Abstract
Objective: To explore the expression and clinical significance of Gal-3 and NFκB pathway related factors in epithelial ovarian carcinoma cells. Methods: 99 histologic specimens of epithelial ovarian cancer and 20 normal ovarian histologic specimens were collected, and the expressions of Gal-3, IκB and p65 were detected by immunohistochemistry. Their relationship with clinical characteristics was analyzed. Results: The expression of Gal-3 and p65 was negatively correlated with the overall survival rate (P<0.05), while the expression of IκB was positively correlated with the overall survival rate (P<0.05). Expression of Gal-3, p65 and IκB were found associated with EOC platinum resistance (P<0.05), and expression of Gal-3 and p65 correlated with pathologic grading (P<0.05). IκB and Gal-3 were associated with the recurrence of EOC (P<0.05). IκB may be related to clinical stage (P<0.05). Multivariate analysis results showed that abnormal expression of Gal-3 may be an independent prognostic risk factors for the drug resistance to platinum-based chemotherapy (95% CI=5.336~34.112, P<0.05). The expression of Gal-3, p65, and IκB can be clinical immunohistochemical indicators that determine the prognosis of EOC, but the amount of Gal-3 expression was related to the epithelial ovarian cancer’s pathologic type and overall survival, which suggested that Gal-3 can be used as a prognostic factor in epithelial ovarian cancer. Conclusion: Targeted therapy of Gal-3 may become an effective potential new method against epithelial ovarian cancer.
Keywords: Galectin-3, epithelial ovarian cancer, p65, IκB, clinical prognosis
Introduction
Ovarian cancer (OC) is one of the more common malignant tumors in women [1]. Because of the characteristics of distant metastasis and surrounding infiltration, as well as lack of effective diagnostic methods and clinical prognostic index, OC is the deadliest malignant tumor in women [2]. Epithelial ovarian cancer (EOC) is the commonest type among ovarian cancer, and the one with worst prognosis [3]. According to statistics, the survival rate of early ovarian epithelial malignant tumor in 5-years can reach 70%~90%, but in the late stage, the 5-year survival rate is only 20% [4]; therefore early diagnosis and effective prognosis have great significance for treatment.
Recently, studies have found that certain functional proteins and signaling pathways may play a role in the origin and development of EOC [5]. Galectin-3 is the only protein with mosaic type of galactose lectin family, which plays an important role in a variety of biological actions [6]. Studies have shown that Gal-3’s expression correlation with the occurrence, development and metastasis with a variety of cancers such as gastric cancer, esophageal cancer, and cervical cancer [7]. Liu et al. have demonstrated that Galectin-3 is closed related to a poor prognosis in serous EOC [8]. However, the mechanism of how Gal-3 affects the biologic behavior in EOC at a clinical level is uncertain.
Nuclear factor-κB (NFκB) proteins are important transcription factors that regulate the expression of genes in a wide range of cell processes [9]. Activated NFκB enters the nucleus from cytoplasm and combines with start region of the specific gene, which starts the gene transcription, therefore affecting cell proliferation, apoptosis, and participation in the development of tumor [10,11]. NFκB is a key factor in a wide variety of cancers such as lung cancer, breast cancer, and liver cancer [12-14]. In EOC, NFκB also plays a greatly significant role in terms of cell proliferation, invasion, and migration [15,16].
Gal-3 is able to affect the migratory capabilities and chemotherapy sensitivity of EOC cancer cells through the NFκB pathway [17]. However, the role of Gal-3 and NFκB signaling pathway in occurrence and development of EOC is still unclear. To shed light on potential effects of Gal-3 on the origin and development of EOC, we first analyzed its expression levels in EOC. Furthermore, statistical analysis was conducted to investigate the correlation between the expression of Gal-3 and NFκB pathway related factors in EOC as well as platinum-based drugs resistance and prognosis. Our clinical research had demonstrated that Gal-3 expression can be a prognostic factor for progression-free survival (PFS) and may be involved in regulating the response to paclitaxel-based chemotherapy in the treatment of EOC.
Materials and methods
Human samples
99 cases of epithelial ovarian cancer patients receiving surgical treatment at Sun Yat-sen Memorial Hospital, Sun Yat-sen University from January 2009 to June 2014 were collected. Basic information, important examination results and prognosis of the patients were considered in our study (Table 1). All patients were diagnosed with epithelial ovarian cancer at Sun Yat-sen Memorial Hospital of Sun Yat-sen University. Chemotherapy was needed after surgery, and the chemotherapy was based on platinum. These patients had no other history of cancer. 20 patients were selected randomly as a control group from the department of gynecology of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. Patients in this group underwent the total hysterectomy due to benign lesions such as uterine fibroids, endometrial polyps, adenomyosis, dysfunction uterine bleeding, or CIN3. Postoperative pathology confirmed that there were no pathologic changes in the ovary. This study was approved by the ethics committee of Sun Yat-sen Memorial Hospital.
Table 1.
General information of patients with epithelial ovarian cancer
| Variable | Case number | Percentage % | Death | Survival | Survival rate | P |
|---|---|---|---|---|---|---|
| Age | ||||||
| >50 | 53 | 53.5 | 16 | 37 | 69.8 | 0.253 |
| ≤50 | 46 | 46.4 | 9 | 37 | 80.4 | |
| Pathologic type | ||||||
| Serous | 69 | 69.7 | 17 | 52 | 75.4 | 0.495 |
| Mucous | 6 | 6 | 3 | 3 | 50 | |
| Clear cell | 11 | 11.1 | 3 | 8 | 72.7 | |
| EA1 | 13 | 13.1 | 2 | 11 | 84.6 | |
| Clinical stage | ||||||
| I | 21 | 21.2 | 1 | 20 | 95.2 | 0.032* |
| II | 9 | 9.1 | 1 | 8 | 88.9 | |
| III | 56 | 56.6 | 19 | 37 | 66.1 | |
| IV | 13 | 13.1 | 4 | 9 | 69.2 | |
| Pathologic grade | ||||||
| G1 | 20 | 20.3 | 7 | 13 | 65 | 0.248 |
| G2 | 37 | 37.4 | 11 | 26 | 70 | |
| G3 | 42 | 42.4 | 7 | 35 | 80.3 | |
| Relapse | ||||||
| Yes | 50 | 50.5 | 25 | 25 | 50 | 0.000** |
| No | 49 | 49.5 | 0 | 49 | 100 | |
| Cisplatin-resistant | ||||||
| Yes | 22 | 22.2 | 17 | 5 | 22.7 | 0.000** |
| No | 77 | 77.8 | 8 | 69 | 89.6 |
EA: endometrial adenocarcinoma;
P<0.05;
P<0.01.
Immunohistochemistry
Paraffin sections gained from pathology department of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The producers of sections were ignorant of the clinical outcomes of samples. All staining procedures referred to the instructions of SP immunohistochemical kit and DAB chromogenic reagent kit. The sections were incubated with primary antibodies overnight at 4°C (Mouse anti-human Galectin-3/p65/IκB monoclonal antibody, Abcam, America). Then Mouse/rabbit universal Streptavidin-HRP kit was used as secondary antibody (Dako Denmark) for 1 h at room temperature. Mayer’s hematoxylin was employed to nucleus staining.
Evaluation of survival
All patients enrolled were followed-up. The definition of overall survival (OS) was the period between pathologic diagnosis and the day death from ovarian cancer or the final follow-up. The cutoff date was June 2016.
Statistical analysis
SPSS19.0 statistical analysis software package was used to process data. The expression difference between Gal-3, p65 and IκB was compared by the Chi-square test. Kaplan-Meier method was used for prognostic single factor analysis, and log-rank test was used in comparison between groups. The analysis of prognostic factors was carried out by Cox proportional risk model. Spearman rank correlation analysis was used for correlation analysis. P<0.05 was considered significant. Graph was made by GraphPad Prism 5.
Results
General information of patients with epithelial ovarian cancer
The basic information of 99 patients enrolled is listed in the Table 1; clinical data collected mainly include age, pathologic type, stage, pathologic classification, relapse, and sensitivity to platinum-based chemotherapy. Classification of clinical stage referred to the standards of The International Union of Gynecology and Obstetrics (FIGO) and The National Comprehensive Cancer Network (NCCN); Scores of pathologic grading were by the standards of FIGO. The mean survival time of patients followed up was 39.3 months, median survival time was 37 months; the shortest follow-up period was 6 months. The longest follow-up period was 72 months.
Expression of Gal-3, p62, and IκB in epithelial ovarian cancer and normal ovarian epithelium
The IHC results showed that Gal-3 protein expressed in cytoplasmic (Figure 1). Among 99 patients with epithelial ovarian cancer, there were 76 cases (76.8%) showing positive expression, among which, there were 45 (45.5%) cases of weak positive expression, and 31 cases (31.3%) of strong positive expression. Normal ovarian epithelium did not express Gal-3 protein (Table 2). The p65 protein is mainly expressed in the cytoplasm and nucleus (Figure 2). 74 (84.4%) cases expressed p65, and there were 38 cases (48.0%) of weak positive expression, and 36 cases (36.4%) of strong positive expression. Normal ovarian epithelium did not express p65 protein (Table 2). IκB was expressed in the cell membrane and cytoplasm (Figure 3). There were 58 cases (58.6%) were negative and 41 (41.4%) positive expressions. As for samples of normal ovarian epithelium, 2 cases (10%) show negative results, and 18 cases (90%) gained positive expression, among which there were 4 cases (20%) of weak positive expression, and 14 cases (70%) of strong positive expression. There were 24 cases (24.2%) of weak positive expression, and 17 cases (17.2%) of strong positive expression. As for samples of normal ovarian epithelium, 2 cases (10%) showed negative results, 18 cases (90%) had positive expression, among which, there were 4 cases (20%) of weak positive expression, and 14 cases (70%) of strong positive expression (Table 2). These results illustrated that the expression rate of Gal-3, p65, and IκB in epithelial ovarian cancer were significantly different from normal ovarian epithelium.
Figure 1.
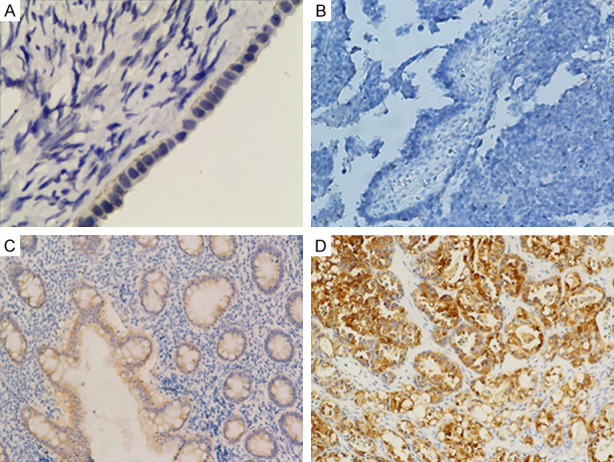
Expression of Gal-3 in normal ovarian epithelium and epithelial ovarian cancer. (×200). A. Normal ovarian epithelial cells; B. Epithelial ovarian cancer cells that are Gal-3 (-); C. Epithelial ovarian cancer cells that are Gal-3 (+); D. Epithelial ovarian cancer cells that are Gal-3 (++).
Table 2.
Expression of Gal-3, p65, and IκB in normal ovarian epithelium and EOC
| Group | Num | Gal-3 | p65 | IκB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| - | + | ++ | P | - | + | ++ | P | - | + | ++ | P | ||
| Normal | 20 | 20 | 0 | 0 | 0.000 | 20 | 0 | 0 | 0.000 | 2 | 4 | 14 | 0.000 |
| EOC | 99 | 23 | 45 | 31 | 25 | 38 | 36 | 58 | 24 | 17 | |||
Figure 2.
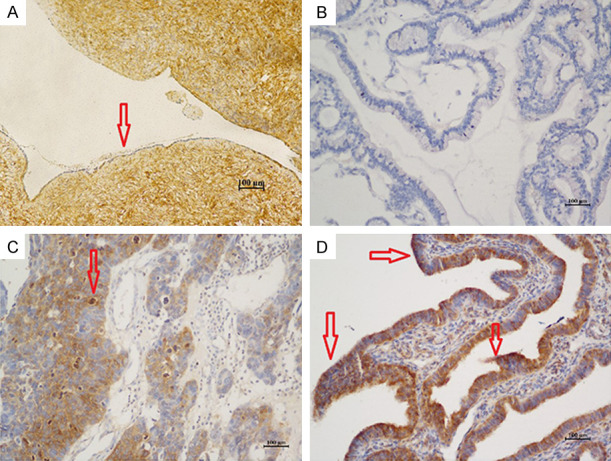
Expression of p65 in normal ovarian epithelium and epithelial ovarian cancer. (×200). A. Normal ovarian epithelial cells; B. Epithelial ovarian cancer cells that are p65 (-); C. Epithelial ovarian cancer cells that are p65 (+); D. Epithelial ovarian cancer cells that are p65 (++).
Figure 3.
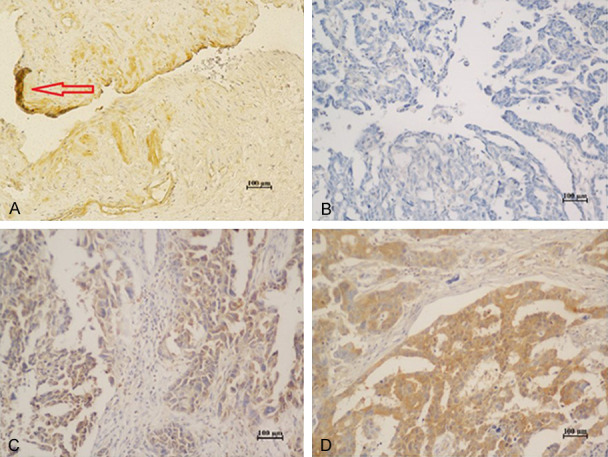
Expression of IκB in normal ovarian epithelial and epithelial ovarian cancer. (×200). A. Normal ovarian epithelial cells; B. Epithelial ovarian cancer cells that are IκB (-); C. Epithelial ovarian cancer cells that are IκB (+); D. Epithelial ovarian cancer cells that are IκB (++).
Relationship between expressions of Gal-3, p65 and IκB in EOC
Spearman correlation test was performed on the data above, and the results showed that the expression of Gal-3 and p65 in epithelial ovarian cancer were positively correlated. The correlation coefficient is 0.556 (r=0.556), P=0.000. This means that the stronger the expression of Gal-3, the stronger the expression of p65. While Gal-3 wsas expressed as weak-positive or negative, the expression of p65 was also attenuated or became negative. By contrast, the expression of Gal-3 and IκB was negatively correlated (r=-0.367), P=0.000, and the difference was statistically significant, which means the stronger the expression of Gal-3, the weaker the expression of IκB. If Gal-3 was expressed as weak-positive or negative, then the expression of IκB was strongly positive. The expression of p65 and I kappa B were negatively correlated (r=-0.469), P=0.000. The stronger the expression of p65, the weaker the expression of IκB. When p65 was expressed as weak-positive or negative, the expression of IκB was strongly positive (Table 3).
Table 3.
Relationship between Gal-3, p65, and IκB in EOC
| Gal-3 | p65 | IκB | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| r | P | r | P | r | P | |
| Gal-3 | 0.556 | 0.000 | -0.367 | 0.000 | ||
| p65 | 0.556 | 0.000 | -.469 | 0.000 | ||
| IkB | -0.367 | 0.000 | -0.469 | 0.000 | ||
Relationship between expression of Gal-3, p65, IκB and survival time
The Kaplan-Meier survival curve was plotted to describe the relationship between the Gal-3, p65, IκB and the survival time of patients, respectively. As shown in Figure 4A and 4B, the survival time of Gal-3and p65 strong positive group was significantly lower than that of the negative group. However, the survival time of IκB negative group was significantly lower than that of the weak positive group and the strong positive group (Figure 4C).
Figure 4.
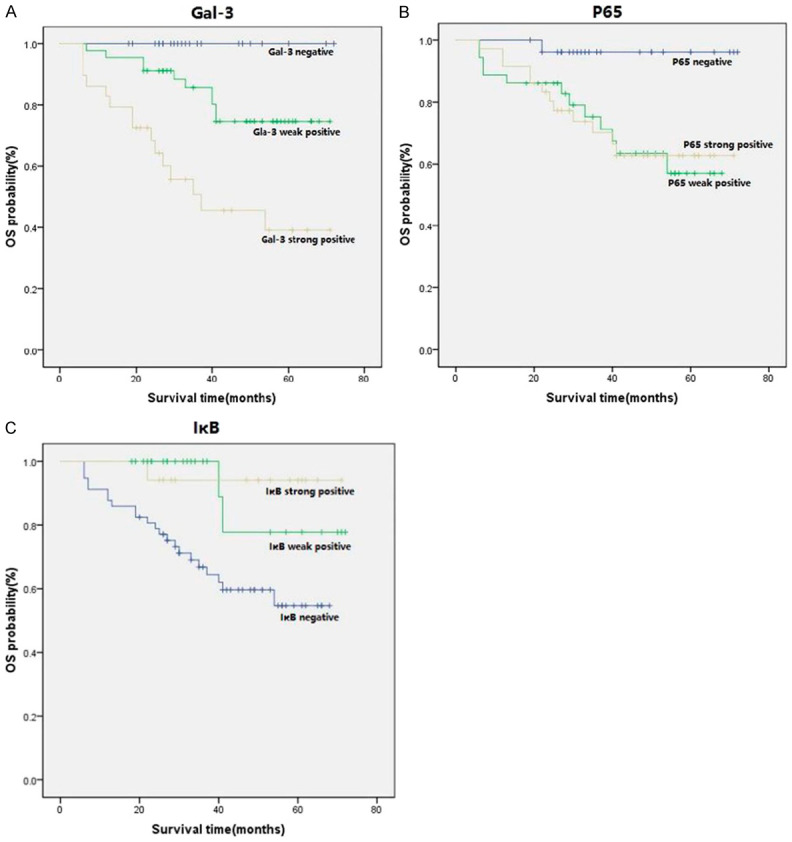
A. Survival curve of IHC positive expression of Gal-3 and survival rate. B. Survival curve of IHC positive expression of p65 and survival rate. C. Survival curve of IHC positive expression of IκB and survival rate.
Analysis of Gal-3, p65 and IκB expression in epithelial ovarian cancer patients and clinical features
Gal-3, p65, and IκB expression by IHC and clinicopathologicfeatures are shown in Table 4. Gal-3 had no difference in the distribution of age, pathologic type, or clinical stage (P>0.05), however, it was correlated with pathologic grade, recurrence, and platinum resistance with statistically significant differences (P<0.05). Protein p65 had no significant differences in age, pathologic type, clinical stage and recurrence (P>0.05), but it did correlate with pathologic grade and platinum resistance (P<0.05). As for IκB, there was no difference in age, pathologic type, or pathologic grade distribution (P>0.05); when compared with clinical stage, recurrence, and platinum resistance, the difference was statistically significant, (P<0.05).
Table 4.
Relationship between Gal-3, p65 and IκB and clinicopathologic data
| Variable | Case number | Gal-3 | p65 | IκB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| - | + | ++ | P | - | + | ++ | P | - | + | ++ | P | ||
| Age | |||||||||||||
| >50 | 53 | 13 | 27 | 13 | 0.436 | 16 | 19 | 18 | 0.768 | 29 | 14 | 4 | 0.854 |
| ≤50 | 46 | 12 | 18 | 16 | 11 | 17 | 18 | 28 | 11 | 7 | |||
| Pathologic type | |||||||||||||
| Serous | 69 | 19 | 32 | 18 | 0.268 | 19 | 21 | 29 | 0.215 | 38 | 18 | 13 | 0.230 |
| Mucous | 6 | 0 | 3 | 3 | 0 | 4 | 2 | 6 | 0 | 0 | |||
| Clear cell | 11 | 1 | 7 | 3 | 5 | 4 | 2 | 7 | 4 | 0 | |||
| EA1 | 13 | 5 | 3 | 5 | 3 | 7 | 3 | 6 | 3 | 4 | |||
| Clinical stage | |||||||||||||
| I | 21 | 9 | 8 | 4 | 0.482 | 10 | 7 | 4 | 0.065 | 9 | 10 | 2 | 0.010* |
| II | 9 | 1 | 5 | 3 | 3 | 3 | 3 | 2 | 3 | 4 | |||
| III | 56 | 11 | 26 | 19 | 9 | 24 | 23 | 38 | 8 | 10 | |||
| IV | 13 | 4 | 6 | 3 | 5 | 2 | 6 | 8 | 4 | 1 | |||
| Pathologic grade | |||||||||||||
| G1 | 20 | 8 | 5 | 7 | 0.034* | 8 | 8 | 4 | 0.023* | 11 | 7 | 2 | 0.412 |
| G2 | 37 | 4 | 23 | 10 | 6 | 19 | 12 | 22 | 6 | 9 | |||
| G3 | 42 | 13 | 17 | 12 | 13 | 9 | 20 | 24 | 12 | 6 | |||
| Relapse | |||||||||||||
| Yes | 50 | 8 | 20 | 22 | 0.003* | 10 | 18 | 22 | 0.186 | 36 | 8 | 6 | 0.021* |
| No | 49 | 17 | 25 | 7 | 17 | 18 | 14 | 21 | 17 | 11 | |||
| Cisplatin-resistant | |||||||||||||
| Yes | 22 | 0 | 8 | 14 | 0.000** | 1 | 9 | 12 | 0.011* | 19 | 3 | 0 | 0.004* |
| No | 77 | 25 | 37 | 15 | 26 | 27 | 24 | 38 | 22 | 17 | |||
EA: endometrial adenocarcinoma.
P<0.05;
P<0.01.
Analysis of prognostic factors in patients with epithelial ovarian cancer
Platinum resistance (P=0.004) and Gal-3 expression (P=0.017) were factors that affected overall survival postoperatively in patients with epithelial ovarian cancer (P<0.05). However, there was no significant correlation between age, pathologic type, pathologic grade, stage, recurrence, p65 and I kappa B expression and overall survival rate post operation in patients with epithelial ovarian cancer (P>0.05). Further multivariate analysis showed that platinum resistance (HR: 13.491, P=0.000) and Gal-3 expression (HR: 3.331, P=0.003) were significantly correlated with prognosis (Table 5).
Table 5.
Relationship between clinicopathologic data and prognosis (Cox regression)
| Variable | Single factor analysis | Multifactor analysis | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| X2 | HR | 95% CI | P | X2 | HR | 95% CI | P | |
| Age | 1.281 | 1.893 | 0.627~5.714 | 0.258 | ||||
| Pathologic type | 0.270 | 1.154 | 0.672~1.980 | 0.604 | ||||
| Clinical stage | 0.012 | 0.954 | 0.409~2.223 | 0.913 | ||||
| Relapse | 0.010 | 166729 | 0.0~6.5*109 | 0.922 | ||||
| Cisplatin-resistant | 0.010 | 5.360 | 1.699~16.909 | 0.004* | 30.226 | 13.491 | 5.336~34.112 | 0.000** |
| Gal-3 | 5.464 | 3.621 | 1.25~10.46 | 0.017* | 8.614 | 3.331 | 1.491~7.439 | 0.003* |
| p65 | 0.202 | 0.803 | 0.309~2.089 | 0.653 | ||||
| IκB | 1.712 | 0.471 | 0.152~1.455 | 0.191 | ||||
P<0.05;
P<0.01.
Discussion
Epithelial ovarian cancer (EOC) is the main form of ovarian cancer, accounting for about 90% of all cases of ovarian cancer [18]. The expression of Gal-3 was related to the development of multiple tumors including EOC [19,20]. However, the mechanism of Gal-3 promoting the occurrence and development of EOC is still unclear. NFκB participates in gene transcription regulation to promote protein expression, thus influencing a wide range of cell processes such as inflammation, cell proliferation, and apoptosis [20]. Gal-3 acts on NFκB signaling pathway, leading to the occurrence and development of ovarian epithelial malignant cancer [21]. Our previous study indicates that in EOC, Gal-3 may affect the migratory and invasive capabilities of cancer cells as well as the sensitivity of cancer cells to carboplatin by acting through the NFκB pathway [17]. We did not answer the question of how the Gal-3 and NFκB pathway affect clinical indicators.
In this study we found that p65 did not express in EOC cells, while IκB was highly expressed, which may indicate that NFκB signaling pathway was in a resting state most of the time in normal ovarian epithelium as well as this result is similar to previous study [22]. However, in EOC cells, p65 is highly expressed while the expression of IκB is low, suggesting that IκB may be phosphorylated and degraded, while p65 and p50 could be released, and resulted in activating the NFκB signaling pathway [23]. Many studies have demonstrated that Gal-3 could activate the NFκB signaling pathway. Zhou et al. found that Gal-3 could promote lung cancer by targeting NFκB [24]. Knockdown of Gal-3 lead to the inhibition of NFκB activation in the latently infected cells [25]. Depending on these messages, we conducted a two-variable Spearman correlation analysis of Gal-3, p65 and IκB respectively, and found that Gal-3 and p65 were positively correlated. Nevertheless, the expression of Gal-3 and IκB negatively correlated as the same as the relationship between the p65 and IκB. The above results may suggest that Gal-3 would lead to the hydrolysis of IκB phosphorylation and the increase of p65 nuclear expression, thus activating the NFκB signaling pathway in EOC [26].
Combining with the previous studies, we analyzed the relationship between IHC results of Gal-3, p65, IκB predominate expression and clinical characteristics of EOC patients. We found that expression of Gal-3, p65, and IκB significantly affected the survival rate of patients with EOC. Only IκB expression positively correlated with survival. Renal cell carcinoma patients show a worse survival rate when they have higher expression of Gal-3 [27]. In serous epithelial ovarian cancer, high expression of Gal-3 is related to a low survival rate and indicates a poor prognosis [8]. Li et al. found that the 5-year survival rate of patients expressing p65 were lower than those who do not express p65 [28]. Our further correlation analysis showed that Gal-3, p65, and IκB expression were associated with platinum resistance. It has been proven that Gal-3 was involved in the taxane resistance process of prostate cancer cells [29].
The expression of Gal-3 and p65 correlated with pathologic grading. Expression of I kappa B and Gal-3 associated with the recurrence of EOC. IκB was associated with clinical stage. Combined with the relationship between Gal-3 and p65 and I kappa B mentioned above, it seems these three markers may influence each other, and influence platinum chemotherapy drug resistance and clinical prognosis of EOC. These are likely to become important immunohistochemical indexes to determine the prognosis. The multi-factorial analysis result indicates that Gal-3 is an independent risk factor affecting the prognosis of patients with EOC, and probably can be used as an indicator of platinum chemotherapy drug resistance.
Conclusion
Although the number of samples and research design is insufficient, our results illustrate that Gal-3 can cause abnormal activation of the NF-kappa B signaling pathway by adjusting I kappa B and p65 in the cytoplasm, resulting in the development process of EOC. The strength of Gal-3 expression was related to the pathologic type and overall survival rate of epithelial ovarian cancer, suggesting that Gal-3 can be used as a prognostic factor, as well as an independent risk factor of platinum chemotherapy drug resistance. Targeted therapy for Gal-3 may be an effective method against ovarian epithelial cancer.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NO. 81602290) and Science and Technology Planning Project of Guangzhou (NO. 201601020102).
Disclosure of conflict of interest
None.
References
- 1.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Travers M, Brown S, Dunworth M, Holbert C, Casero R, Zahnow C. DFMO and 5-azacytidine increase M1 macrophages in the tumor microenvironment of an ovarian cancer mouse model. In.: AACR. 2019 doi: 10.1158/0008-5472.CAN-18-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katabuchi H. Frontiers in ovarian cancer science. Springer; 2017. [Google Scholar]
- 4.Nezhat FR, Apostol R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213:262–7. doi: 10.1016/j.ajog.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Sereni MI, Baldelli E, Gambara G, Deng J, Zanotti L, Bandiera E, Bignotti E, Ragnoli M, Tognon G, Ravaggi A, Meani F, Memo M, Angioli R, Liotta LA, Pecorelli SL, Petricoin E 3rd, Pierobon M. Functional characterization of epithelial ovarian cancer histotypes by drug target based protein signaling activation mapping: implications for personalized cancer therapy. Proteomics. 2015;15:365–73. doi: 10.1002/pmic.201400214. [DOI] [PubMed] [Google Scholar]
- 6.Funasaka T, Raz A, Nangia-Makker P. Galectin-3 in angiogenesis and metastasis. Glycobiology. 2014;24:886–91. doi: 10.1093/glycob/cwu086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song L, Tang JW, Owusu L, Sun MZ, Wu J, Zhang J. Galectin-3 in cancer. Clin Chim Acta. 2014;431:185–91. doi: 10.1016/j.cca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Xie L, Wang D, Li D, Xu G, Wang L, Zhou H, Yu Y, Lin Z, Lu H. Galectin-3 and β-catenin are associated with a poor prognosis in serous epithelial ovarian cancer. Cancer Manag Res. 2018;10:3963–3971. doi: 10.2147/CMAR.S171146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borg NA, Dixit VM. In encyclopedia of immunobiology. Academic Press; 2016. Ubiquitin signaling to NF-κB; pp. 51–64. [Google Scholar]
- 10.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 12.Wang SH, Ma SB, Yan Y, Cheng ZK, Zhang YC, Liu TX. Effects of total flavonoids from Cycas Revolute on expression of VEGF, bFGF, HIF-1α and NF-κB in model mice of Lewis lung cancer. Chin J Immunol. 2017;33:1029–1034. [Google Scholar]
- 13.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, Zeng M, Song E. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–81. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D, Breuhahn K, Conner EA, Galle PR, Andersen JB, Factor VM, Thorgeirsson SS. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J Hepatol. 2015;63:661–9. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–53. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutta S, Wang FQ, Wu HS, Mukherjee TJ, Fishman DA. The NF-κB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC) Gynecol Oncol. 2011;123:129–37. doi: 10.1016/j.ygyno.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Liu Y, Wang D, Wang L, Zhou H, Xu G, Xie L, Wu M, Lin Z, Yu Y, Li G. Galectin-3 regulates metastatic capabilities and chemotherapy sensitivity in epithelial ovarian carcinoma via NF-κB pathway. Tumour Biol. 2016;37:11469–77. doi: 10.1007/s13277-016-5004-3. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Qian Z, Ma X, Lin X, You Y, Li Y, Chen T, Jiang H. MiR-628-5p decreases the tumorigenicity of epithelial ovarian cancer cells by targeting at FGFR2. Biochem Biophys Res Commun. 2018;495:2085–2091. doi: 10.1016/j.bbrc.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 19.Farhad M, Rolig AS, Redmond WL. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology. 2018;7:e1434467. doi: 10.1080/2162402X.2018.1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossein G, Halvaei S, Heidarian Y, Dehghani-Ghobadi Z, Hassani M, Hosseini H, Naderi N, Sheikh Hassani S. Pectasol-C modified citrus pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019;8:4315–4329. doi: 10.1002/cam4.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7–20. doi: 10.1006/abbi.1997.0447. [DOI] [PubMed] [Google Scholar]
- 22.Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad Sci U S A. 1998;95:2307–12. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirotkin AV, Dekanová P, Harrath AH, Alwasel SH, Vašíček D. Interrelationships between sirtuin 1 and transcription factors p53 and NF-κB (p50/p65) in the control of ovarian cell apoptosis and proliferation. Cell Tissue Res. 2014;358:627–32. doi: 10.1007/s00441-014-1940-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Chen X, Hu Q, Chen X, Chen Y, Huang L. Galectin-3 activates TLR4/NF-κB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer. 2018;18:580. doi: 10.1186/s12885-018-4461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto M, Hidaka A, Toyama M, Baba M. Galectin-3 is involved in HIV-1 expression through NF-κB activation and associated with tat in latently infected cells. Virus Res. 2019;260:86–93. doi: 10.1016/j.virusres.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Nangia-Makker P, Balan V, Raz A. Galectin-3 binding and metastasis. Methods Mol Biol. 2012;878:251–66. doi: 10.1007/978-1-61779-854-2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang CS, Tang SJ, Lee MH, Chang Wang CC, Sun GH, Sun KH. Galectin-3 promotes CXCR2 to augment the stem-like property of renal cell carcinoma. J Cell Mol Med. 2018;22:5909–5918. doi: 10.1111/jcmm.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YZ, Zhao P. Expressions and clinicopathologic significance of Id2 and NF-κB/P65 in gastric cancer. Zhonghua Yi Xue Za Zhi. 2018;98:846–850. doi: 10.3760/cma.j.issn.0376-2491.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Fukumori T, Daizumoto K, Tsuda M, Ozaki K, Kusuhara Y, Mori H, Fukawa T, Yamamoto Y, Yamaguchi K, Takahashi M. MP81-12 galectin-3 is involved in the tumor progression and drug resistance induced by taxane chemotherapy and poly (adenosine diphosphate [adp] -ribose) polymerase (parp) inhibitor in castration-resistant prostate cancer. 2019;201:e1176–e1176. [Google Scholar]


