Abstract
Background: Increasing evidence suggests that circRNAs are involved in the pathogenesis of multiple kinds of cancer. Nevertheless, the differential expression of circRNAs in prostate cancer (PCA) is rarely reported. Material/Method: In our present analyses, circRNAs expression profiles were identified in PCA, based on 5 pairs of PCA and matched non-PCA tissues using circRNA chips. Results: A number of 749 differential circRNAs were expressed between PCA tumor and paracancerous tissues (Fold Change, FC ≥ 2.0 and P < 0.05): 261 were upregulated, whereas 487 were downregulated in PCA tissues. Gene ontology and KEGG pathway analyses indicated that many of the circRNAs are related to carcinogenesis. Circ_0033074 and circ_0016064 both showed changes of maximum magnitude among differentially expressed circRNAs. Conclusions: Our study detected a relative comprehensive differential map of circRNAs in PCA, which may become novel biomarkers for diagnosis, treatment and follow-up in the future.
Keywords: Prostate cancer, circRNA, microarray, expression
Introduction
The latest forecast data estimated that 174,650 new cases of prostate cancer (PCA) were diagnosed in the USA in 2019, causing 31,620 related deaths [1]. With dramatic economic growth and socio-cultural changes leading to an increased life expectancy and westernized lifestyle, the incidence and mortality frequency of PCA in China have shown a rapid growth trend [2]. Currently PCA has already ranked the sixth in incidence of the most frequent cancers, with about 72 thousand cases in 2015, and the tenth in cancer-related death, with about 31 thousand cases.
The androgen receptor (AR) pathway plays an essential role in the early stage of PCA. The androgen deprivation therapy (ADT) is effective for more than 80% patients, but after a median time of 14-30 months, lesions in almost all patients will gradually develop into an androgen independent state, namely castration resistant prostate cancer (CRPC) [3], which is a major cause of death in patients with advanced PCA with a median survival time less than 20 months. The mechanism of CRPC is very complicated, and recent studies revealed that the AR pathway still plays an important role [4]; in addition, the tumor related pathways, such as PI3K/AKT, RAS/MAPK, TGF-β were also reported in the process of CRPC [5-7]. Although docetaxel/prednisone and AR blocking drugs (such as abiraterone, enzalutamide) can delay the development of CRPC [8,9], the clinical effect is limited, and the development of effective biomarkers for early detection and targeted treatment is receiving much attention.
So far, many novel biomarkers, including DNA, proteins, non-coding RNAs, and exosomes, have been reported [10-13]. Among them, the role of non-coding RNAs in cancer has been under wide consideration, with miRNAs, lncRNA, and circRNAs identified in the last five years [14,15]. Accumulated evidence has suggested a close association between miRNAs/lncRNAs and the proliferation, invasion, and progression of cancer. Recent studies have focused on the circRNAs in cancer development [16,17]. However, studies about the association between circRNAs and PCA risk have not been large enough to reach a definitive conclusion.
CircRNAs are a class of RNA molecules that lack 5’-3’ ends and poly A tail and covalently form closed loops [18]. The advantage of circRNAs, rather than miRNAs and lncRNAs, is that they exist stably and are not easily degraded by exonuclease RNase R in the cells [19]. Growing evidence has shown that circRNAs are involved in the pathogenesis of variety of diseases, such as diabetes, Alzheimer’s disease, and cancer through corresponding miRNAs [20-22]. Moreover, the stability and specificity of circRNAs in body fluids have made them new molecular biomarkers for cancer diagnosis and monitoring [23,24]. Still, the expression and latent roles of circRNAs in PCA are still little understood. In the current analyses, we found a differential expression of circRNAs in PCA tissues, to identify several significant and potential biomarkers.
Materials and methods
Tissue samples
A total of 5 pairs of PCA and matched non-tumor normal tissues were collected from Huashan Hospital, Fudan University. Our study was approved by the ethics committee of Huashan Hospital, Fudan University, and written informed consent was acquired from all patients. All tissue was histologically identified, diagnosed as prostate adenocarcinoma, and the Gleason score, PSA value, TNM stage, and recurrence were according to the NCCN guidelines. The initial screening step (Table 1) was processed by microarray chip assay.
Table 1.
Basic information of patients with PCA included in our study
| NO | Gender | Age (Years) | Histologic type | Initial total PSA | Gleason score | TNM stage |
|---|---|---|---|---|---|---|
| 13 | Man | 64 | Adenocarcinoma | 9.39 | 3+4 | T2cN0M0 |
| 24 | Man | 50 | Adenocarcinoma | 15.84 | 4+3 | T3bN0M0 |
| 26 | Man | 62 | Adenocarcinoma | 14 | 4+3 | T3bN0M1 |
| 45 | Man | 54 | Adenocarcinoma | 9.13 | 4+3 | T2cN0M0 |
| 67 | Man | 62 | Adenocarcinoma | 54.66 | 5+4 | T3bN1M1 |
RNA extraction and purification
MirVana™ miRNA Isolation Kit without phenol (Ambion, Austin, TX, US) was applied to extracted and purified total RNA, following the manufacturer’s instructions and Agilent Bioanalyzer 2100 was put to use to check for a RIN number to inspect RNA integration (Agilent Technologies, Santa Clara, US).
RNA labeling
Low Input Quick Amp WT Labeling Kit (Agilent Technologies, Santa Clara, US) was used to amplify and label the rRNA. Immediately afterwards, RNeasy mini kit was carried out to purify the labeled cRNA (QIAGEN, Germany).
Array hybridization
Gene Expression Hybridization Kit was used to hybridize each slide with 1.65 μg Cy3-labeled cRNA in Hybridization Oven (Agilent Technologies, Santa Clara, US). After hybridization, staining dishes (Thermo Shandon, Waltham, US) and Gene Expression Wash Buffer Kit (Agilent Technologies, Santa Clara, US) were employed to wash the slides. Differentially expressed circRNAs were analyzed with independent samples by t-test. Any fold change (FC) and P-value for circRNAs that were more than 2 or less than 0.05, respectively, were considered as significant differential expression.
Data acquisition
Agilent Microarray Scanner was applied to scan the slides (Agilent Technologies, Santa Clara, US) with default settings, Dye channel: Green, Scan resolution = 3 μm, PMT 100%, 20 bits. Feature Extraction software 10.7 was applied to extract data (Agilent technologies, Santa Clara, US). Raw data were normalized by Quantile algorithm (Limma packages in R).
Bioinformatics analysis
Differentially expressed circRNAs identified with profiling data were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses, and whose targeted miRNAs were predicted by miRanda software (http://miranda.org.uk/) coupled with statistical analysis substantially. The circRNAs expression profile microarray chip assay, plus data and bioinformatics analysis were produced, tested and analyzed by Shanghai Biotechnology Corporation (Shanghai, China).
Results
CircRNAs expression profiles in PCA
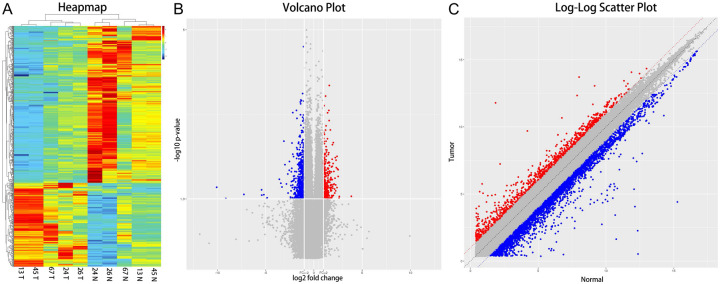
The microarray testing identified 88,750 kinds of circRNAs in PCA and or non-PCA tissues. As illustrated in Figure 1, 749 circRNAs were differentially expressed between PCA tumor and paracancerous tissues (FC ≥ 2.0 and P < 0.05) (Table S1): among which 261 were upregulated, and the other 487 were downregulated in PCA tissues. The circRNAs with the highest differential expression were the has_circ_0016064 among downregulated circRNAs (FC = 0.00656, P = 0.0399) and the has_circ_0033074 among upregulated circRNAs (FC = 14.85488, P = 0.0439), respectively. Hierarchical clustering (Figure 1A), volcano plot (Figure 1B), and scatter plots (Figure 1C) showed that the different expression profiles of circRNAs between PCA and non-PCA tissues were diverse. The top each twenty up- and down-regulated circRNAs are listed in Table 2.
Figure 1.
Hierarchical clustering, volcano plots, and scatter plots displayed the differentially expressed circRNAs in PCA tissues compared to paracancerous tissues. A. Hierarchical clustering: numbers were the samples used for the microarray assay. T: PCA tissues, N: paracancerous tissues. B. Differentially expressed circRNAs are shown as volcano plots. The blue and red parts indicate (FC more than 2 folds) downregulated and upregulated expression circRNAs in PCA tissues, respectively (P < 0.05). C. Differentially expressed circRNAs shown by scatter plots. The green and red parts indicate (FC more than 2 folds) downregulated and upregulated expression circRNAs in PCA tissues, respectively (P < 0.05).
Table 2.
The top twenty kinds of decreased and increased differentially expressed circRNAs in PCA tissues compared to those in non-cancerous tissues and their highest frequency of MREs through sponge adsorption
| ProbeName | P-values | Fold change | Regulation | Circ_chrom | Hostgene | CircRNA | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MRE1$ (combine bit points) | MRE2$ (combine bit points) | MRE3$ (combine bit points) | ||||||
| hsa_circ_0033074 | 0.043966188 | 14.85487618 | Up | chr14 | SERPINA3 | hsa-miR-6889-3p$2 | hsa-miR-1273e$2 | hsa-miR-296-5p$1 |
| hsa_circ_0057549 | 0.044251247 | 6.259110255 | Up | chr2 | TMEFF2 | hsa-miR-6838-5p$4 | hsa-miR-195-5p$4 | hsa-miR-16-5p$4 |
| hsa_circ_0076303 | 0.011520736 | 5.749538882 | Up | chr6 | PGC | hsa-miR-198$2 | hsa-miR-4779$2 | hsa-miR-520a-5p$2 |
| hsa_circ_0076304 | 0.016135493 | 5.747004796 | Up | chr6 | PGC | hsa-miR-6799-3p$2 | hsa-miR-6789-3p$2 | hsa-miR-3173-5p$2 |
| hsa_circ_0076305 | 0.011034354 | 5.461548042 | Up | chr6 | PGC | hsa-miR-6865-5p$3 | hsa-miR-6815-5p$3 | hsa-miR-936$3 |
| hsa_circ_0040583 | 0.00586104 | 5.295847499 | Up | chr16 | CENPN | hsa-miR-619-5p$4 | hsa-miR-6506-5p$4 | hsa-miR-661$4 |
| hsa_circ_0024925 | 0.028275165 | 5.28313187 | Up | chr11 | ACAD8 | hsa-miR-7112-5p$1 | hsa-miR-6828-3p$1 | hsa-miR-3615$1 |
| hsa_circ_0008053 | 0.01034582 | 5.025655906 | Up | chr6 | PAK1IP1 | hsa-miR-4252$2 | hsa-miR-3144-5p$1 | hsa-miR-6810-5p$1 |
| hsa_circ_0007405 | 0.007097955 | 4.940850802 | Up | chr16 | CENPN | hsa-miR-7110-3p$2 | hsa-miR-554$1 | hsa-miR-1185-5p$1 |
| hsa_circ_0062627 | 0.024770482 | 4.810985476 | Up | chr22 | GGT1 | hsa-miR-3605-5p$2 | hsa-miR-3065-3p$2 | hsa-miR-3679-5p$2 |
| hsa_circ_0004390 | 0.03341108 | 4.801446853 | Up | chr1 | LPAR3 | hsa-miR-198$3 | hsa-miR-711$2 | hsa-miR-6512-3p$2 |
| hsa_circ_0040578 | 0.006241681 | 4.676950834 | Up | chr16 | CENPN | hsa-miR-619-5p$5 | hsa-miR-6506-5p$5 | hsa-miR-146a-3p$4 |
| hsa_circ_0070475 | 0.017644399 | 4.574571538 | Up | chr4 | PDLIM5 | hsa-miR-4480$2 | hsa-miR-5695$2 | hsa-miR-8485$2 |
| hsa_circ_0005917 | 0.030493374 | 4.458396676 | Up | chr6 | PAK1IP1 | hsa-miR-572$1 | hsa-miR-324-5p$1 | hsa-miR-6501-5p$1 |
| hsa_circ_0070466 | 0.023657495 | 4.306640303 | Up | chr4 | PDLIM5 | hsa-miR-544b$4 | hsa-miR-378g$3 | hsa-miR-4324$3 |
| hsa_circ_0013059 | 0.042001336 | 4.089111212 | Up | chr1 | LPAR3 | hsa-miR-198$4 | hsa-miR-6720-5p$3 | hsa-miR-6512-3p$3 |
| hsa_circ_0062625 | 0.002930265 | 4.055779738 | Up | chr22 | GGT1 | hsa-miR-4778-3p$3 | hsa-miR-4469$2 | hsa-miR-324-5p$2 |
| hsa_circ_0075601 | 0.024302633 | 3.988707364 | Up | chr6 | PAK1IP1 | hsa-miR-572$1 | hsa-miR-324-5p$1 | hsa-miR-6501-5p$1 |
| hsa_circ_0004646 | 0.009677779 | 3.774169933 | Up | chr1 | UAP1 | hsa-miR-6772-3p$3 | hsa-miR-6801-3p$2 | hsa-miR-6810-3p$2 |
| hsa_circ_0072904 | 0.044529246 | 3.764492045 | Up | chr5 | MCCC2 | hsa-miR-1306-5p$4 | hsa-miR-6514-3p$2 | hsa-miR-3160-5p$2 |
| hsa_circ_0020064 | 0.048931868 | 0.236283669 | Down | chr10 | ABLIM1 | hsa-miR-4731-5p$5 | hsa-miR-3972$5 | hsa-miR-1202$5 |
| hsa_circ_0090179 | 0.005928275 | 0.232902566 | Down | chrX | DMD | hsa-miR-4635$3 | hsa-miR-378g$2 | hsa-miR-3664-3p$2 |
| hsa_circ_0090180 | 0.005242068 | 0.220318677 | Down | chrX | DMD | hsa-miR-4687-3p$1 | hsa-miR-7113-5p$1 | hsa-miR-3664-3p$1 |
| hsa_circ_0057896 | 0.047006842 | 0.214726487 | Down | chr2 | NRP2 | hsa-miR-23b-5p$1 | hsa-miR-23a-5p$1 | hsa-miR-454-5p$1 |
| hsa_circ_0029996 | 0.028345901 | 0.214352917 | Down | chr13 | DCLK1 | hsa-miR-3189-5p$1 | hsa-miR-4434$1 | hsa-miR-5703$1 |
| hsa_circ_0032813 | 0.012751171 | 0.198553084 | Down | chr14 | NRXN3 | hsa-miR-181a-5p$2 | hsa-miR-181b-5p$2 | hsa-miR-181d-5p$2 |
| hsa_circ_0020060 | 0.025564507 | 0.192676458 | Down | chr10 | ABLIM1 | hsa-miR-3972$4 | hsa-miR-1202$4 | hsa-miR-3194-5p$4 |
| hsa_circ_0074026 | 0.047967039 | 0.188441006 | Down | chr5 | PITX1 | hsa-miR-6784-5p$4 | hsa-miR-1292-3p$4 | hsa-miR-4747-3p$4 |
| hsa_circ_0032812 | 0.007589741 | 0.172980926 | Down | chr14 | NRXN3 | hsa-miR-181a-5p$2 | hsa-miR-181b-5p$2 | hsa-miR-181d-5p$2 |
| hsa_circ_0087142 | 0.011816017 | 0.17097621 | Down | chr9 | PIP5K1B | hsa-miR-7110-3p$4 | hsa-miR-6873-3p$4 | hsa-miR-6817-3p$4 |
| hsa_circ_0087140 | 0.008909913 | 0.125440984 | Down | chr19 | HSPB6 | hsa-miR-6861-5p$2 | hsa-miR-1229-5p$2 | hsa-miR-5589-5p$2 |
| hsa_circ_0087144 | 0.002724689 | 0.116309931 | Down | chr9 | PIP5K1B | hsa-miR-6735-3p$2 | hsa-miR-100-3p$2 | hsa-miR-3141$1 |
| hsa_circ_0007499 | 0.02749466 | 0.101227605 | Down | chr3 | VEPH1 | hsa-miR-216b-5p$3 | hsa-miR-6511a-3p$2 | hsa-miR-6511b-3p$2 |
| hsa_circ_0067802 | 0.022712773 | 0.093496121 | Down | chr3 | VEPH1 | hsa-miR-211-3p$3 | hsa-miR-6754-5p$3 | hsa-miR-4270$3 |
| hsa_circ_0067804 | 0.018611184 | 0.083830657 | Down | chr3 | VEPH1 | hsa-miR-8070$2 | hsa-miR-653-3p$2 | hsa-miR-4666b$2 |
| hsa_circ_0057223 | 0.047480592 | 0.034978971 | Down | chr2 | TTN | hsa-miR-4659a-3p$17 | hsa-miR-4659b-3p$17 | hsa-miR-4778-3p$14 |
| hsa_circ_0057213 | 0.040500989 | 0.027514635 | Down | chr2 | TTN | hsa-miR-6715b-5p$32 | hsa-miR-4269$32 | hsa-miR-4266$20 |
| hsa_circ_0057224 | 0.041513118 | 0.023651164 | Down | chr2 | TTN | hsa-miR-4660$10 | hsa-miR-5581-5p$8 | hsa-miR-4474-3p$8 |
| hsa_circ_0057222 | 0.031686665 | 0.023389371 | Down | chr2 | TTN | hsa-miR-4659a-3p$14 | hsa-miR-4659b-3p$14 | hsa-miR-4778-3p$11 |
| hsa_circ_0016064 | 0.03996311 | 0.006560389 | Down | chr1 | MYBPH | hsa-miR-1911-3p$2 | hsa-miR-6753-5p$2 | hsa-miR-516b-5p$2 |
FC: Fold changes. MRE: microRNA response element. $: the number of combination sites.
Bioinformatics analysis
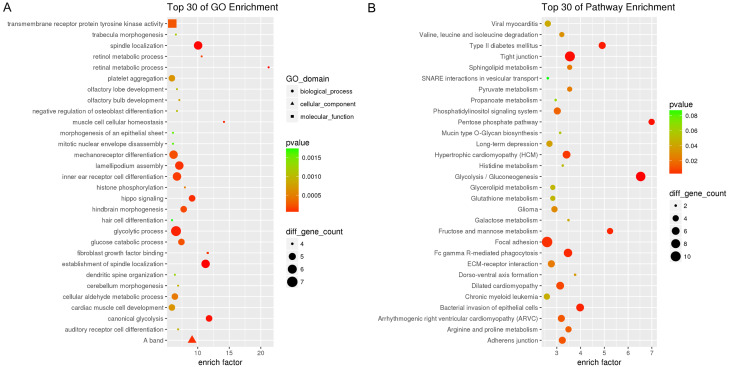
All differentially expressed circRNAs could be located to all chromosomes, except for chromosomes 21 and Y (Table S1). The top each twenty up- and down-regulated circRNAs and corresponding three kinds of sponges of microRNAs are shown in Table 2. Moreover, each Top 30 enrichments according to GO and KEGG analyses suggested that these differentially expressed circRNAs were relevant to several vital physiologic processes, such as transmembrane receptor protein tyrosine kinase activity, spindle localization, glycolytic process, phosphatidylinositol signaling system, and gluconeogenesis. Many common pathways associated with invasion and metastasis of PCA, such as tight junction, focal adhesion, adherens junction, ECM-receptor interatom, and SNARE interactions in vesicular transport were also implicated (Figure 2A, 2B).
Figure 2.
GO and KEGG pathway analysis of differentially expressed circRNAs. A. GO enrichment terms of top 30 classes. B. KEGG pathway enrichment terms of top 30 classes.
Discussion
The most the popular biomarker for early detection of PCA is the PSA value. However, the specificity and stability of PSA are relatively low. Therefore, it is necessary to find new PCA diagnostic and monitoring markers for early screening of PCA [25,26]. CircRNAs are natural endogenous RNAs, widely studied in recent five years. The mechanism of circRNAs is to function as miRNA sponges like competitive endogenous RNA molecules [27]. miRNAs are important regulators in gene expression and play a crucial role in cancer progression. Based on its stable properties, it may become a biomarker in many samples: such as tissues, blood, urine, saliva, secretions, and feces.
Xia et al. screened differentially expressed circRNAs using SBC-ceRNA array in 4 pairs of prostate tumor and paracancerous tissues [28]. 1021 differentially expressed circRNAs were identified. They demonstrated that combination of PSA level and two differentially expressed circ_0057558 and circ_0062019 showed significantly increased AUC, sensitivity, and specificity compared to PSA alone. However, the clinical information about PSA value, Gleason score, and TNM stage were missing, so heterogeneity may be enlarged. Zhang et al. analyzed differential circRNAs among three kinds of PCA cells (RWPE-1, 22RV1 and PC-3) by high-throughput circRNAs sequencing [29]. 9545 circRNAs were detected and hundreds of differentially expressed circRNAs were recognized. Our study is a timely and updated study combining gene chip and bioinformatic analyses. In this study, we identified 749 circRNAs differentially expressed between PCA and non-cancerous tissues by circRNAs chips. Has_circ_0033074 had the highest magnitude of upregulation, whereas has_circ_0016064 had the lowest expression in PCA tissue compared to the corresponding normal tissue.
SERPINA3 (serpin family A member 3), locates on 14q32.13 is the host gene for circ_0033074, which is a plasma protease inhibitor and member of the serine protease inhibitor class, which acts as an oncogene based on previous studies. Kulesza et al. reported SERPINA3 was a novel STAT3 target gene, involved in regulation of melanoma migration and invasion [30]. Cao et al. showed that SERPINA3 silencing may inhibit the migration, invasion, and liver metastasis of colon cancer cells [30]. Yang et al. provided insight that SERPINA3 promoted endometrial cancer cell growth by regulating cell cycle checkpoint and inhibiting apoptosis [31]. Based on the lowest expression of circ_0016064, MYBPH (myosin binding protein H) is its host gene, located at the 1q32.1 position. Hosono et al. validated that MYBPH, as a transcriptional target of TTF-1, could inhibit ROCK1 and reduce cell motility and metastasis in lung adenocarcinoma [32]. In addition, Zhu et al. found MYBPH could inhibit vascular smooth muscle cell migration and attenuate neointimal hyperplasia in a rat carotid balloon-injury model [33]. In summary, MYBPH acts as a anti-cancer molecule. Bioinformatics analyses of the trends for both circ_0033074 and circ_0016064 are according to the function of their host genes.
CircRNAs in PCA act as a double-edged sword. On one hand, circRNAs are proven to be related to the behavior of cancer cells’ tumorigenesis and malignancy, such as proliferation, migration, and invasion. For example, Chen et al. illustrated that circHIPK3 was overexpressed in PCA tissue, and its higher expression was associated with tumor stage. Additionally, circHIPK3/miR-193a-3p-MCL1 signaling promoted PCA development and progression [34]. On the other hand, circRNAs have been identified to play vital roles in suppressing cell proliferation and arresting tumor progression. For instance, Huang et al. considered that circ-ITCH was downregulated in PCA tissue, and its low expression correlated with clinical characteristics, such as advanced pathologic T stage, high lymph mode metastasis risk, and poor overall survival [34]. Moreover, Song et al. revealed circ_0001206 played a suppressive role in the pathogenesis of PCA [35].
The current study used the combination of circRNAs chips with bioinformatic analyses of PCA tissues. There remain several limitations to the study. First, the top 20 differentially expressed circRNAs should be verified by real-time PCR. Second, the clinical PCA patients undergoing radical prostatectomy should be increased to compare the significant circRNAs to corresponding non-tumor tissues. Other evaluation-indicators such as overall survival, disease-free survival, Gleason score, surgical margin status, and lymph node metastasis, should be included in the analysis. Third, some PCA cells should be added to evaluate to the function and deep mechanism of significant circRNAs, so that they may be become novel biomarkers for diagnosis and treatment.
Conclusion
Our study provided a new landscape of circRNA differential expression in PCA vs. benign tissue. Further studies are required to show their potential functions as biomarkers for PCA.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 81372316, 81802576), the Science and Technology Development Fund of Wuxi (no. WX18IIAN024, CSE31N1605) and the Wuxi Health and Family Planning Commission (no. Q201746, J201803, jzyx03, Z201712, T201713, J201810, ZM001), Youth talent project of Wuxi Commission of Health and Family Planning (no. QNRC043), Traditional Chinese Medicine Administration of Jiangsu Province (no. YB201827). In addition, we are grateful for the guidance of Professor Guowei Xia and Affiliated Hospital of Jiangnan University.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Tilki D, Schaeffer EM, Evans CP. Understanding mechanisms of resistance in metastatic castration-resistant prostate cancer: the role of the androgen receptor. Eur Urol Focus. 2016;2:499–505. doi: 10.1016/j.euf.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crumbaker M, Khoja L, Joshua AM. AR signaling and the PI3K pathway in prostate cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimbrough-Allah MN, Millena AC, Khan SA. Differential role of PTEN in transforming growth factor beta (TGF-beta) effects on proliferation and migration in prostate cancer cells. Prostate. 2018;78:377–389. doi: 10.1002/pros.23482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 10.Brikun I, Nusskern D, Freije D. An expanded biomarker panel for the detection of prostate cancer from urine DNA. Exp Hematol Oncol. 2019;8:13. doi: 10.1186/s40164-019-0137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intasqui P, Bertolla RP, Sadi MV. Prostate cancer proteomics: clinically useful protein biomarkers and future perspectives. Expert Rev Proteomics. 2018;15:65–79. doi: 10.1080/14789450.2018.1417846. [DOI] [PubMed] [Google Scholar]
- 12.Panigrahi GK, Deep G. Exosomes-based biomarker discovery for diagnosis and prognosis of prostate cancer. Front Biosci (Landmark Ed) 2017;22:1682–1696. doi: 10.2741/4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song C, Chen H, Song C. Research status and progress of the RNA or protein biomarkers for prostate cancer. Onco Targets Ther. 2019;12:2123–2136. doi: 10.2147/OTT.S194138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinge CM. Non-coding RNAs: long non-coding RNAs and microRNAs in endocrine-related cancers. Endocr Relat Cancer. 2018;25:R259–R282. doi: 10.1530/ERC-17-0548. [DOI] [PubMed] [Google Scholar]
- 15.Lim MCJ, Baird AM, Aird J, Greene J, Kapoor D, Gray SG, McDermott R, Finn SP. RNAs as candidate diagnostic and prognostic markers of prostate cancer-from cell line models to liquid biopsies. Diagnostics (Basel) 2018;8 doi: 10.3390/diagnostics8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: a new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–388. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Yang J, Zhou P, Le Y, Zhou C, Wang S, Xu D, Lin HK, Gong Z. Circular RNAs in cancer: novel insights into origins, properties, functions and implications. Am J Cancer Res. 2015;5:472–480. [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Guo S, Li W, Yu P. The circular RNA Cdr1as, via miR-7 and its targets, regulates insulin transcription and secretion in islet cells. Sci Rep. 2015;5:12453. doi: 10.1038/srep12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Alexandrov PN, Jaber V, Lukiw WJ. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer’s disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7) Genes (Basel) 2016;7 doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH, Pandolfi PP. Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Memczak S, Papavasileiou P, Peters O, Rajewsky N. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS One. 2015;10:e0141214. doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J, Wildhagen MF, Roobol MJ, Bangma CH, Bartsch G. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol. 2010;57:921–927. doi: 10.1016/j.eururo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Romero Otero J, Garcia Gomez B, Campos Juanatey F, Touijer KA. Prostate cancer biomarkers: an update. Urol Oncol. 2014;32:252–260. doi: 10.1016/j.urolonc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J, Ao Y. Circular RNA related to the chondrocyte ECM regulates MMP13 expression by functioning as a MiR-136 ‘sponge’ in human cartilage degradation. Sci Rep. 2016;6:22572. doi: 10.1038/srep22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Q, Ding T, Zhang G, Li Z, Zeng L, Zhu Y, Guo J, Hou J, Zhu T, Zheng J, Wang J. Circular RNA expression profiling identifies prostate cancer- specific circRNAs in prostate cancer. Cell Physiol Biochem. 2018;50:1903–1915. doi: 10.1159/000494870. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Xiong J, Yang Q, Wang Y, Shi H, Tian Q, Huang H, Kong D, Lv J, Liu D, Gao X, Zi X, Sun Y. Profiling and bioinformatics analyses of differential circular RNA expression in prostate cancer cells. Future Sci OA. 2018;4:FSOA340. doi: 10.4155/fsoa-2018-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulesza DW, Ramji K, Maleszewska M, Mieczkowski J, Dabrowski M, Chouaib S, Kaminska B. Search for novel STAT3-dependent genes reveals SERPINA3 as a new STAT3 target that regulates invasion of human melanoma cells. Lab Invest. 2019;99:1607–1621. doi: 10.1038/s41374-019-0288-8. [DOI] [PubMed] [Google Scholar]
- 31.Yang GD, Yang XM, Lu H, Ren Y, Ma MZ, Zhu LY, Wang JH, Song WW, Zhang WM, Zhang R, Zhang ZG. SERPINA3 promotes endometrial cancer cells growth by regulating G2/M cell cycle checkpoint and apoptosis. Int J Clin Exp Pathol. 2014;7:1348–1358. [PMC free article] [PubMed] [Google Scholar]
- 32.Hosono Y, Yamaguchi T, Mizutani E, Yanagisawa K, Arima C, Tomida S, Shimada Y, Hiraoka M, Kato S, Yokoi K, Suzuki M, Takahashi T. MYBPH, a transcriptional target of TTF-1, inhibits ROCK1, and reduces cell motility and metastasis. EMBO J. 2012;31:481–493. doi: 10.1038/emboj.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu T, He Y, Yang J, Fu W, Xu X, Si Y. MYBPH inhibits vascular smooth muscle cell migration and attenuates neointimal hyperplasia in a rat carotid balloon-injury model. Exp Cell Res. 2017;359:154–162. doi: 10.1016/j.yexcr.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Lu X, Yang F, Xing N. Circular RNA circHIPK3 promotes cell proliferation and invasion of prostate cancer by sponging miR-193a-3p and regulating MCL1 expression. Cancer Manag Res. 2019;11:1415–1423. doi: 10.2147/CMAR.S190669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Z, Zhuo Z, Ma Z, Hou C, Chen G, Xu G. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47:2449–2464. doi: 10.1080/21691401.2019.1626866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.