Abstract
Background: Xerostomia is the main manifestation from patients with Sjögren syndrome (SS). However, traditional immunosuppressive agents are nearly invalid due to complicated etiopathogenesis in salivary glands, including aberrant immune dysregulation, epithelial structure destruction, and diminished secretory function. Objective: To investigate the therapeutic effect of murine embryonic mesenchymal stem cells (ME-MSCs) on salivary glandular epithelium structure and secretory function in Sjögren-like mice. Methods: Salivary flow rate (SFR), blood glucose, and body weight was weekly monitored among treatment group, disease group, and health control group. ME-MSCs were used to treat NOD mice via tail vein injection. HE staining and transmission electron microscope was used to evaluate the structure of salivary gland epithelial cells (SGEC). TUNEL fluorescence staining and PCNA immumohistochemical staining was used to evaluate the SGEC apoptosis and proliferation. The SGEC secretory function was tested by PAS staining and amylase immumohistochemical staining. Results: ME-MSC treatment could elevate SFR, restore the acini and micromorphologies, promote the SGEC proliferation, and suppress the SGEC apoptosis in NOD mice, but not restore to that in health control group. The SGEC structure was more intact in treatment group. Mucopolysaccharide and amylase of salivary acinar cells in treatment group was better than that in disease group, although transmission electron microscopy showed secretory granules were lower than those in healthy control. Conclusion: ME-MSCs demonstrated its potential as a candidate treatment for xerostomia due to some effects on salivary flow rate in NOD mice by restoring the SGEC impairment and secretory function.
Keywords: Mesenchymal stem cells, Sjögren syndrome, xerostomia, salivary gland epithelium cells
Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease that affects exocrine glands such as salivary glands and lacrimal glands, as well as multiple visceral systems and organs. The typical manifestations are xerostomia and keratoconjunctivitis sicca, but the mechanism is still obscure. Recent studies indicated that salivary gland epithelial cells (SGEC), and their interaction with cells of innate and adaptive immune system might play a vital part in autoimmune epithelitis. After an initial stimulus, SGEC became apoptotic and aberrant autoantigens are expressed, which may result in autoreactive T cells [1]. Moreover, SGEC have the unique capacity to express proinflammatory molecules, i.e. HLA-DR, CD80, CD86, CD40, CCL17, CCL19, CCL21, CCL22, interferons, and other cytokines, and cause complex interactions [2]. Therefore, SGEC seem to be the nidus of pathogenetic events in SS, and as antigen presenting cells leads to lymphocytic infiltration. The chronic proliferation of T and B cells secrete interferon gamma and tumor necrosis factor alpha which also induce Fas expression and mediate the apoptosis of SGEC [3]. The resulting autoimmune epithelitis is responsible not only for altered glandular homeostasis, but have also been implicated in aberrant acinar cells and ductal cells, and secretory dysfunction or xerostomia, maybe prior to onset of inflammation [4].
There are two kinds of treatment to xerostomia in SS: saliva replacement and stimulation of salivary flow with secretagogues. Traditional disease-modifying antirheumatic drugs and biologic agents is nearly invalid due to aberrant SGEC and secretory dysfunction [5]. Mesenchymal stem cells (MSCs) have their potential clinical benefits with immunomodulatory function and multi-directional differentiation into adipocyte, osteocyte, myocyte, and other cells under specific culture condition [6]. Studies had proven that MSCs could migrate to damaged salivary glands through various pathways such as stromal cell-derived factor-1 (SDF-1)/C-X-C chemokine receptor 4 (CXCR4) signal pathway [7]. Our previous research had indicated that treating NOD/Ltj mice with MSCs could improve salivary flow rate (SFR) and alleviate lymphocyte infiltration in submandibular glands. Levels of serum IL-6, hepatocyte growth factor (HGF), IL-10, prostaglandin E2 (PGE2), and transforming growth factor beta 1 (TGF-beta 1) were elevated in treatment group. Meanwhile levels of IL-2 and IFN-γ were decreased [8].
However, few studies had investigated the specific SGEC variation after MSC treatment. In this study, the mesenchymal stem cells (ME-MSCs) were isolated, identified, and infused into NOD mice through the tail vein. The SGEC proliferation, apoptosis, and cellular substructure, as well as saliva secreting function in NOD/Ltj mice would be evaluated after ME-MSC treatment.
Material and methods
Animals
Four-week-old female NOD/Ltj (n=30, ~15 g) and ICR mice (n=15, ~15 g) were bought from Model Animal Research Center of Nanjing University (Nanjing, China). Female NOD/Ltj mice were served as SS animal model which were randomly divided into treatment group (n=11) and disease group (n=9), while ICR mice were the healthy controls (n=3). All the mice were maintained under specific pathogen-free conditions with a 12/12 h light/dark cycle (22±3°C, 50% humidity) in the Animal Experimental Center of Tongji University (Shanghai, China).
Isolation and enrichment of ME-MSCs
Female ICR mice at 12.5 days gestation period (n=2) were sacrificed by cervical dislocation. Embryo mice were taken out from the womb and their limbs were gently removed so that the trunk part could be used to extract stem cells. Embryo mouse trunk was cut into pieces and spread on culture flasks. After cultured at 37°C overnight, primary stem cells were achieved. All the MSCs used in this research were derived from passage 3-5.
Identification of ME-MSCs
Primary stem cells were cultured to the third passage then identified by flow cytometry (Becton, Dickinson and Company, USA) to detect cell surface antigen (MSCs were positive for CD29, CD44, and Sca-1 and negative for CD34, CD11b, and CD45). In addition, osteogenic, and adipogenic differentiation base medium (Biotowntek Co., LTD., China) was used to induce stem cells respectively differentiate into osteocyte and adipocyte in about 20 days. Calcium deposits were detected in osteogenesis differentiation through alizarin red S staining (Sigma, USA), and lipid droplets were detected for adipogenic differentiation by oil red O (Sigma, USA) staining.
Measurements of blood glucose and SFR of NOD/Ltj mice
To evaluate if NOD mice were valid, blood was acquired using a sterilized needle to stab the tail-vein and glucose levels were measured by a blood glucometer (Sannuo, Changsha Sinocare, Inc., Changsha, China) once a week. The mice, which were hyperglycemic, would be injected with long-acting insulin once a day to keep the blood glucose level in normal ranges. SFR was monitored once a week by intramuscular injection of pilocarpine (Yangzhou Aoxin Chemical Co., Ltd., Yangzhou, China; 0.5 mg/kg body weight) in order to stimulate saliva secretion. Using 4% chloral hydrate (Solarbio, Beijing, China) to anaesthetize the mice (the dose was 400 mg chloral hydrate per kg animal body weight), then put the cotton sliver into their mouths to collect saliva in 15 minutes. The whole saliva secretory volume was measured gravimetrically by weighing cotton sliver before and after saliva collection. SFR was detected since mice were 5 weeks old.
ME-MSC transplantation
After 3-5 passage, ME-MSCs were diluted to 4×105/mlin PBS (Gibco, USA). The 12 week old NOD/Ltj mice (n=11) were infused with freshly collected ME-MSCs (1×105/mouse) through tail-vein injection. This treatment was performed 2 times a week and lasted for 2 weeks.
Hematoxylin-eosin (HE) and periodic acid-schiff (PAS) staining
All the mice in the three groups were sacrificed at the age of 19 week old. Submandibular glands were immediately removed to 4% paraformaldehyde then fixed at 4°C for 24 h, then embedded in paraffin. The tissue sections were deparaffinized, rehydrated, and stained with the HE staining kit and PAS staining kit (Sigma-Aldrich, INC, USA). Finally the ratio of the surface area filled with PAS positive cells to the total measured area was quantified from five random fields under 400× magnification with a light microscope. The measurement was performed in three randomly selected sections by using Image J software (NIH, USA).
Detection of SGEC apoptosis and proliferation
An In Situ Cell Death Detection Kit (Roche, USA) was used based on the TUNEL assay, to detect SGEC apoptosis according to the manufacturer’s instructions. As for detection of SGEC proliferation, a Zymed PCNA staining kit (Roche, USA) was used according to the manufacturer’s instructions based on ABC method. The amount of PCNA positive cells was counted in five random fields per section under light microscopy (400× magnification) and three randomly selected sections were chosen for analysis.
Transmission electron microscopy (TEM)
The sections were fixed in 2.5% glutaraldehyde solution for 12 h and post-fixed in 1% osmium tetroxide for 2 h, then dehydrated and embedded in epoxy resin.The specimens were cut into thin sections and examined with a transmission electron microscope (JEM-1230; Jeol, Tokyo, Japan).
Immunohistochemical staining of amylase
Amylase production was a vital index analyzing SGEC secreting function. Staining was performed with a commercially available kit (Abcam Inc., Cambridge, MA, USA) based on the avidin-biotin-peroxidase complex (ABC) method. Tissue sections were deparaffinized, rehydrated, incubated three times for 5 min in microwave oven, washed with Tris-buffered saline (TBS), covered with 1% H2O2 for 8 min, added normal goat serum for 20 min, and incubated with anti-amylase antibodies (Abcam Inc., Cambridge, MA, USA) overnight (except the negative control sections). Tissue sections were washed with PBS for 5 min then incubated with biotinylated IgG and peroxidase-marked avidin-biotin complex respectively for 30 min. Finally, peroxidase was visualized by DAB reaction. The measurement of positive cell was performed randomly in five fields within three randomly selected sections by using Image J software (NIH, USA).
Statistical analysis
All data were presented in the form of mean ± standard deviation. ANOVA was used for the comparison between multiple groups. All the experimental data were analyzed with SPSS21.0 statistical software (IBM SPSS, Armonk, NY, USA) and set p-value <0.05 as statistical difference.
Results
Identification of ME-MSCs
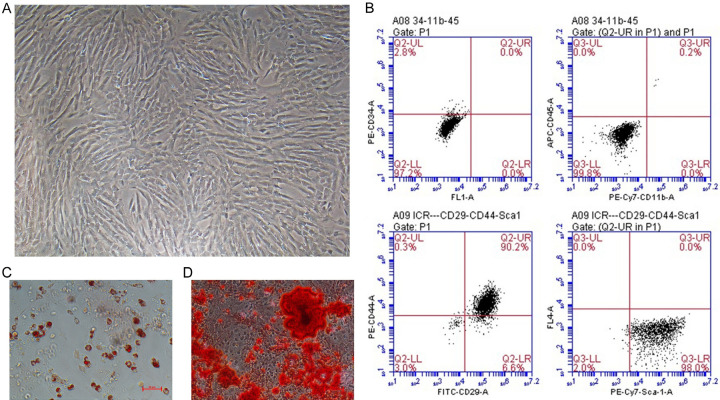
Cultivating the primary stem cells acquired from embryo mice to passage 3-5. ME-MSCs displayed spindle-shaped and fibroblast-like cell outline (Figure 1A). Passage 3-5 ME-MSCs were identified by flow cytometry with the results showing that stem cells were positive for CD29, CD44, and Sca-1, but negative for CD34, CD11b, and CD45 (Figure 1B). In addition, stem cells could respectively differentiate into osteocyte and adipocyte in vitro in about 20 days, as calcium deposits were detected in osteogenesis differentiation through alizarin red S staining (Sigma, USA) (Figure 1C) and lipid droplets were detected in adipogenic differentiation by oil red O (Sigma, USA) staining (Figure 1D). All the results indicated that the stem cells acquired from embryo mice were confirmed to the characteristics of MSCs.
Figure 1.
ME-MSC culture and identification. A. ME-MSCs displayed spindle-shaped and fibroblast-like morphology under the microscope. (magnifcation, ×100). B. Identification of ME-MSCs cell surface markers by flow cytometry. ME-MSCs were negative for CD34, CD11b, and CD45, but positive for CD29, CD44, and Sca-1. C. ME-MSCs could respectively in vitro differentiate into osteocyte detected through alizarin red S staining. D. In vitro differentiate into adipocyte detected by oil red O staining.
ME-MSC transplantation ameliorates SFR in NOD/Ltj mice
NOD/Ltj mice became hyperglycemic since the 10-week-old, and the highest blood glucose was 13.7 mmol/L at 12-week-old. 11 NOD mice became hyperglycemic in treatment group and 9 in disease group at the 12-week-old (Figure 2A), and insulin was used at a dose of 0.5 UI/kg to control blood glucose each day. SFR in NOD/Ltj mice decreased since the 9-week-old, and the SFR value at the 12-week-old was: treatment group (117.75±31.24) μg/min, disease group (115.82±25.24) μg/min, healthy control group (272.19±12.76) μg/min. SFR in both treatment group and disease group from NOD/Ltj mice with hyperglycemia was obviously decreased compared with that in ICR mice from 9-week-old to 12-week-old (P<0.05, Figure 2B). These results showed that NOD/Ltj mice with hyperglycemia became disorder in autoimmunity and had low SFR.
Figure 2.
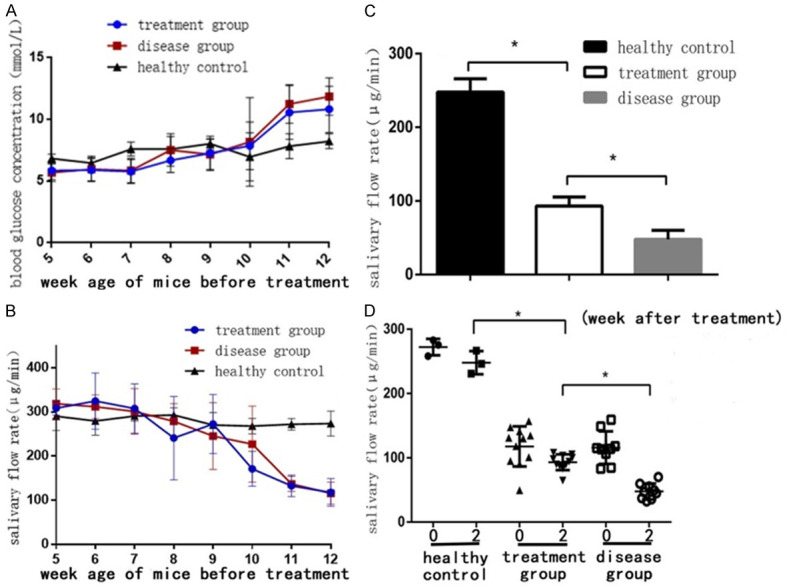
ME-MSC transplantation ameliorated SFR in NOD/Ltj mice. A. Blood glucose levels of mice 12 weeks before ME-MSC treatment. NOD/Ltj mice became hyperglycemia since the 10-week-old, while blood glucose concentration in ICR mice was normal. B. SFR of mice 12 weeks before ME-MSC treatment. SFR in NOD/Ltj mice decreased since the 9-week-old compared with that in ICR mice (P<0.05). C. SFR of mice 2 weeks after ME-MSC treatment. 12-week-old NOD mice were injected intraperitoneally with. 2 weeks after ME-MSC treatment, the mouse SFR in disease group [(47.8±12.4) μg/min, n=9] was distinctly decreased compared with that in the treatment group [(93.1±12.2) μg/min, n=11] (P<0.05), but SFR in treatment group was still lower than that in the healthy control group [(247.8±18.0) μg/min, n=3] (P<0.05). D. SFR of mice 2 weeks after ME-MSC treatment. After ME-MSC treatment, SFR in treatment group was slightly decreased. However, SFR in treatment group was still better than that in disease group. *P<0.05.
SFR value after ME-MSCs treatment for 2 weeks was: treatment group (93.1±12.2) μg/min, disease group (47.8±12.4) μg/min, healthy control group (247.8±18.0) μg/min, respectively. SFR value of disease group were distinctly decreased compared with treatment group (P<0.05), but the treatment group SFR level was still lower than that of healthy controls (P<0.05, Figure 2C). Although SFR in the treatment group still declined after ME-MSCs treatment for 2 weeks, the decrease was less than that in the disease group (P<0.05, Figure 2D), demonstrating that ME-MSC treatment could improve the SFR in NOD mice.
ME-MSC treatment could restore the acini and micromorphologies in salivary gland from NOD mice
HE staining revealed that there were relatively well preserved acini and micromorphologies that could be found not in the disease group, but in the health control group and in the treatment group. Although acini and micromorphologies in treatment group could not restore to that in health control group, it was alleviated compared with that in disease group (Figure 3A). The results showed that ME-MSC treatment could protect the acini and micromorphologies in salivary gland from NOD mice.
Figure 3.
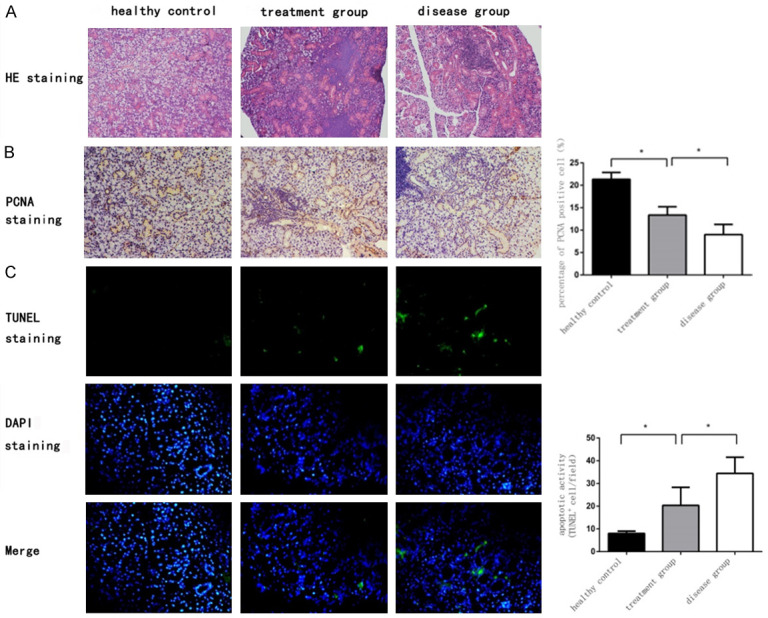
ME-MSC treatment could restore the acini and micromorphologies, promote the SGEC proliferation, and suppress the SGEC apoptosis from NOD mice. A. HE staining of submandibular glands in mice (magnification, ×200). Relatively well preserved acini and micromorphologies could be found not in the disease group, but in the health control group and in the treatment group. B. PCNA immunohistochemical staining in SGEC in health control group, treatment group, and disease group. The percentage of proliferation cells in disease group [(9.0±2.3%), n=9] was lower than that of treatment group [(13.4±1.9%), n=11]. *P<0.05. C. Apoptosis cells in salivary glands were stained by TUNEL fluorescence staining in health control group, treatment group, and disease group. The percentage of apoptosis cells in treatment group [(20.4±7.9/field), n=11] was lower than that in disease group [(34.4±7.1/field), n=9]. *P<0.05.
ME-MSC infusion could promote the SGEC proliferation while suppress the SGEC apoptosis in salivary gland from NOD mice
We further explored the effects of ME-MSC infusion on SGEC proliferation in NOD mice. Proliferative nuclear antigen (PCNA) is mainly located in cell nucleus, and the cell nucleus in salivary gland epithelial with biologically proliferation activity that can be brown/yellow stained by PCNA immunohistochemical staining. Stained intensity in treatment group (13.4±1.9%) was higher than that in disease group (9.0±2.3%) (P<0.05), but the intensity in both treatment group and disease group was lower than that in healthy control group (21.3±1.5%) (P<0.05, Figure 3B), which indicated that ME-MSC treatment was able to promote the SGEC proliferation in NOD mice.
Meanwhile, the SGEC apoptosis was detected by TUNEL fluorescence staining. The number of apoptotic epithelial cells in salivary gland in treatment group (20.4±7.9/field) was less than that in disease group (34.4±7.1/field) (P<0.05), but the number of apoptotic cells in both treatment group and disease group was more than that in healthy control group (8.0±1.1)/field (P<0.05, Figure 3C), which indicated that ME-MSC treatment could inhibit the SGEC apoptosis from NOD mice.
Intravenous ME-MSCs protected SGEC morphological structure from NOD mice
Transmission electron microscope revealed that an intact nucleus with condensed chromatin at its periphery could be seen in healthy control group, but SGEC in disease group had malformed nuclei and an obviously decreased amount of secretory granules. While in treatment group, SGEC were nearly restored to normal in appearance. However, the amounts of secretory granules in the treatment group were lower than that in healthy controls (Figure 4). Results above indicated that ME-MSCs might contribute to protect the SGEC morphological integrity.
Figure 4.
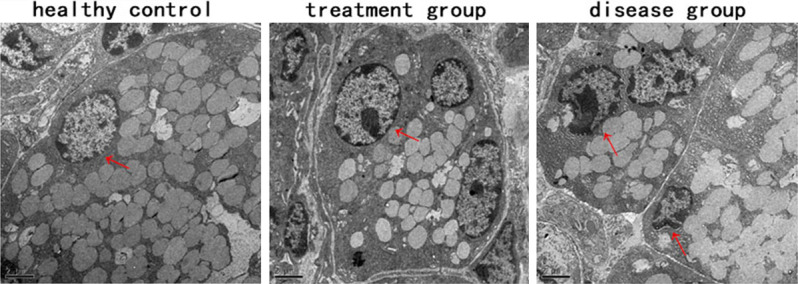
ME-MSC treatment protected SGEC morphological strucrure from NOD mice. SGEC ultrastructure by TEM (Scale bars=2 μm). SGEC in healthy control group, where an intact nucleus with condensed chromatin at its periphery could be seen (arrow). SGEC in treatment group, where cell nuclei were still normal in appearance, however, the amount of secretory granules was lower than that in healthy control. In disease group, SGEC had malformed nuclei and an obviously decreased amount of secretory granules.
ME-MSCs by intravenous infusion improved the SGEC secretory function
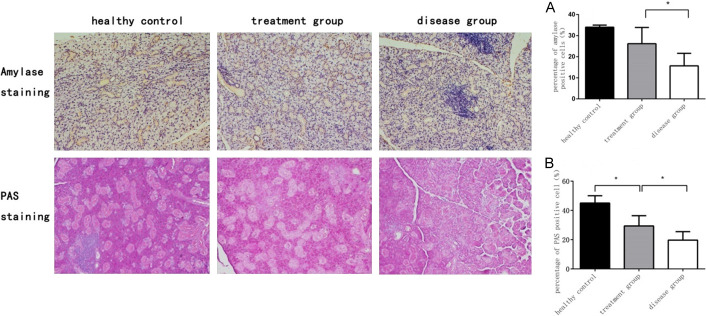
Amylase and mucopolysaccharide was the main ingredients in saliva, which was detected by amylase immunohistochemical staining and PAS staining respectively. The percentage of salivary amylase positive cells in healthy control group, treatment group, and disease group was (34.5±1.2)%, (26.2±7.7)%, and (15.7±5.9)%, and salivary amylase content in disease group was lower than that in treatment group (P<0.05, Figure 5A). Concomitantly, the percentage of PAS positive cells in healthy control group, treatment group and disease group was (45.0±5.5)%, (29.4±7.1)%, and (19.7±5.8)%, and the function of glycogen secretion in treatment group was better than that in the disease group (P<0.05, Figure 5B). The results revealed that ME-MSC transplantation could effectively revive the SGEC secretory function from NOD mice.
Figure 5.
ME-MSC improved the SGEC secretory function from NOD mice. Amylase immunohistochemical staining of submandibular glands in health control group, treatment group and disease group. The percentage of salivary amylase positive cells in disease group [(15.7±5.9)%, n=9] was lower than that in treatment group [(26.2±7.7)%, n=11]. *P<0.05 (A). PAS staining of submandibular glands in health control group, treatment group, and disease group. The percentage of PAS positive cells in disease group [(19.7±5.8)%, n=9] was less than that in treatment group [(29.4±7.1)%, n=11]. *P<0.05 (B).
Discussion
In this study, we observed improvement on SFR, SGEC structure, and secretory function from the Sjögren-like NOD mice, which were injected with MSCs from ICR fetal mice through the caudal vein injection for two weeks. As a result, the average static SFR was higher in MSC treatment group compared with that in disease group. We observed less destructed tissue structure of SGEC in treatment group than that in disease group through HE staining, transmission electron microscopy experiments, TUNEL fluorescence assay for apoptosis, and PCNA immunohistochemical staining for proliferation. In the treatment group, the SGEC nucleus displayed an almost commonly shaped and totally different from the nucleus phenotype in disease group with deformation atrophy and abnormal deposition of chromatin. The mice in both groups have decreasing secretion granules compared with healthy control. Besides, the SGEC in disease group have higher apoptosis activity and lower proliferation activity versus MSCs treatment group. Salivary amylase immunohistochemical experiment and PAS glycogen staining showed more intact salivary gland secretion for amylase and mucopolysaccharide in treatment group compared with disease group, but both groups displayed lower secretion level versus healthy controls. All the above results indicated that ME-MSC treatment has a protective effect on SGEC damage from NOD mice.
To our surprise, SFR in treatment group was restored evidently compared with that in disease group, which is hard to improve via traditional disease-modifying antirheumatic drugs and biologic agents due to aberrant SGEC and secretory dysfunction [5]. More and more evidence supports a immunosuppressive action for MSCs on T cells, B cells, dendritic cells (DC), macrophages, and natural killer T cells (NK) through cell to cell contact or release of soluble factors such as prostaglandin E2 (PGE2), interleukin- (IL-) 10, IL-6, in doleamine 2,3-dioxygenase (IDO), and etc [9]. Our previous study had indicated that BMMSCs prevented SFR decline and lymphocyte infiltration in the salivary glands of NOD mice, maybe through PGE2, IL-10, IL-6, transforming growth factor-beta (TGF-β), and hepatic growth factor (HGF) [5]. Activated lymphocyte infiltration produced a variety of inflammatory mediators and cytokines originated from chronic and progressive acini and ducts destruction, and in turn led to catheter obstruction, acinar atrophy, and impaired exocrine gland function [10]. MSC treatment ameliorated sialadenitis in the SS-like mouse and in pSS patients partly through decreasing T cell activation, and increasing Tregs [11,12]. Therefore, in our study, ME-MSC treatment might revive SFR by lessening lymphocyte infiltration focal in submandibular glands. In addition, Xu also found infused MSCs could migrate toward the inflammatory salivary gland in a stromal cell-derived factor-1-dependent manner [12], and interestingly, it has been demonstrated when cocultured with SGEC, MSCs could transdifferentiate into SGEC showing comparable cellular structures such as tight junctions and some secretory granules, and expressed several salivary genes such as aquaporin 5, α-amylase, and E-cadherin [13]. Accordingly, in our study, ME-MSC infusion might restore salivary gland destruction via transdifferentiating into SGEC.
However, the SFR was not elevated to the level of health control, consistent with the recent report [14,15], on account that MSC treatments promoted tissue proliferation in the glands significantly higher than the disease group and their rates were comparable to the ICR group by immunofluorescence staining of submandibular glands for proliferation protein Ki-67. Meantime, MSC treatments could decrease a specific Caspase-3 gene in gland apoptosis by quantitative RT-PCR in submandibular glands. Our study further validated ME-MSC treatment could facilitate SGEC proliferation by PCNA immunohistochemical staining and prohibit SGEC apoptosis by TUNEL fluorescence assay, in spite of not restoring to healthy control. SGEC apoptosis and proliferation, which is induced by paracrine mechanisms, has been found to be unusual in MSG biopsy of patients with SS. The cause of dysfunction is probably multifactorial, potentially including altered glandular homeostasis in the preimmune and non-immune phase [16], which are difficult to remit by ME-MSC treatment. MSC treatment may retard the declining speed of SFR compared with disease group. As a result, the treatment was to slow down the progress of salivary gland damage, which may be related to the MSC treatment initiation. Our previous studies had confirmed that MSC treatment in 22-week-old NOD mice, which were at the terminal stage of SS, could only slow down SFR decline instead of improving SFR to healthy control [8]. In this study, we started MSC treatment in 12-week-old NOD mice, which were at the middle disease stage, showing that MSC treatment can slow down the process of decreased SFR.
While ME-MSC treatment could ameliorate the SFR from the Sjögren-like NOD mice, no study has demonstrated that traditional disease-modifying antirheumatic drugs, such as hydroxychloroquine [17], methotrexate [18], leflunomide [19], mycophenolate [20], azathioprine [21], and cyclosporinA [22] could reverse glandular dysfunction and, therefore, cure xerostomia [23]. Salivary amylase and glycogen was the main secretory biomarkers of SGEC. In this study, we found the treatment group had higher amylase and mucopolysaccharide content in SGEC compared with disease group. The protective function of MSCs in SGEC secretion may relate to a potential MSCs differentiation to SGEC. One study noted that α-amylase expression in the MSCs of labial glands from SS patients was lower than that from healthy individuals by Real-time polymerase chain reaction and immunofluorescence staining. Thus, MSCs in the labial glands from SS patients could lack salivary α-amylase secretion [24]. Another recent study showed the migration of BM-MSCs in the basal membrane of the ducts and acinar cells obviously damaged by radiation exposure, and there was significant increase in the expression of α-amylase after transplantation [25], indicating MSCs could improve the damaged salivary glands by multiplying the vitality of the α-amylase except for MSC immunoregulation.
In conclusion, this study confirmed that the ME-MSCs had a significant effect on impaired SGEC structure and secretory function from Sjögren-like NOD mice. However, the uderlying mechanism and signaling pathways need further exploration.
Acknowledgements
This study was funded by the projects from National Natural Science Foundation of China grants (grant number: 81273295, 81671598, 81801601), China International Medical Exchange Foundation (grant number: Z-2014-06-2-1620), Shanghai Wu Mengchao Medical Foundation (grant number: 17YF1417200), Clinical research key program of Tongji Hospital Tongji University (grant number: ITJZD1909), Research training foundation of Tongji Hospital (grant number: GJPY1805). We would like to thank technicians in Shanghai Tongji Hospital Stem Cell Research Center for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Sun Y, Zhang W, Li B, Zou Z, Selmi C, Gershwin ME. The coexistence of Sjogren’s syndrome and primary biliary cirrhosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;48:301–15. doi: 10.1007/s12016-015-8471-1. [DOI] [PubMed] [Google Scholar]
- 2.Sandhya P, Kurien BT, Danda D, Scofield RH. Update on pathogenesis of Sjogren’s syndrome. Curr Rheumatol Rev. 2017;13:5–22. doi: 10.2174/1573397112666160714164149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varin MM, Guerrier T, Devauchelle-Pensec V, Jamin C, Youinou P, Pers JO. In Sjogren’s syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun Rev. 2012;11:252–8. doi: 10.1016/j.autrev.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Pérez Leirós C. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142:411–8. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivino FB, Bunya VY, Massaro-Giordano G, Johr CR, Giattino SL, Schorpion A, Shafer B, Peck A, Sivils K, Rasmussen A, Chiorini JA, He J, Ambrus JL Jr. Sjogren’s syndrome: an update on disease pathogenesis, clinical manifestations and treatment. Clin Immunol. 2019;203:81–121. doi: 10.1016/j.clim.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 6.BaghabanEslaminejad M, Malakooty Poor E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J Stem Cells. 2014;6:344–54. doi: 10.4252/wjsc.v6.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–24. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 8.Ruan GF, Zheng L, Huang JS, Huang WX, Gong BD, Fang XX, Zhang XY, Tang JP. Effect of mesenchymal stem cells on Sjogren-like mice and the microRNA expression profiles of splenic CD4+ T cells. Exp Ther Med. 2017;13:2828–2838. doi: 10.3892/etm.2017.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Yu Y, Ma J, Olsen N, Lin J. Mesenchymal stem cells in primary Sjogren’s syndrome: prospective and challenges. Stem Cells Int. 2018;2018:4357865. doi: 10.1155/2018/4357865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8:60–9. doi: 10.1016/s1542-0124(12)70070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluri HS, Samizadeh M, Edman MC, Hawley DR, Armaos HL, Janga SR, Meng Z, Sendra VG, Hamrah P, Kublin CL, Hamm-Alvarez SF, Zoukhri D. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of Sjogren’s syndrome. Stem Cells Int. 2017;2017:3134543. doi: 10.1155/2017/3134543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, Chen W, Sun L, Wang S. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–51. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maria OM, Tran SD. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev. 2011;20:959–67. doi: 10.1089/scd.2010.0214. [DOI] [PubMed] [Google Scholar]
- 14.Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD. Mesenchymal stem cells extract (MSCsE)-based therapy alleviates xerostomia and keratoconjunctivitis sicca in Sjogren’s syndrome-like disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili S, Faustman DL, Liu Y, Sumita Y, Blank D, Peterson A, Kodama S, Tran SD. Treatment for salivary gland hypofunction at both initial and advanced stages of Sjogren-like disease: a comparative study of bone marrow therapy versus spleen cell therapy with a 1-year monitoring period. Cytotherapy. 2014;16:412–23. doi: 10.1016/j.jcyt.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Sandhya P, Kurien BT, Danda D, Scofield RH. Update on pathogenesis of Sjogren’s syndrome. Curr Rheumatol Rev. 2017;13:5–22. doi: 10.2174/1573397112666160714164149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottenberg JE, Ravaud P, Puéchal X, Le Guern V, Sibilia J, Goeb V, Larroche C, Dubost JJ, Rist S, Saraux A, Devauchelle-Pensec V, Morel J, Hayem G, Hatron P, Perdriger A, Sene D, Zarnitsky C, Batouche D, Furlan V, Benessiano J, Perrodeau E, Seror R, Mariette X. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA. 2014;312:249–58. doi: 10.1001/jama.2014.7682. [DOI] [PubMed] [Google Scholar]
- 18.Skopouli FN, Jagiello P, Tsifetaki N, Moutsopoulos HM. Methotrexate in primary Sjögren’s syndrome. Clin Exp Rheumatol. 1996;14:555–558. [PubMed] [Google Scholar]
- 19.van Woerkom JM, Kruize AA, Geenen R, van Roon EN, Goldschmeding R, Verstappen SM, van Roon JA, Bijlsma JW. Safety and efficacy of leflunomide in primary Sjögren’s syndrome: a phase II pilot study. Ann Rheum Dis. 2007;66:1026–32. doi: 10.1136/ard.2006.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willeke P, Schlüter B, Becker H, Schotte H, Domschke W, Gaubitz M. Mycophenolate sodium treatment in patients with primary Sjögren syndrome: a pilot trial. Arthritis Res Ther. 2007;9:R115. doi: 10.1186/ar2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price EJ, Rigby SP, Clancy U, Venables PJ. A double blind placebo controlled trial of azathioprine in the treatment of primary Sjögren’s syndrome. J Rheumatol. 1998;25:896–9. [PubMed] [Google Scholar]
- 22.Drosos AA, Skopouli FN, Galanopoulou VK, Kitridou RC, Moutsopoulos HM. Cyclosporin a therapy in patients with primary Sjögren’s syndrome: results at one year. Scand J Rheumatol Suppl. 1986;61:246–9. [PubMed] [Google Scholar]
- 23.Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, Fisher BA, Gottenberg JE, Hernandez-Molina G, Kocher A, Kostov B, Kruize AA, Mandl T, Ng WF, Retamozo S, Seror R, Shoenfeld Y, Sisó-Almirall A, Tzioufas AG, Vitali C, Bowman S, Mariette X EULAR-Sjögren Syndrome Task Force Group. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79:3–18. doi: 10.1136/annrheumdis-2019-216114. [DOI] [PubMed] [Google Scholar]
- 24.Wang SQ, Wang YX, Hua H. Characteristics of labial gland mesenchymal stem cells of healthy individuals and patients with Sjögren’s syndrome: a preliminary study. Stem Cells Dev. 2017;26:1171–1185. doi: 10.1089/scd.2017.0045. [DOI] [PubMed] [Google Scholar]
- 25.Mulyani SWM, Astuti ER, Wahyuni OR, Ernawati DS, Ramadhani NF. Xerostomia therapy due to ionized radiation using preconditioned bone marrow-derived mesenchymal stem cells. Eur J Dent. 2019;13:238–242. doi: 10.1055/s-0039-1694697. [DOI] [PMC free article] [PubMed] [Google Scholar]