Abstract
Lung cancer has the highest mortality and recurrence rate among cancers in the world. Receptor tyrosine kinase-like orphan receptor 1 (ROR1) has been widely recognized for its role in promoting the growth and metastasis of lung cancer, but its comprehensive role and molecular mechanisms in regulating cell cycle, apoptosis, and autophagy remain unclear. In this study, a series of ROR1-stably silenced monoclonal clones from lung adenocarcinoma cell lines PC9, PC9erlo, and NCI-H1975 were successfully selected and confirmed by qRT-PCR, western blot, and flow cytometry, and used as cell models in the following assays. Our study clearly shows that blocking ROR1 significantly downregulates cell cycle-inducing molecules such as CDK4 and Cyclin E1, and anti-apoptotic molecules such as Bcl-XL and Bcl-2, while it markedly upregulates pro-apoptotic molecules such as Bak, Caspase-3, and Caspase-7, which extends our previous observation on the molecular mechanism of ROR1-mediated tumor growth in lung adenocarcinoma. Our data also show that silencing ROR1 promotes autophagy since the key molecules involved in autophagy including ATG7, ATG12, BNIP3L, LC3A, LC3B, and NBS1 were up-regulated. We further screened key phosphokinase signaling pathways downstream of ROR1 in lung adenocarcinoma by a human phospho-kinase array. Our data indicate that blocking ROR1 could deactivate Akt, then activate GSK-3α/β by de-phosphorylation, and finally deactivate mTOR. In this way blocking ROR1 could effectively regulate the cell cycle, apoptosis, and autophagy in lung cancer.
Keywords: ROR1, lung adenocarcinoma, apoptosis, cell cycle, autophagy
Introduction
Lung cancer remains the leading cause of cancer morbidity and mortality [1]. In 2018, 2.1 million new lung cancer cases were reported, accounting for nearly a fifth (18.4%) of cancer deaths [2]. Although until now ten types of standard therapeutic options including surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy [3-7] are available for patients with lung cancer and shown exciting treatment efficacy in small portion of patients, especially those with late-stage metastasis, the overall 5-year survival rate is still poor, which impels more study on the molecular and pathogenetic mechanisms of lung cancer [8].
Oncogene receptor tyrosine kinase-like orphan receptor 1 (ROR1) is expressed during embryonic development [9,10], but found to be overexpressed in several types of hematologic neoplasms including chronic lymphocytic leukemia (CLL) [11,12] and acute lymphoblastic leukemia (ALL) [13], and a variety of solid tumors such as breast cancer [14-16], colorectal carcinoma [17], ovarian carcinoma [18], melanoma [19,20], as well as lung adenocarcinoma [21,22]. ROR1 has been widely known for its vital roles in promoting the growth and metastasis of tumor, inducing drug-resistance and enhancing apoptosis-resistance, which makes it a feasible target for tumor therapy [23,24], but little is known about its roles and downstream signaling cascades in regulating the cell cycle and autophagy in lung adenocarcinoma.
Autophagy is a cell mechanism by which cellular components are subjected to the organized degradation and recycling, which has raised great interest in its role in tumorigenesis [25,26]. Moreover, increasing studies have shown that cell cycle, apoptosis and autophagy are closely linked and may positively and negatively regulate each other [27], so it is interesting to know whether ROR1 plays a role in regulating the cell cycle and autophagy. Based on our previous work which showed that ROR1 protein is widely expressed in both tissues and cell lines of lung adenocarcinoma, and that silencing ROR1 with small interfering RNA (siRNA) can induce tumor cell death and apoptosis [22,23,28], here in this study we continue to investigate the underlying molecular mechanism of ROR1 in regulating the cell cycle and autophagy in ROR1-stably silenced lung adenocarcinoma cell lines.
Materials and methods
Cell Lines and cell culture
Three human lung adenocarcinoma cell lines were used in our study: the erlotinib-sensitive cell line PC9 was kindly provided by Dr. Jun Zhang of Shanghai Pulmonary Hospital; the primary erlotinib-resistant cell line NCI-H1975 was obtained from the stem cell bank of Chinese Academy of Sciences. Those two cell lines were cultured in RPMI-1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Transgen, Beijing, China) and 1% penicillin/streptomycin (HyClone, Logan, UT, USA). The acquired erlotinib-resistant cell line PC9erlo was established in our laboratory as reported before [29] and cultured in complete RPMI-1640 culture medium containing 2.5 μM erlotinib to maintain drug resistance. All the cells were incubated at 37°C in a 95% humidified air incubator (Panasonic, Ehime, Japan) containing 5% CO2.
Infection with lentivirus-mediated small-hairpin RNA (shRNA)
The packaged lentiviruses containing shRNA with a specific human ROR1 targeting sequence [23,25,26] (Sense 5’-GUACUGCGAUGAAACUUCATT-3’; antisense 5’-UGAAGUUUCAUCGCAGUACGG-3’) (HBLV-H-ROR1-shRNA1-GFP-PURO, Lv-shROR1, titer 1 × 108 TU/mL) or nonrelated shRNA (HBLV-GFP-PURO, Lv-shCon, titer 2 × 108 TU/mL) were purchased from HanBio Biotechnology Co. (Shanghai, P. R. China). Lung adenocarcinoma cell lines PC9, PC9erlo, and NCI-H1975 were infected with Lv-shROR1 or Lv-shCon as below: cells were seeded in 96-well plates at a density of 3 × 103 cells/well and infected with lentiviruses at various multiplicity of infection (MOI) (PC9 MOI 10, PC9erlo MOI 10 and NCI-H1975 MOI 20) with 5 μg/mL polybrene for 24 h. 1.5 μg/mL puromycin was used 48 h later to maintain the selection pressure. The efficiency of lentivirus infection was determined by green fluorescent protein (GFP) fluorescence intensity measured under inverted fluorescence microscope (Nikon ECLIPSE Ti, Tokyo, Japan). Monoclonal cells (clones) were selected by finite dilution method, and PC9 and NCI-H1975 clones were subcultured in complete RPMI-1640 culture medium containing 1.5 μg/mL puromycin, and PC9erlo clones were subcultured in complete RPMI-1640 culture medium containing 2.5 μM erlotinib and 1.5 μg/mL puromycin.
Flow cytometry
Cells were collected and washed with phosphate-buffered saline (PBS) at 300 g for 5 min, then cells were incubated with 5 μg/mL chimeric rabbit/human anti-ROR1 monoclonal antibody R12 as reported before [30] ornormal human IgG antibodies (Code#: 009-000-003, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min at 4°C. After washing with flow cytometry buffer (PBS+10% FBS), cells were further incubated with HA-Tag (6E2) mouse mAb (Alexa Fluor® 647 Conjugate) (Cat#: 3444, 1:1000,Cell Signaling Technology, Danvers, MA, USA) for 30 min at 4°C. Cells were captured on NovoCyte flow cytometry (ACEA Biosciences, Inc., San Diego, California, USA) and analyzed with FlowJo-V10 software (FlowJo LLC, Ashland, OR, USA). The formula used to calculate the inhibition rate of ROR1 expression level in different cell lines was: [value (Lv-shCon) - value (background)] - [value (Lv-shROR1) - value (background)]/[value (Lv-shCON) - value (background)] × 100% (value is the median fluorescence intensity of ROR1 in different groups).
Western blot analysis
Protein extraction from cell lines was prepared using RIPA lysis buffer (Beyotime Institute of Biotechnology, Hangzhou, China) containing 1% proteinase inhibitor (Millipore, Bedford Park, IL, USA) and 10% phosphatase inhibitor(Roche Diagnostics, Basel, Switzerland) and the concentration of protein was analyzed by BCA protein assay kit(Beyotime Institute of Biotechnology, Hangzhou, China). Protein was separated by SDS-PAGE and transferred to 0.45 μM PVDF membrane (Millipore, Bedford Park, IL, USA). After blocking with 5% skim milk at 37°C for 1 h, membranes were incubated with antibodies. The first antibodies used in these studies were ROR1 (D6T8C) (Cat#: 16540, 1:1000), Akt (Cat#: 2920S, 1:2000), P-Akt (S473) (Cat#: 4060S, 1:1000), GSK-3α/β (D7503) (Cat#: 5676S, 1:1000), P-GSK-3α/β (S21/9) (Cat#: 8566S, 1:1000), mTOR (Cat#: 2972S, 1:1000), P-mTOR (Ser2448) (Cat#: 5536S, 1:1000), CDK4 (D9G3E) (Cat#: 12790, 1:1000), Cyclin E1 (D7T3U) (Cat#: 20808, 1:1000), Bcl-XL (54H6) (Cat#: 2764, 1:1000), Bak (D4E4) (Cat#: 12105, 1:1000), Caspase-3 (8G10) (Cat#: 9665, 1:1000), Caspase-7 (D2Q3L) (Cat#: 12827, 1:1000), β-actin (Cat#: HC201, Mouse, 1:1000) (Cell Signaling Technology, Danvers, MA, USA) and Bcl-2 (Cat#: 51-6511GR, 1:500; BD Biosciences, Franklin Lake, New Jersey, USA). The secondary antibodies used in these studies were horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (Cat#: HS101-01, 1:5000) and HRP-conjugated anti-mouse antibody (Cat#: HS201-01, 1:5000;Transgen Biotech Co., Ltd., Beijing, China). Chemiluminescence was detected using enhanced Pierce ECL kit (Foregene Company Limited, Chengdu, China). Images were captured by molecular imager ChemiDocTM XRS+ Imaging system (Bio-Rad, Laboratories, Hercules, CA, USA) and analyzed by Image Lab 6.0 software (Bio-Rad, Laboratories, Hercules, CA, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Cells were seeded in six-well plates and total RNA was extracted by Trizol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and transcribed into cDNA by SuperScriptTM III reverse transcriptase (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The predesigned ROR1 primers and human GAPDH endogenous reference genes primers used in the study were purchased from Shanghai Bioengineering Co., Ltd. (Shanghai, China) with the primer sequences: ROR1 upstream: 5’-CAGACACAGGCTACTTCCAGTGC-3’, ROR1 downstream: 5’-CTCCATATAGACGGTGCGGTTGC-3’. The reaction condition of PCR with PowerUp SYBR Green master mix (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was as follows: 94°C for 2 min followed by 35 cycles of denaturation 94°C for 3 s, and annealing/extension 60°C for 30 s. The relative gene expression was normalized to internal GAPDH, and the fold difference in the expression of ROR1 was calculated by Livak method (2-ΔΔCt). All samples were done in triplicate.
Human autophagy array
Protein extraction from cell lines was performed as mentioned for western blot assay and 20 human proteins related to autophagy were semi-quantitatively determined by RayBio® C-Series Human Autophagy Array 1 (Cat#: AAH-ATG-1-8, RayBiotech, Inc., Atlanta, GA, USA) following the protocol provided by the manufacturer. Briefly, 500 μg of cell lysate protein was incubated with specific antibodies precoated on the membrane at 4°C overnight. After washing, the membrane was first incubated with biotinylated antibody cocktail at room temperature (RT) for 2 h, then incubated with HRP-conjugated streptavidin for 1 h at RT. The membrane was washed and chemiluminescence was developed by enhanced Pierce ECL kit (Foregene Company Ltd., Chengdu, China). The density of each spot pixel on the membrane was determined by image lab 6.0 software (Bio-Rad, Laboratories, Hercules, CA, USA) and calculated by AAH-ATG-1 analysis tools provided by RayBiotech, Inc. (Atlanta, USA).
Human phospho-kinase array
The relative levels of protein phosphorylation were determined by proteome profiler human phospho-kinase array kit (Cat#: ARY003B, R&D Systems, Minnesota, USA). Protein extraction was performed as mentioned in western blot assay. Briefly, 400 μg of cell lysate protein was incubated with the microarrays which have been precoated with antibodies against 43 kinase phosphorylation sites and 2 related total proteins at 4°C overnight. After washing, the microarrays were incubated with biotin-labeled antibodies at RT for 2 h, then microarrays were incubated with HRP-conjugated streptavidin at RT for 30 min on a rocking platform. Chemiluminescence and Density of spot pixels was measured as mentioned in a human autophagy array.
Statistical analysis
Quantitative data were expressed as mean ± SEM of at least three independent experiments. Data were analyzed by one-way ANOVA (LSD method) or Student t test using Graphpad prism 7.0a software (San Diego, CA, USA). A value of P<0.05 was considered significant.
Results
Silencing ROR1 with lentivirus-mediated shRNA in lung adenocarcinoma cell lines
In our previous study, we investigated the role of ROR1 by transiently blocking ROR1 expression by siRNA. Here we aimed to establish ROR1-stably-silenced clones from PC9, PC9erlo, and NCI-H1975 cell lines, and study the long-term effect of ROR1 blocking in lung adenocarcinoma. Our data showed that cells were successfully infected with Lv-shROR1 and Lv-shCon as demonstrated by GFP expression under a fluorescence microscope (Figure 1A). Meanwhile, the ROR1-blocking efficacy of Lv-shROR1 was verified by flow cytometry with inhibition rates of 55% in PC9, 50% in PC9erlo, and 35% in NCI-H1975, respectively (Figure 1B).
Figure 1.
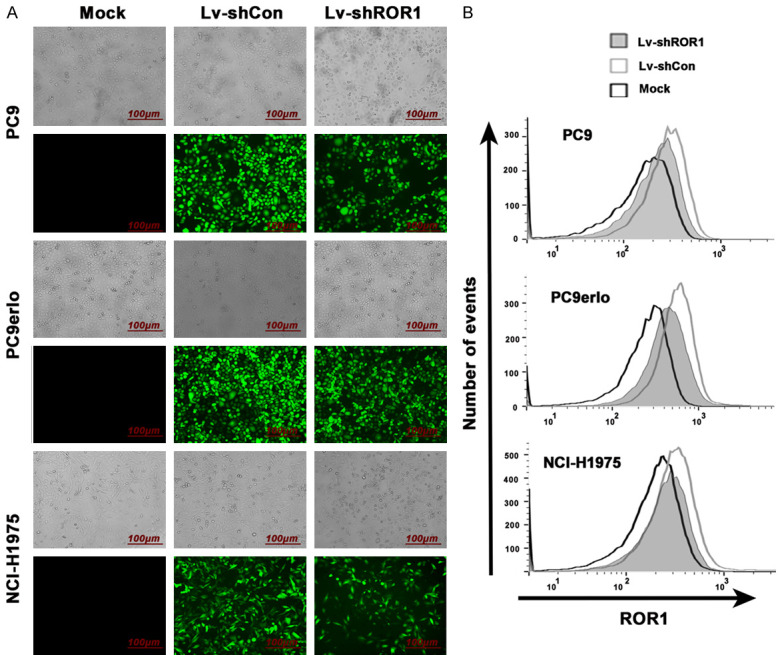
Silencing of ROR1 with lentivirus-mediated shRNA in lung adenocarcinoma cell lines. The infection efficiency was demonstrated by GFP fluorescence intensityunder fluorescence microscope (A) and the relative expression levels of ROR1 were analyzed by flow cytometry (B). Mock, cells cultured with RPMI-1640 culture medium with 10% FBS alone; Lv-shCon, cells infected with lentivirus containing non-related shRNA; Lv-shROR1, cells infected with lentivirus containing ROR1-specific shRNA.
Establishment of ROR1-stably-silenced clones from lung adenocarcinoma cell lines
The ROR1-stably-silenced clones were screened from cell lines PC9, PC9erlo, and NCI-H1975 which have been infected with Lv-shROR1 through the finite dilution method. In total, 13 clones (4 clones from PC9, 4 clones from PC9erlo, and 5 clones from NCI-H1975) were selected, expanded, and prepared for identification. Firstly, the protein expression level of ROR1 was detected by western blot which indicated that the ROR1-inhibition rates were higher in clones R3 and R4 from PC9, clones R1 and R2 from PC9erlo, and clones R3 and R5 from NCI-H1975 (Figure 2). Next, those six clones (Clones R3 and R4 from PC9, clones R1 and R2 from PC9erlo, and clones R3 and R5 from NCI-H1975) were selected and we further detected ROR1 mRNA and protein expressions by qRT-PCR (Figure 3B) and flow cytometry assay (Figure 3C), respectively. Our results showed that compared with clones infected with Lv-shCon (MN), the inhibition rates of surface ROR1 in clones R3 and R4 from PC9 were 65% and 89%, respectively, clones R1 and R2 from PC9erlo were 80% and 80%, respectively, and clones R3 and R5 from NCI-H1975 were 42% and 33%, respectively. The data from qRT-PCR also had similar results which further confirmed that we successfully established clones with ROR1 stably-silenced from lung adenocarcinoma cell lines. The fluorescence images of the six clones are shown in Figure 3A.
Figure 2.
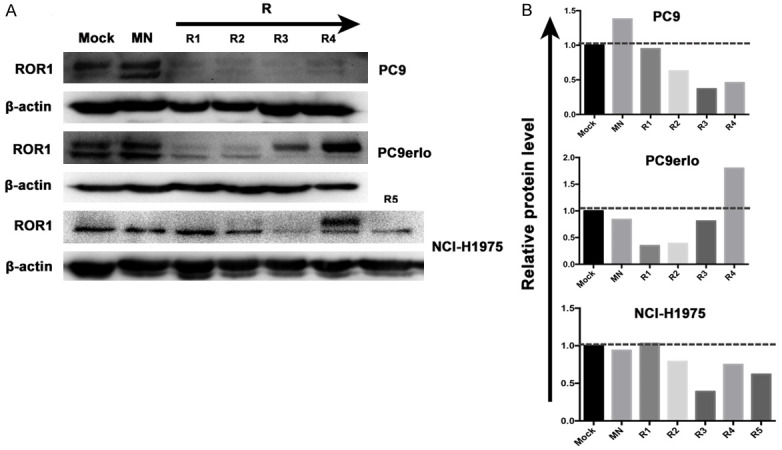
Selection of ROR1 stably-silenced clones from lung adenocarcinoma cell lines by western blot. Clones (From left to right: R1, R2, R3, R4 and R5) were screened from cell lines PC9, PC9erlo, and NCI-H1975 which have been infected with Lv-shROR1 by finite dilution method (A). The integrated density analysis showed the changes in expression (B). Mock, cells cultured with RPMI-1640 culture medium with 10% FBS alone; MN, clones selected from cells infected with Lv-shCon; R, clones selected from cells infected with Lv-shROR1.
Figure 3.
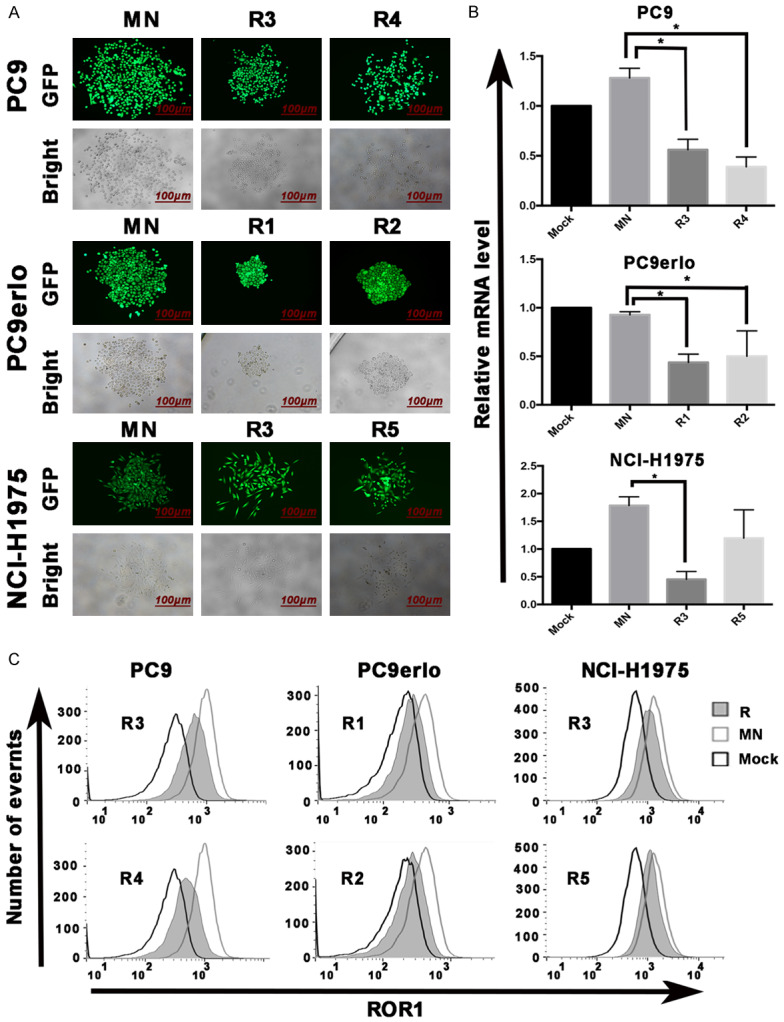
Identification of clones with ROR1 stably-silenced by RT-PCR and flow cytometry. The infection efficiency of Lv-shROR1 in selected clones (R3 and R4 from PC9, R1 and R2 from PC9erlo, R3 and R5 from NCI-H1975) was demonstrated by GFP fluorescence intensity under fluorescence microscope (A) and the relative expression levels of ROR1 were analyzed by qRT-PCR (B) and flow cytometry (C), respectively. Asterisks indicate statistically significant differences compared with the non-related shRNA group (*P<0.05). Statistical analyses was performed using one-way ANOVA (LSD method) (R3 and R4 from PC9, R1 and R2 from PC9erlo, R3 and R5 from NCI-H1975 compared with MN). Mock, cells cultured with RPMI-1640 culture medium with 10% FBS alone; MN, clones selected from cells infected with Lv-shCon; R, clones selected from cells infected with Lv-shROR1.
Silencing ROR1 downregulates the expression of cell cycle-related molecules
We and several other groups have shown that the signal from ROR1 sustains tumor cell proliferation in lung adenocarcinoma. We wondered whether ROR1 also plays a role in regulating the cell cycle, thus enhancing tumor cell growth. Key regulators in the cell cycle were analyzed by western blot in clone R1 from cell line PC9erlo. Compared with clone infected with Lv-shCon (MN), the expression levels of Cyclin E1 and CDK4, two key players in DNA synthesis and G1/S-phase progression, were markedlydown-regulated (Figure 4A, 4B) which supported our hypothesis that ROR1 might play an essential role in lung adenocarcinoma growth by upregulation of cell cycle related proteins.
Figure 4.
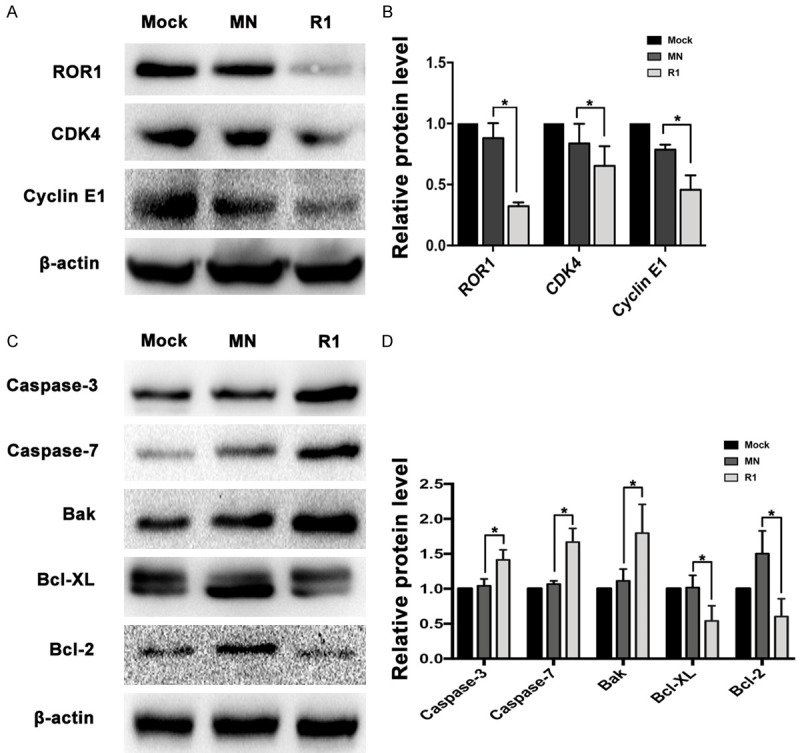
ROR1 regulates cell cycle- and apoptosis-related molecules. The expression levels of ROR1 and cell cycle-related molecules (A) and apoptosis-related molecules (C) and β-actin were detected by western blot. The integrated density analysis showed the changes of expression (B, D). Asterisks indicate statistically significant differences compared with the non-related shRNA group (*P<0.05). Statistical analyses were performed using Student’s T test (R1 compared with MN). Mock, cells cultured with RPMI-1640 culture medium with 10% FBS alone; MN, clone selected from PC9erlo cell lines infected with Lv-shCon; R1, clone 1 selected from PC9erlo cell lines infected with Lv-shROR1.
Silencing ROR1 upregulates the expression of pro-apoptotic molecules
Our previous data have shown that silencing ROR1 by siRNA induces tumor cell apoptosis in lung adenocarcinoma [23]. Here, we further assessed the expression levels of pro- or anti-apoptotic molecules in clone R1 from PC9erlo. We found that silencing ROR1 noticeably decreased the expression of anti-apoptotic molecules such as Bcl-2 and Bcl-XL, while it upregulated pro-apoptotic molecules such as Caspase-3, Caspase-7, and Bak (Figure 4C, 4D). Our results reinforced our previous data that ROR1 induces apoptosis-resistance by regulating the activity of pro- or anti-apoptotic players in lung adenocarcinoma cell lines.
Silencing ROR1 upregulates the expression of molecules involved in autophagy
Autophagy is an evolutionarily-conserved and genetically programmed lysosomal degradation pathway, but its role in cancer still needs to be clarified. Increasing evidence demonstrates the intimate interactions among the cell cycle, apoptosis, and autophagy. Hence, it is interesting to know whether blocking ROR1 can regulate autophagy. In this study, we screened 20 autophagy-related proteins in clone R1 from PC9erlo by a commercially available human autophagy array kit and found that several players in the autophagy process such as autophagy-related gene 7 (ATG7), ATG12, LC3A, LC3B, BNIP3L, and NBS1 were relatively upregulated after ROR1 was silenced (Figure 5).
Figure 5.
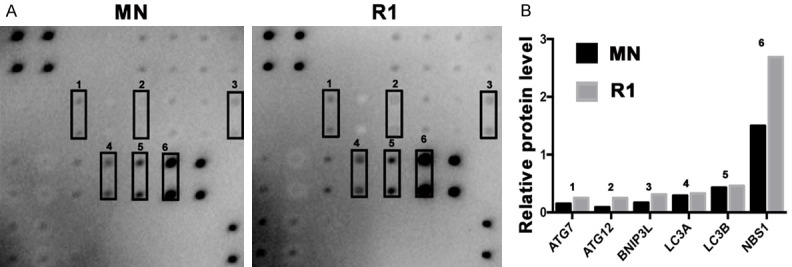
ROR1-silenced induces autophagy. Twenty autophagy-related proteins were screened in clone R1 from PC9erlo by a commercially available human autophagy array kit (A). The relative expression levels of autophagy-related proteins were graphed (B). MN, clone selected from PC9erlo cell lines infected with Lv-shCon; R1, clone 1 selected from PC9erlo cell lines infected with Lv-shROR1.
Screening of ROR1-mediated phospho-kinase signaling pathways in lung adenocarcinoma cell lines
Although the downstream signal cascades of ROR1 have been reported in several groups including ours [22,23,28,31], the overall screening of key phospho-kinase signaling pathways downstream of ROR1 in lung adenocarcinoma has not been reported yet. In this study, we use a human phospho-kinase array to simultaneously detect the relative phosphorylation levels of 43 human protein kinases in clone R1 from PC9erlo cell lines. We found that after ROR1 blocking, the phosphorylation levels of GSK-3α/β (S21/S9) and Akt123 (S473) were down-regulated (Figure 6A, 6B). We further detected the activity of key molecules in the Akt/GSK-3α/β/mTOR pathway by western blot assay, and found that the phosphorylation levels of GSK-3α/β (S21/S9), Akt1/2/3 (S473), and mTOR (Ser2448) were significantly reduced after ROR1-silencing (Figure 6C).
Figure 6.
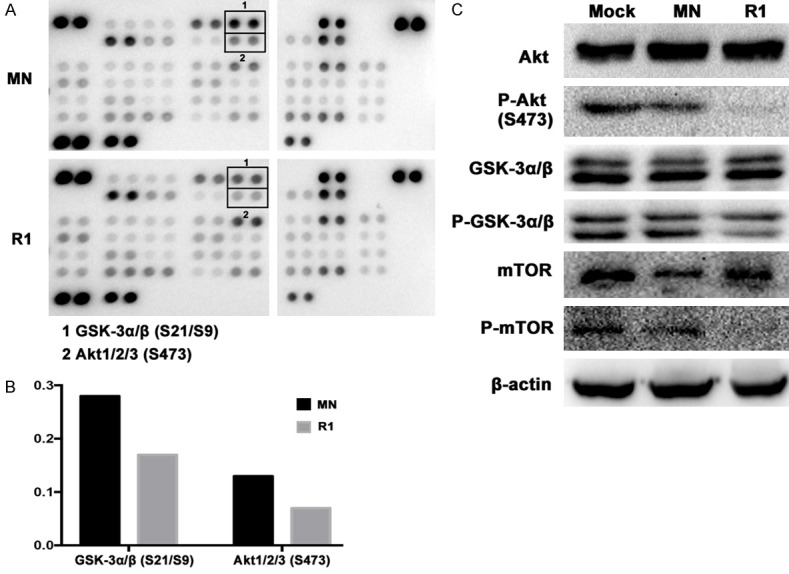
Screening ROR1-mediated phospho-kinase signaling pathways. Detecting the relative phosphorylation levels of 43 human protein kinases in clone R1 from PC9erlo cell lines by a human phospho-kinase assay kit (A). The relative expression levels of Akt and GSK-3α/β were graphed (B). The phosphorylation levels of Akt, GSK-3α/β, mTOR and β-actin were detected by western blot (C). MN, clone selected from PC9erlo cell lines infected with Lv-shCon; R1, clone 1 selected from PC9erlo cell lines infected with Lv-shROR1.
Discussion
Although it is well known that ROR1 plays important roles in tumor growth, metastasis, and drug-resistance, most of those studies employed siRNA strategy to silence ROR1 transiently, thus, not reflecting the long-term effects of ROR1 blocking in tumors [23,28]. Therefore, in this study we used lentivirus-mediated shRNA instead of siRNA to silence ROR1 permanently and a series of ROR1-stably-silenced clones from lung adenocarcinoma cell lines PC9, PC9erlo, and NCI-H1975 were selected, identified, and used as cell models for follow-up functional study.
Our previous data have demonstrated the cell-proliferating role of ROR1 in lung adenocarcinoma, but little is known about the expression patterns of apoptotic- and cell cycle-related molecules after ROR1 blocking [23]. In this study, the data clearly showed that pro-apoptotic molecules including Bak, Caspase-3, and Caspase-7, were significantly upregulated, while anti-apoptotic molecules including Bcl-2 and Bcl-XL, were markedly downregulated in ROR1-stably-silenced clones, which extend our previous observations and further explore the molecular mechanism of ROR1-mediated apoptosis-inhibition in lung adenocarcinoma. We also detected the expression levels of key cell cycle regulators and found that the expression levels of CDK4 and Cyclin E1 were significantly reduced after ROR1 blocking. It is well known that CDK4 associates with cyclin D along with its inhibitors. After stimulation by extracellular mitogenic stimuli, the inhibitor will be released and the active complex of CDK4/cyclin D can phosphorylate retinoblastoma protein (Rb), allowing subsequent activation of the Cyclin E/CDK2 complex [32,33]. Cyclin E/CDK2 further phosphorylates Rb to activate G1/S-phase gene expression. Our study indicates that ROR1 enhances tumor cell proliferation partly by upregulating the activity of CDK4 and Cyclin E1 which sequentially removes the inhibitors involved in transcription of genes required for DNA replication, thereby allowing cell survival and proliferation [34].
Autophagy is a ubiquitous highly conserved pathway in eukaryotic cells whereby cytoplasmic components are catabolically degraded in lysosomes for recycling [25,26]. Autophagy may function as a tumor-suppressive mechanism during early tumorigenesis [26], but its role in advanced tumors is dependent on the context [35]. Increasing studies have suggested that autophagy, the cell cycle, and apoptosis are coordinated and reciprocally regulated. It was reported that blocking CDK4/6 by siRNA or chemical inhibitors promotes autophagy in multiple cancer cells [36,37] and autophagy plays important roles in regulation of cell death, especially apoptosis-signaling pathways [38,39]. For example, anti-apoptotic Bcl-2 proteins (Bcl-2, Bcl-XL) interact with Beclin-1 to impede autophagy [40-42]. As we have shown above that ROR1 upregulated the cell cycle but downregulated apoptosis, it would be interesting to know whether ROR1 participates in the process of autophagy and how it works in lung tumorigenesis and progression. In this study we first screened 20 human autophagy-related molecules by a commercially available human autophagy array, and found that autophagy-related molecules including ATG7, ATG12, BNIP3L, LC3A, LC3B and NBS1 were mildly or significantly increased in ROR1-stably-silenced cell lines. Although until now we still cannot explain the exact role of ROR1-mediated autophagy in the pathogenesis of lung cancer, its occurrence is to a certain extent in accordance with ROR1-mediated anti-apoptotic and proliferation-enhancing roles which deserve further study.
ROR1-mediated downstream signaling cascades in tumors have been reported in several studies including ours [22,23,28,31], but the overall screening of key phosphokinase signaling pathways downstream of ROR1 in lung adenocarcinoma has not been reported yet. In this study, we analyzed the signaling consequences of ROR1-silencing in PC9erlo cells with antibody-based phospho proteomics. We found that the phosphorylation levels of GSK-3α/β (S21/S9) and Akt1/2/3 (S473) were significantly decreased which was further verified in ROR1-knockout cell line PC9erlo by western blot assay. GSK-3 is a serine/threonine kinase which has two isoforms GSK-3α and GSK-3β [43]. GSK-3 was originally demonstrated to play an important role in regulating glycogen synthesis, but recent studies found that GSK-3 also regulates the cell cycle, apoptosis and autophagy [44,45]. In our previous studies, we have shown that the Akt/mTOR signaling cascade is one of the key pathways downstream of ROR1. It is well known that mTOR is the most extensively investigated down-regulator of autophagy [46,47] which could partly explain the autophagy-enhancing role of ROR1-silencing in our observation. Here our data extend our previous study and further indicate that blocking ROR1 could deactivate Akt, then activate GSK-3α/β via de-phosphorylation, and finally deactivate mTOR. In this way blocking ROR1 could effectively regulate cell cycle, apoptosis, and autophagy in lung cancer as shown (Figure 7).
Figure 7.
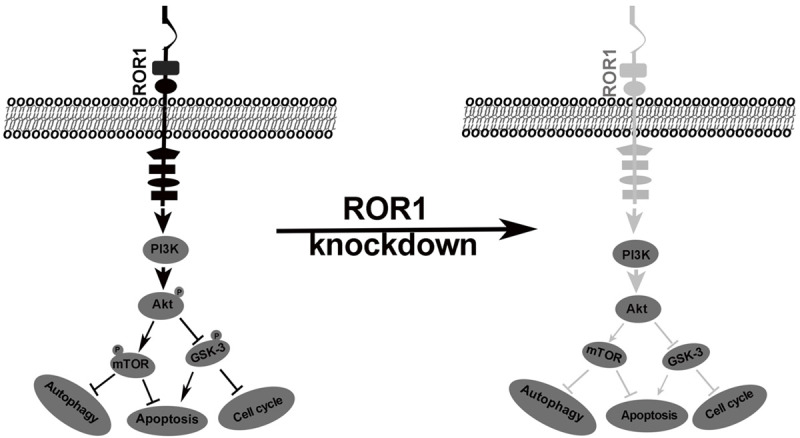
Schematic representation of the proposed mechanism of ROR1 in lung adenocarcinoma cell lines.
In summary, we have successfully established a batch of ROR1-stable-silenced clones with lentivirus-mediated shRNA instead of siRNA from lung adenocarcinoma cell lines PC9, PC9erlo, and NCI-H1975 which provides an effective cell model to comprehensively study the molecular mechanisms of ROR1 in lung adenocarcinoma. We found that ROR1 enhances lung adenocarcinoma growth by regulating the activity of key molecules regulating the cell cycle, apoptosis and autophagy, and that the Akt/GSK-3α/β/mTOR signaling cascade is the central pathway involved in ROR1-mediated pathogenesis which deserves further clarification and at the same time, paves a path for development of small molecules against the key molecules downstream of ROR1 in lung adenocarcinoma.
Acknowledgements
We wish to thank Dr. Jun Zhang (Shanghai Pulmonary Hospital, Shanghai, China) for providing the lung cancer cell line PC9. This study was supported by the grants from the National Natural Science Foundation of China (No. 81401904, http://www.nsfc.gov.cn), Science & Technology Department of Sichuan Province, P. R. China (No. 2018HH0005, http://www.scst.gov.cn), and Xinglin Scholar Discipline Talents Scientific Research Promotion Program (No. XSGG2018004). Dr. Jiahui Yang received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- ROR1
receptor tyrosine kinase-like orphan receptor 1
- siRNA
small interfering RNA
- shRNA
small-hairpin RNA
- PBS
phosphate-buffered saline
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- MOI
multiplicity of infection
- GFP
green fluorescent protein
- qRT-PCR
quantitative real-time polymerase chain reaction
- Akt
protein kinase B
- GSK-3
glycogen synthase kinase 3
- mTOR
mammalian target of rapamycin
- STAT3
signal transducer and activator of transcription 3
- ATG
autophagy-related gene
- Bcl-2
B-cell lymphoma-2
- Bcl-xl
B-cell lymphoma-extra-large
- Caspase
cysteinyl aspartate specific proteinase
- Bak
Bcl-2 associated k protein
- CDK4
cyclin-dependent kinase 4
- HRP
horseradish conjugated peroxidase
- NBS1
Nijmegen breakage syndrome 1
- BNIP3L
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like
- LC3
light chain 3
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Riaz MK, Zhang X, Wong K, Chen H, Liu Q, Chen X, Zhang G, Lu A, Yang Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int J Nanomedicine. 2019;14:2879–2902. doi: 10.2147/IJN.S192219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren XC, Liu YE, Li J, Lin Q. Progress in image-guided radiotherapy for the treatment of non-small cell lung cancer. World J Radiol. 2019;11:46–54. doi: 10.4329/wjr.v11.i3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi: 10.1016/j.csbj.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park S, Park IK, Kim ER, Hwang Y, Lee HJ, Kang CH, Kim YT. Current trends of lung cancer surgery and demographic and social factors related to changes in the trends of lung cancer surgery: an analysis of the national database from 2010 to 2014. Cancer Res Treat. 2017;49:330–337. doi: 10.4143/crt.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Q, Wang Q, Li D, Wei X, Jia Y, Zhang Z, Ai B, Cao X, Guo T, Liao Y. Co-administration of 20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI resistance by decreasing SCD1 induced lipid accumulation in non-small cell lung cancer. J Exp Clin Cancer Res. 2019;38:129. doi: 10.1186/s13046-019-1120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi M, Bahrami T, Salmaninejad A, Nosrati R, Ghaffari P, Ghaffari SH. Lung cancer-associated brain metastasis: molecular mechanisms and therapeutic options. Cell Oncol (Dordr) 2017;40:419–441. doi: 10.1007/s13402-017-0345-5. [DOI] [PubMed] [Google Scholar]
- 9.Oishi I, Takeuchi S, Hashimoto R, Nagabukuro A, Ueda T, Liu ZJ, Hatta T, Akira S, Matsuda Y, Yamamura H, Otani H, Minami Y. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells. 1999;4:41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Borcherding N, Kusner D, Liu GH, Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014;5:496–502. doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan MK, Yu J, Chen L, Cui B, Widhopf Ii GF, Rassenti L, Shen Z, Briggs SP, Kipps TJ. Wnt5a induces ROR1 to complex with HS1 to enhancemigration of chronic lymphocytic leukemia cells. Leukemia. 2017;31:2615–2622. doi: 10.1038/leu.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CL, Goswami S, Frissora F, Xie Z, Yan P, Bundschuh R, Walker L, Huang X, Mani R, Mo X, Baskar S, Rader C, Phelps M, Marcucci G, Byrd J, Lee L, Muthusamy N. ROR1-targeted delivery of miR-29b induces cell cycle arrest and therapeutic benefit in vivo in CLL mouse model. Blood. 2019;134:432–444. doi: 10.1182/blood.2018882290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bicocca VT, Chang BH, Masouleh BK, Muschen M, Loriaux MM, Druker BJ, Tyner JW. Crosstalk between ROR1 and the Pre-B cell receptor promotes survival of t (1;19) acute lymphoblastic leukemia. Cancer Cell. 2012;22:656–667. doi: 10.1016/j.ccr.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Chen L, Cui B, Chuang H, Yu J, Wang-Rodriguez J, Tang L, Chen G, Basak GW, Kipps TJ. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey G, Borcherding N, Kolb R, Kluz P, Li W, Sugg S, Zhang J, Lai DA, Zhang W. ROR1 potentiates FGFR signaling in basal-like breast cancer. Cancers (Basel) 2019;11:718. doi: 10.3390/cancers11050718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Zhang H, Ghia EM, Huang J, Wu L, Zhang J, Lam S, Lei Y, He J, Cui B, Widhopf GF 2nd, Yu J, Schwab R, Messer K, Jiang W, Parker BA, Carson DA, Kipps TJ. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci U S A. 2019;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Zheng Y, Liu X, Gou Q, Ma R, Guo C, Croce CM, Liu L, Peng Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget. 2017;8:32864–32872. doi: 10.18632/oncotarget.15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Cui B, Lai H, Liu G, Ghia EM, Widhopf GF 2nd, Zhang Z, Wu CCN, Chen L, Wu R, Schwab R, Carson DA, Kipps TJ. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A. 2014;111:17266–17271. doi: 10.1073/pnas.1419599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich BE, Hojjat-Farsangi M, Ghaemimanesh F, Daneshmanesh AH, Bayat AA, Mahmoudian J, Jeddi-Tehrani M, Rabbani H, Mellstedt H. Inhibition of the receptor tyrosine kinase ROR1 by anti-ROR1 monoclonal antibodies and siRNA induced apoptosis of melanoma cells. PLoS One. 2013;8:e61167. doi: 10.1371/journal.pone.0061167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.O’Connell MP, Marchbank K, Webster MR, Valiga AA, Kaur A, Vultur A, Li L, Herlyn M, Villanueva J, Liu Q, Yin X, Widura S, Nelson J, Ruiz N, Camilli TC, Indig FE, Flaherty KT, Wargo JA, Frederick DT, Cooper ZA, Nair S, Amaravadi RK, Schuchter LM, Karakousis GC, Xu W, Xu X, Weeraratna AT. Hypoxia induces phenotypic plasticity and therapy resistance in melanoma via the tyrosine kinase receptors ROR1 and ROR2. Cancer Discov. 2013;3:1378–1393. doi: 10.1158/2159-8290.CD-13-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng YZ, Ma R, Zhou J, Guo C, Wang Y, Li ZG, Liu L, Peng Y. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci Rep. 2016;6:36447. doi: 10.1038/srep36447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Lu C, Ida L, Yanagisawa K, Usukura J, Cheng J, Hotta N, Shimada Y, Isomura H, Suzuki M, Fujimoto T, Takahashi T. ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nat Commun. 2016;7:10060. doi: 10.1038/ncomms10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Yang H, Chen T, Luo Y, Xu Z, Li Y, Yang J. Silencing of receptor tyrosine kinase ROR1 inhibits tumor-cell proliferation via PI3K/AKT/mTOR signaling pathway in lung adenocarcinoma. PLoS One. 2015;10:e0127092. doi: 10.1371/journal.pone.0127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daneshmanesh AH, Hojjat-Farsangi M, Ghaderi A, Moshfegh A, Hansson L, Schultz J, Vagberg J, Bystrom S, Olsson E, Olin T, Osterborg A, Mellstedt H. A receptor tyrosine kinase ROR1 inhibitor (KAN0439834) induced significant apoptosis of pancreatic cells which was enhanced by erlotinib and ibrutinib. PLoS One. 2018;13:e0198038. doi: 10.1371/journal.pone.0198038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng H, Ge F, Du L, Zhang Z, Liu D. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4. J Cell Mol Med. 2019;23:5282–5291. doi: 10.1111/jcmm.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C, Kato S, Tomida S, Suzuki M, Osada H, Takahashi T. NKX2-1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Wang HL, Liu YC, Long MP, Zheng C, Yang JH. Blocking ROR1 enhances the roles of erlotinib in lung adenocarcinoma cell lines. Oncol Lett. 2019;18:2977–2984. doi: 10.3892/ol.2019.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS One. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. The ROR1 pseudokinase diversifies signaling outputs in MET-addicted cancer cells. Int J Cancer. 2014;135:2305–2316. doi: 10.1002/ijc.28879. [DOI] [PubMed] [Google Scholar]
- 32.Takaki T, Fukasawa K, Suzuki-Takahashi I, Semba K, Kitagawa M, Taya Y, Hirai H. Preferences for phosphorylation sites in the retinoblastoma protein of D-type cyclin-dependent kinases, Cdk4 and Cdk6, in vitro. J Biochem. 2005;137:381–6. doi: 10.1093/jb/mvi050. [DOI] [PubMed] [Google Scholar]
- 33.Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D: Cdk4. Proc Natl Acad Sci U S A. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan SM, Lents NH, Baldassare JJ. Expression of cyclin E renders cyclin D-CDK4 dispensable for inactivation of the retinoblastoma tumor suppressor protein, activation of E2F, and G1-S phase progression. J Biol Chem. 2004;279:5387–5396. doi: 10.1074/jbc.M310383200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Tang B, Xie X, Xiao YF, Yang SM, Zhang JW. The interplay between DNA repair and autophagy in cancer therapy. Cancer Biol Ther. 2015;16:1005–1013. doi: 10.1080/15384047.2015.1046022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumi NJ, Kuenzi BM, Knezevic CE, Remsing Rix LL, Rix U. Chemoproteomics reveals novel protein and lipid kinase targets of clinical CDK4/6 inhibitors in lung cancer. ACS Chem Biol. 2015;10:2680–2686. doi: 10.1021/acschembio.5b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iriyama N, Hino H, Moriya S, Hiramoto M, Hatta Y, Takei M, Miyazawa K. The cyclin-dependent kinase 4/6 inhibitor, abemaciclib, exerts dose-dependent cytostatic and cytocidal effects and induces autophagy in multiple myeloma cells. Leuk Lymphoma. 2018;59:1439–1450. doi: 10.1080/10428194.2017.1376741. [DOI] [PubMed] [Google Scholar]
- 38.Kim KY, Park KI, Kim SH, Yu SN, Park SG, Kim YW, Seo YK, Ma JY, Ahn SC. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18:1088. doi: 10.3390/ijms18051088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sophia J, Kowshik J, Dwivedi A, Bhutia SK, Manavathi B, Mishra R, Nagini S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/GSK-3beta signalling pathway in oral cancer. Cell Death Dis. 2018;9:1087. doi: 10.1038/s41419-018-1126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong L, Shu W, Dai W, Gao B, Xiong S. Reactive oxygen species-mediated c-Jun NH2-terminal kinase activation contributes to hepatitis B virus X protein-induced autophagy via regulation of the beclin-1/Bcl-2 interaction. J Virol. 2017;91:e00001–17. doi: 10.1128/JVI.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang WC, Wei Y, Kuo YC, Wei S, Zhou A, Zou Z, Yehl J, Ranaghan MJ, Skepner A, Bittker JA, Perez JR, Posner BA, Levine B. High-throughput screens to identify autophagy inducers that function by disrupting beclin 1/Bcl-2 binding. ACS Chem Biol. 2018;13:2247–2260. doi: 10.1021/acschembio.8b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv X, Wang K, Tang W, Yu L, Cao H, Chi W, Wang B. miR-34a-5p was involved in chronic intermittent hypoxia-induced autophagy of human coronary artery endothelial cells via Bcl-2/beclin 1 signal transduction pathway. J Cell Biochem. 2019;120:18871–18882. doi: 10.1002/jcb.29207. [DOI] [PubMed] [Google Scholar]
- 43.Lal H, Ahmad F, Woodgett J, Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116:138–149. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long Q, Li X, He H, He D. Autophagy activation protects shock wave induced renal tubular epithelial cell apoptosis may through modulation of Akt/GSK-3β pathway. Int J Biol Sci. 2016;12:1461–1471. doi: 10.7150/ijbs.16864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du J, Zhu X, Guo R, Xu Z, Cheng FF, Liu Q, Yang F, Guan L, Liu Y, Lin J. Autophagy induces G0/G1 arrest and apoptosis in menstrual blood-derived endometrial stem cells via GSK3-beta/beta-catenin pathway. Stem Cell Res Ther. 2018;9:330. doi: 10.1186/s13287-018-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82:1245–1266. doi: 10.1111/bcp.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson CE, Tee AR. Exploiting cancer vulnerabilities: mTOR, autophagy, and homeostatic imbalance. Essays Biochem. 2017;61:699–710. doi: 10.1042/EBC20170056. [DOI] [PubMed] [Google Scholar]


