Abstract
Background: This study provided a reliable experimental basis for exploring the pathogenesis of PTSD-induced memory impairment, fear abnormalities, and affective disorders, aiming to facilitate new thinking for the prevention, treatment, and drug development of clinical PTSD. Material and methods: A rat model of PTSD was established by continuous single stress stimulation method. The Morris water maze was used to detect the learning and spatial memory exploration abilities of rats. The autonomic motion behavior, fear, and anxiety of rats in each group was detected by the elevated plus maze test and the open field test. The immunofluorescence method was employed to observe and detect the changes of autophagy in mPFC neurons of PTSD rats. Western blotting was used to detect the expressions of autophagy-related genes Beclin-1 and LC3, autophagy substrate p62 protein, and apoptosis-related factors Bcl-2 and Bax. Result: Gastrodin could improve the learning and spatial memory abilities of PTSD-SPS rats, the reduction of active movement and inquiry behavior, and the autophagy of mPFC neurons, and also increase the expressions of Beclin-1, LC3-I, LC3-II, and Bax proteins, as well as decrease the expressions of Bcl-2 and p62. Conclusions: Gastrodin is effective in the treatment of PTSD-induced memory impairment, fear abnormalities, and affective disorders. The mechanism is related to autophagy in mPFC neurons.
Keywords: Gastrodin, PTSD, medial prefrontal cortex, autophagy
Introduction
Post-traumatic stress disorder (PTSD) is a mental disorder caused by a serious or fatally stressful event or situation. Memory impairment is one of the main symptoms and clinical diagnostic criteria of PTSD. Most patients with PTSD who have experienced severe and fatally stressful events have significant memory impairments, such as invasive memory, fear memory, abnormal declarative memory, and traumatic forgetting [1]. Studies have shown that memory impairment in PTSD patients is closely related to changes in the structure and function of the central nervous system [2]. Medial prefrontal cortex (mPFC) is one of the important marginal systems, and is also sensitive to stress response. mPFC can regulate the response of amygdala to fear stimuli. The structural and functional changes of mPFC caused by PTSD can reduce the regulation and control ability of amygdala, leading to excessive response of amygdala to fear stimuli. This may also be the cause of fearful reactions in patients with PTSD that cannot be resolved for a long time or even a lifetime. At the same time, the unbalanced communication between mPFC, hippocampal, and amygdala loops have further led to memory impairment in patients with PTSD [3].
Autophagy is a conserved subcellular structure degradation process of eukaryotic cells. It is characterized by a bilayer membrane structure that encapsulates the substances to form autophagosomes, bind to lysosomes, and degradation. The process of autophagy is precisely regulated by a series of autophagy-related genes. Beclin-1 and microtubule-associated protein 1 light chain 3 (MAP1-LC3/LC3) are mammalian homologs of Atg6 and Atg8, respectively. It plays a vital role in the process of phagocytosis and autophagy development [4].
Materials and methods
Establishment of rat PTSD-SPS model
The PTSD-SPS rat model was established according to the SPS method determined by the International PTSD Scientific Conference held by the Ministry of Education, Japan. The specific steps were as follows: The rats were imprisoned for 7 h after 7 days of adaptive feeding. Following this, the rats were forced to swim for 20 min (water depth 40 cm, water temperature 25°C). After 15 minutes of break, the rats were anesthetized to unconsciousness, then put back in the cage and routinely raised. The rats were randomly divided into three groups: the Control group, the SPS group, and the SPS + Drug group. The drug was intravenously injected once a day, 20 mg/kg, for 7 days.
Morris water maze test
The Morris water maze pool has a diameter of 160 cm and a depth of 50 cm. The platform has a diameter of 10 cm. The water surface is about 2 cm above the platform and the water temperature is (22 ± 1)°C. The Morris water maze directional navigation test was continued for 5 days, 4 times for each rat. The rat head was placed in the water toward the pool wall, and the time spent when the rats found the underwater platform was recorded as the escape latency time. Rats were allowed to stay on the platform for 20 s. If the rat failed to find the underwater platform within 120 s, the experimenter guided the rat to the platform, left it on the platform for 20 s, and recorded the escape latency time as 120 s. Each rat was separated by 20 s interval between 4 experiments, and the average value of ELT obtained from the 4 experiments on that day was counted. After the experiment, the rats were returned to the cage for normal feeding.
Open field test
The rats were placed in the center of the open field test box. The automatic video analysis system was used to determine the exercise parameters of the rats within 15 min. The total distance of exercise was used to analyze the motor function. The distance and retention time of the central area were used to reflect the anxious behavior.
Elevated plus maze test
EPMT was conducted in a room with controlled sound and light. The device is 50 cm high above the ground, surrounded by black plexiglass: 2 open arms, 2 closed arms, and one central area. Video analysis was performed on entry times and retention times of open and closed arms, and the corresponding percentage was calculated.
Immunofluorescence staining of mPFC neurons
After perfusion, the rats in the drug-administered group and the model group were perfused with 300 ml of normal saline and then with 4% paraformaldehyde. The rat mPFC tissue was obtained and immersed in 4% paraformaldehyde solution for 3 h, then immersed in Holt’S liquid to the bottom of the bottle. The cryostat was cooled and the mPFC coronal section was made. The sections were air-dried for 2 h, rinsed with PBS for 5 min × 3, and then washed with 0.3% Triton solution for 10 min. The Triton solution was removed. Then 5% BSA was added to block the sections for 20 min. The sections were added successively with rabbit anti-Beclin-1, rabbit anti-LC 3 polyclonal antibody, murine anti-GFAP polyclonal antibody, and murine anti-NeuN polyclonal antibody. Following this, 0.01 mol/L PBS, instead of primary antibody was used as the negative control, and then the sections were incubated overnight at 4°C darkness. After this, the sections were washed with PBS for 5 min × 3. FITC and Cy3 Labeled IgG were added dropwise to incubate the sections overnight at 4°C in darkness. The sections were rinsed in PBS for 5 min × 3, added with DAPI. 30 min later, the sections were rinsed in PBS for 5 min × 3 times. The sections were sealed with glycerol, and observed under a Nikon laser confocal microscope.
Western blotting to detect the expressions of Beclin-1, LC3-I/LC3-II, Bcl-2, Bax, and p62 in the prefrontal cortex
The mPFC tissues of each group rats were obtained and lysed with 100-300 μl of RIPA lysate containing 1:100 diluted protease inhibitor cocktail and phosphatase inhibitor. Protein concentration was determined by BCA protein quantification method, and the sample amount of the western blot groups was calculated. The total protein sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to a PVDF membrane. The primary antibody was added to incubate the tissues at 4°C overnight. The secondary antibody was added to incubate at room temperature for 30 min. The western blot band was colored and photographed by chemiluminescence. The imageJ software was used for strip analysis.
Statistical approach
All data are expressed as mean ± standard deviation (mean ± SD). The t-test was used for comparison between the two groups, and the comparison between multiple groups of data (> 2) was performed using One-way ANOVA. There was a statistical significance at P < 0.05. The data was analyzed using Graphpad Prism 5.0 (Graphpad Software. San Diego, CA).
Results
Morris water maze test of learning and memory ability changes in rats
The results showed that the rats in the Control group were able to find the platform smoothly, while the rats in the SPS group failed to find the platform within the specified time. Moreover, the ELT of the rats in each group from day 1 to day 7 showed a gradual decline trend. After administration, the time was shortened, indicating that Gastrodin can improve the learning and memory ability of rats, as shown in Figure 1.
Figure 1.
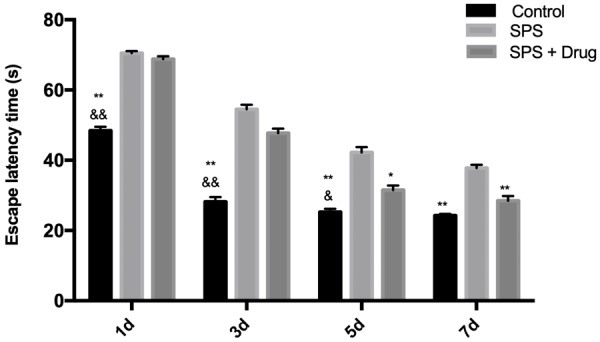
Effect of Gastrodin on results of Morris water maze test. **P < 0.01 vs SPS, *P < 0.05 vs SPS, &&P < 0.01 vs SPS + Drug, &P < 0.05 vs SPS + Drug.
Effects of drugs on SPS-induced PTSD-like behavior
SPS rats showed significantly elevated anxiety levels and cognitive and attention impairment. In EPMT, compared with the control group, the number of open arms and the percentage of staying time in the SPS group decreased, and the drug reduced the anxiety level and cognitive and attention impairment. In the OFT, compared with the control group, the percentage of distance in the central area to the residence time in the SPS group was decreased, while the drug reversed the reduction caused by SPS, as shown in Figure 2.
Figure 2.
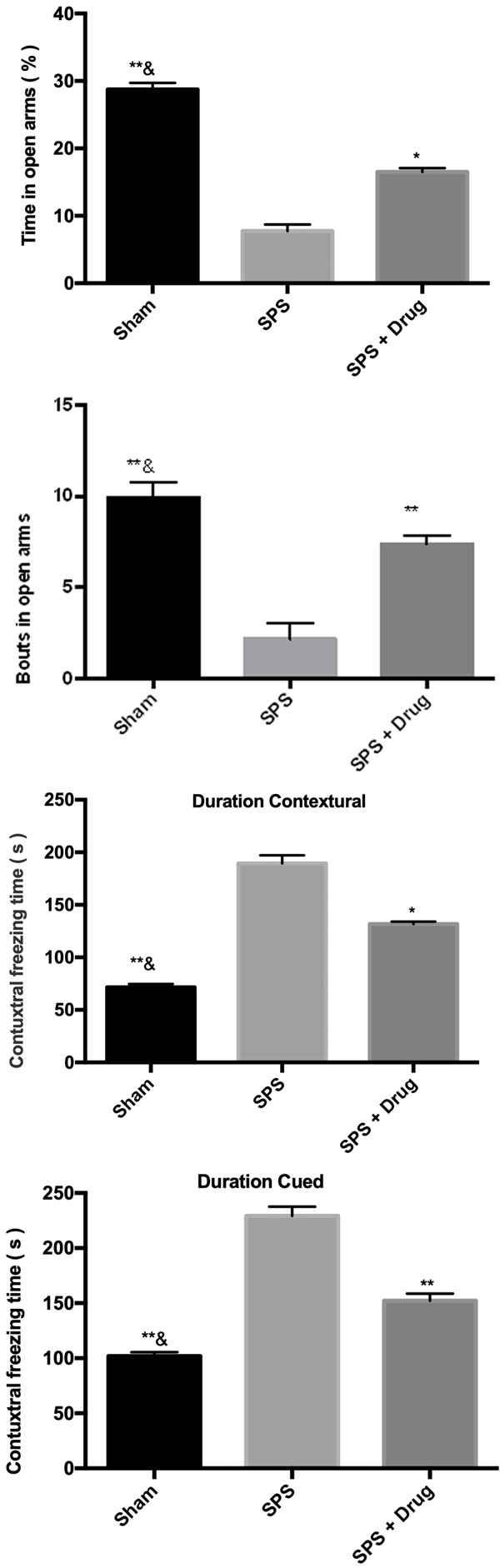
Effect of Gastrodin on SPS-induced PTSD-like behavior. **P < 0.01 vs SPS, *P < 0.05 vs SPS, &&P < 0.01 vs SPS + Drug, &P < 0.05 vs SPS + Drug.
Immunofluorescence detection mPFC neuron autophagy
Beclin-1 and GFAP/NeuN, LC3, and GFAP/NeuN fluorescence double labeling were performed on the mPFC in the drug-administered group and the model group, respectively. It is found that Beclin-1 ir and LC 3 ir were mainly expressed in NeuN-positive cells (i.e. neuronal cells) in mPFC of the two groups of rats. Only a very small number of cells are expressed in GFAP-positive cells (i.e. astrocytes) in mPFC, and drugs can reduce the number of neuronal cell death, as shown in Figure 3.
Figure 3.
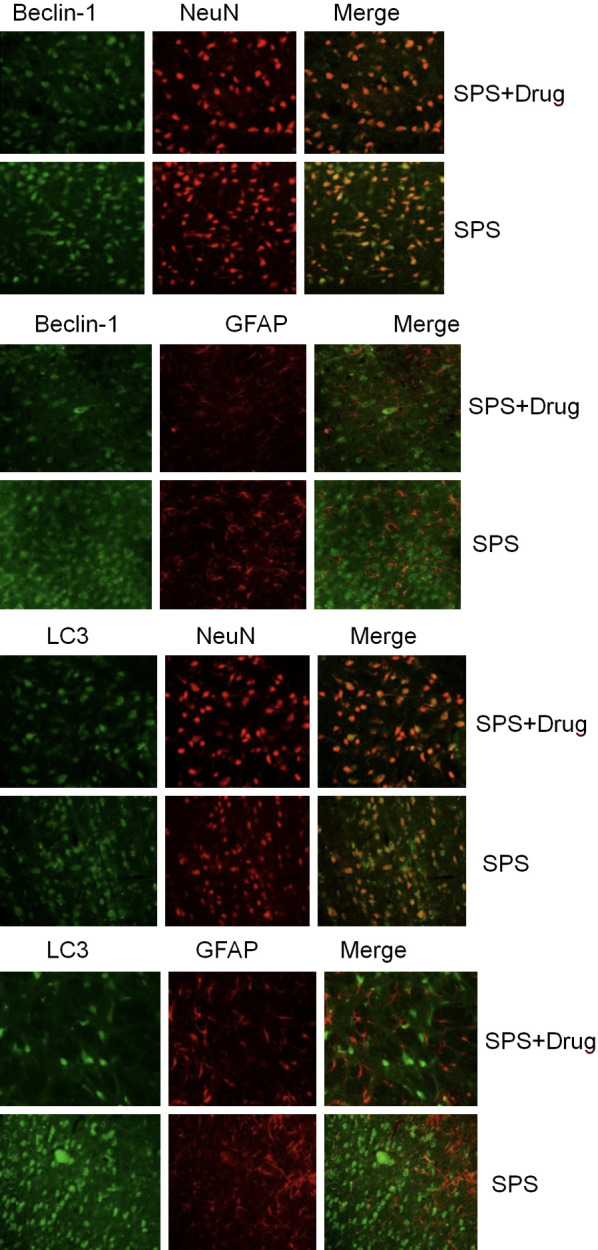
Effect of Gastrodin on the expressions of LC3 and NeuN/GFAP immunofluorescence in rat medial prefrontal cortex.
Western blotting to detect the expressions of various proteins in the medial prefrontal cortex of rats
In the model group, the expressions of LC3-I, LC3-II, Beclin-1, Bax, and Bcl-2 proteins reduced, while the expression of p62 increased, as shown in Figure 4.
Figure 4.
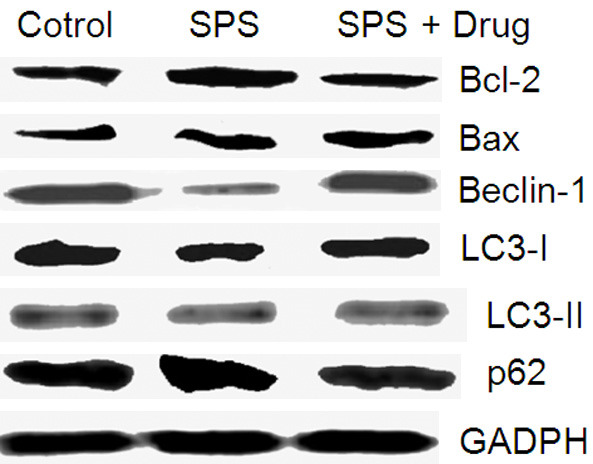
The effect of Gastrodin on various proteins.
Discussion
As the gold standard for autophagy, the structure of bilayer membrane and autophagosome was observed by TEM. Beclin-1 and LC3 are also important autophagy-related genes for measuring autophagy levels. Autophagy is delicately regulated by a series of autophagy-related genes or factors. Among them, Beclin-1 (Atg6) and LC-3 (Atg8) play an important role in the occurrence and development of autophagy, that is, the formation of the early bilayer membrane structure and the extension to form autophagosomes, in which Beclin-1 and LC-3 are important markers of autophagy [5,6].
There are two classical regulatory pathways for autophagy: the first one is the mTOR pathway and the other is the Beclin-1-Vps34-Vps15 complex pathway aforementioned. In addition, Bcl-2 family proteins can play a regulatory role in autophagy through interaction with Beclin-1 [7]. Beclin-1 is a protein containing BH3 fragment. In the early stage of autophagy bilayer membrane formation, Beclin-1 acts as a platform factor to form a compound with Class III PI3k. Under normal conditions, Beclin-1 binds to Bcl-2 family proteins (such as Bcl-2, Bcl-XL, and Bcl-w). Bcl-2 family proteins and Class III PI3k bind to different domains of Beclin-1 respectively. Through inhibiting the expression of Beclin-1, preventing Beclin-1 protein from binding to Class III PI3k, or reducing its phosphorylation activity, autophagy is inhibited [8]. Therefore, the relationship between autophagy and apoptosis, as a programmed cell death, in terms of cellular functions and related factors, is inseparable. Bcl-2, which may act as a bridge between autophagy and apoptosis, has multiple roles and significance in regulating autophagy and apoptosis.
Studies have shown that decreased expression of p62 can cause a decrease in the ability of cells to resist oxidative stress, which in turn leads to abnormal expression of total tau protein and phosphorylated tau protein. Tau protein is hyperphosphorylated to form neurofibrillary tangles, and its dissociation with microtubule leads to microtubule depolymerization, which hinders the transport of substances in neurons and thus affects the normal material and energy supply of neurites and synaptic structures, which may eventually cause neuronal cell death [9]. PTSD-SPS as a stressor can induce endoplasmic reticulum stress in neuronal cells. Previous studies have clarified that factors such as calcium balance disorder in PTSD-SPS rat neuronal cells can aggravate endoplasmic reticulum stress [10]. At the same time, abnormalities were also found in the expressions of tau, p-tau, and microtubule-associated proteins in rat hippocampal neurons after SPS stimulation [11], which may be related to the abnormal expression of p62. Its mechanism needs further study. The p62-conjugated ubiquitinated protein is degraded by the autophagy pathway through its LIR domain and LC3-II, which can alleviate the endoplasmic reticulum stress and help the cell maintain homeostasis. SPS stimulation resulted in a gradual increase in the autophagy flow of rat mPFC from 4 d to 14 d. The increase in autophagy resulted in the elimination of excessive p62, leading to a further decrease in cellular stress, which accelerated changes in neuronal structure and function.
In summary, the general behavior of PTSD rat changes, such as decreased learning and spatial memory ability, decreased exercise and inquiry behavior, anxiety, and fear-like state. Abnormal autophagy exists in rat mPFC neurons. Autophagy-related genes Beclin-1 and LC3 were abnormally expressed in PTSD rat mPFC neurons, and the expression of p62 changed in autophagy-degrading substrate. Gastrodin may have an inhibitory effect on cell death and apoptosis of Beclin-1 and LC3 in PTSD rat mPFC neurons, and thus improves PTSD learning and spatial memory impairment, decreased exercise and inquiry behavior, as well as anxiety and fear in rats.
Disclosure of conflict of interest
None.
References
- 1.Minihan S, Liddell BJ, Byrow Y. Patterns and predictors of posttraumatic stress disorder in refugees: a latent class analysis. J Affect Disord. 2018;232:252–259. doi: 10.1016/j.jad.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Norman SB, Haller M, Kim HM. Trauma related guilt cognitions partially mediate the relationship between PTSD symptom severity and functioning among returning combat veterans. J Psychiatr Res. 2018;100:56–62. doi: 10.1016/j.jpsychires.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Zhu RT, Liu XH, Shi YW. Propranolol can induce PTSD-like memory impairments in rats. Brain Behav. 2018;8:e00905. doi: 10.1002/brb3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han K, Kim J, Choi M. Autophagy mediates phase transitions from cell death to life. Heliyon. 2015;1:e00027. doi: 10.1016/j.heliyon.2015.e00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ynag DS, Stavrides P, Kumar A. Cyclodextrin has conflicting actions on autophagy flux in vivo in brains of normal and Alzheimer model mice. Hum Mol Genet. 2017;26:843–859. doi: 10.1093/hmg/ddx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson KL, Lin WL, Miriyala S. p62 pathology model in the rat substantia nigra with filamentous inclusions and progressive neurodegeneration. PLoS One. 2017;12:e0169291. doi: 10.1371/journal.pone.0169291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song P, Li S, Wu H. Parkin promotes proteasomal degradation of p62: implication of selective vulnerability of neuronal cells in the pathogenesis of Parkinson’s disease. Protein Cell. 2016;7:114–129. doi: 10.1007/s13238-015-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Y, Li B, Han F. Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the medial prefrontal cortex in post-traumatic stress disorder. Mol Med Rep. 2012;6:1140–1144. doi: 10.3892/mmr.2012.1022. [DOI] [PubMed] [Google Scholar]
- 9.Ciechanover A, Kwon YT. Protein quality control by molecular chaperones in neurodegeneration. Front Neurosci. 2017;11:185. doi: 10.3389/fnins.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JY, Gertner M, Pontarelli F. Global ischemia induces lysosomal-mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ. 2017;24:317–329. doi: 10.1038/cdd.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM 1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282:4672–4678. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]


