expression positively correlated with tumor size, tumor stage, differentiation, and LNM and TNM stages. The OS analysis showed that IBC patients expressing Nanog survived for less time than patients who did not express Nanog. Our results suggested that Nanog overexpression is involved in the progression and metastasis of IBC and that Nanog should be considered as a biomarker to predict IBC prognosis.
CD133 is a CSC marker in IBC. Its overexpression can promote tumorigenesis and tumor cell proliferation, invasion, and metastasis [24,25]. In this study, CD133 overexpression was significantly associated with tumor size, tumor stage, differentiation, and LNM and TNM stages, which agrees with the results of previous studies [22,24-26]. The OS analysis indicated that patients whose tumor expressed CD133 survived for less time than those who did not express the protein. These results indicated that CD133 expression plays an important role in IBC progression, invasion, and metastasis and that the protein is associated with poor prognosis, which is consistent with previous studies [22,24-27].
WWOX acts as a tumor suppressor in human cancer, it suppresses tumorigenesis by inducing apoptosis and inhibiting tumor cell proliferation, invasion, metastasis, and angiogenesis [28,29]. Our data demonstrated that WWOX expression was inversely associated with tumor size, tumor stage, differentiation, and LNM and TNM stages. Furthermore, the OS analysis indicated that patients expressing WWOX lived longer than those who did not express the protein. These results suggested that the reduction or loss of WWOX expression promoted IBC progression and metastasis and should be associated with a poor prognosis, which is in accordance with other studies [27,30-32].
The results of our study showed that the expression of Nanog, CD133, WWOX, ER, and HER2 and the TNM stage were independent prognostic factors of OS for patients with IBC. We also demonstrated that WWOX expression was negatively associated with Nanog, CD133, and HER2 expression and positively associated with ER and PR expression and that Nanog expression was positively associated with CD133 expression.
Previous studies have suggested that CSCs promote tumorigenesis [33]. CSCs can induce malignant transformation partly by activating the Wnt/β-catenin signaling pathway [34]. Overexpression of Nanog and CD133 is considered to cause IBC progression and metastasis. WWOX is considered to be associated with hormonal status and breast carcinoma [35]. Normal WWOX can suppress cells’ tumorigenicity and decrease cells invasive ability [36]. Aberrant expression of WWOX can cause cell proliferation, mobility, migration, and metastasis. Therefore, we speculate that the overexpression of Nanog and CD133 and the underexpression of WWOX synergistically promote IBC cell proliferation, progression, and metastasis.
Conclusions
The results of this study suggested that the overexpression of Nanog and CD133 and that underexpression of WWOX could affect the initiation, progression, and metastasis of IBC. Therefore, Nanog, CD133, and WWOX should be considered as valuable biomarkers to predict metastasis and prognosis in IBC patients.
Acknowledgements
This work was supported by the Nature Science Key Program of College and University of Anhui Province (No. KJ2018A0990).
Disclosure of conflict of interest
None.
Abbreviations
- IBC
infiltrating breast carcinoma
- WWOX
WW domain-containing oxidoreductase
- LNM
lymph node metastasis
- TNM
tumor-node-metastasis
- OS
overall survival
- CSCs
cancer stem cells
- ESC
embryonic stem cell
- AJCC
American Joint Committee on Cancer
- HPF
high-power-field
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan PS, Sinyuk M, Turaga SM, Mulkearns-Hubert EE, Hale JS, Rao V, Demelash A, Saygin C, China A, Alban TJ, Hitomi M, Torre-Healy LA, Alvarado AG, Jarrar A, Wiechert A, Adorno-Cruz V, Fox PL, Calhoun BC, Guan JL, Liu H, Reizes O, Lathia JD. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat Commun. 2018;9:578. doi: 10.1038/s41467-018-02938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang P, Andrianakos R, Yang Y, Liu C, Lu W. Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem. 2010;285:9180–9. doi: 10.1074/jbc.M109.077958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X, Piao L, Cavey GS, Old M, Teknos TN, Mapp AK, Pan Q. Phosphorylation of nanog is essential to regulate bmi1 and promote tumorigenesis. Oncogene. 2014;33:2040–52. doi: 10.1038/onc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292–303. doi: 10.1016/j.canlet.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Yu AQ, Ding Y, Li CL, Yang Y, Yan SR, Li DS. TALEN-induced disruption of Nanog expression results in reduced proliferation, invasiveness and migration, increased chemosensitivity and reversal of EMT in HepG2 cells. Oncol Rep. 2016;35:1657–63. doi: 10.3892/or.2015.4483. [DOI] [PubMed] [Google Scholar]
- 8.Kawamura N, Nimura K, Nagano H, Yamaguchi S, Nonomura N, Kaneda Y. CRISPR/Cas9-mediated gene knockout of NANOG and NANOGP8 decreases the malignant potential of prostate cancer cells. Oncotarget. 2015;6:22361–74. doi: 10.18632/oncotarget.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–95. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 10.Chang B, Park MJ, Choi SI, In KH, Kim CH, Lee SH. NANOG as an adverse predictive marker in advanced non-small cell lung cancer treated with platinum-based chemotherapy. Onco Targets Ther. 2017;10:4625–33. doi: 10.2147/OTT.S144895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Zhang F, Yu M, Zhao P, Ji W, Zhang H, Wu B, Wang Y, Niu R. RNA interference-mediated silencing of NANOG reduces cell proliferation and induces G0/G1 cell cycle arrest in breast cancer cells. Cancer Lett. 2012;32:80–8. doi: 10.1016/j.canlet.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Osama A, Sabry D, Hassany SM, Abdelmoneim SS, Sabry A. SIRT-1expression is associated with expression of NANOG in patients with colorectal adenocarcinoma. Cancer Biomark. 2016;17:61183–98. doi: 10.3233/CBM-160626. [DOI] [PubMed] [Google Scholar]
- 13.Haeng RS. Roles of tumor microenvironment in hepatocelluar carcinoma. Current Cancer Therapy Reviews. 2015;11:82–93. [Google Scholar]
- 14.Huang R, Mo D, Wu J, Ai H, Lu Y. CD133 expression correlates with clinicopathologic features and poor prognosis of colorectal cancer patients. An updated meta-analysis of 37 studies. Medicine (Baltimore) 2018;97:e10446. doi: 10.1097/MD.0000000000010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Kim IK, Choi I, Kim SH, Seo HR. Oxytetracycline have the therapeutic efficiency in CD133+ HCC population through suppression CD133 expression by decreasing of protein stability of CD133. Scientific Rep. 2018;8:16100. doi: 10.1038/s41598-018-34301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–5. [PubMed] [Google Scholar]
- 18.Baryla I, Styczen-Binkowska E, Bednarek AK. Alteration of WWOX in human cancer, a clinical view. Exp Bio Med. 2015;240:305–314. doi: 10.1177/1535370214561953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sze CI, Kuo YM, Hsu LJ, Fu TF, Chiang MF, Chang JY, Chang NS. A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency. Cell Death Dis. 2015;6:e1881. doi: 10.1038/cddis.2015.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue X, Zhou L, Song W, et al. ORAOV1 and WWOX are metastatic and prognostic biomarker for infiltrating breast cancer. Int J Clin Exp Med. 2017;10:13607–15. [Google Scholar]
- 21.Del Mare S, Aqeilan RI. Tumor Suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci Rep. 2015;5:12959. doi: 10.1038/srep12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun AX, Liu CJ, Sun ZQ, Wei Z. NANOG: a promising target for digestive malignant tumors. World J Gastroenterol. 2014;20:13071–8. doi: 10.3748/wjg.v20.i36.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Guan H, Liu XD, Xie DF, Wang Y, Ma T, Huang B, Zhou PK. p53 positively regulates the expression of cancer stem cell marker CD133 in HCT116 colon cancer cells. Oncol Lett. 2018;16:431–8. doi: 10.3892/ol.2018.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying X, Wu J, Meng X, Zuo Y, Xia Q, Chen J, Feng Y, Liu R, Li L, Huang W. AC133 expression associated with poor prognosis in stage II colorectal cancer. Med Oncol. 2013;30:356. doi: 10.1007/s12032-012-0356-z. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Zhou L, Wu S, Gong X, Feng Z, Ma L, Zhu B, Yao N, Wang D, Dong H. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J Surg Oncol. 2014;12:118. doi: 10.1186/1477-7819-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu G, Zhou L, Song W, Wu S, Zhu B, Wang D. Expression of ORAOV1, CD133 and WWOX correlate with metastasis and prognosis in gastric adenocarcinoma. Int J Clin Exp Pathol. 2017;10:8916–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Abu-Odeh M, Salah Z, Herbel C, Hofmann TG, Aqeilan RI. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc Natl Acad Sci U S A. 2014;111:E4716–25. doi: 10.1073/pnas.1409252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang R, Song L, Xu Y, Wu Y, Dai C, Wang X, Sun X, Hou Y, Li W, Zhan X, Zhan L. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat Commun. 2018;9:3486. doi: 10.1038/s41467-018-05852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maroni P, Matteucci E, Bendinelli P, Desiderio MA. Functions and epigenetic regulation of wwox in bone metastasis from breast carcinoma: comparison with primary tumors. Int J Mol Sci. 2017;18:75. doi: 10.3390/ijms18010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Płuciennik E, Kusińska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX--the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol. 2006;32:153–7. doi: 10.1016/j.ejso.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat. 2005;89:99–105. doi: 10.1007/s10549-004-1474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 34.Hou MF, Chen PM, Chu PY. LGR5 overexpression confers poor relapse-free survival in breast cancer patients. BMC Cancer. 2018;18:219. doi: 10.1186/s12885-018-4018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer. 2004;100:1605–14. doi: 10.1002/cncr.20137. [DOI] [PubMed] [Google Scholar]
- 36.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian Cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res. 2009;69:4835–42. doi: 10.1158/0008-5472.CAN-08-2974. [DOI] [PubMed] [Google Scholar]