Abstract
Background
Patients increasingly receive home-based outpatient parenteral antimicrobial therapy (OPAT). Understanding which patients might be at higher risk of complications is critical in effectively triaging resources upon and after hospital discharge.
Methods
A prospective cohort of patients discharged from 1 of 2 academic medical centers in Baltimore, Maryland, between March 2015 and December 2018 were consented and randomly divided into derivation and validation cohorts for development of a risk score for adverse OPAT outcomes. Data from the derivation cohort with the primary outcome of a serious adverse outcome (infection relapse, serious adverse drug event, serious catheter complication, readmission, or death) were analyzed to derive the risk score equation using logistic regression, which was then validated in the validation cohort for performance of predicting a serious adverse outcome.
Results
Of 664 patients in the total cohort, half (332) experienced a serious adverse outcome. The model predicting having a serious adverse outcome included type of catheter, time on OPAT, using a catheter for chemotherapy, using a catheter for home parenteral nutrition, being treated for septic arthritis, being on vancomycin, being treated for Enterococcus, being treated for a fungal infection, and being treated empirically. A score ≥2 on the serious adverse outcome score had a 94.0% and 90.9% sensitivity for having a serious adverse outcome in the derivation and validation cohorts, respectively.
Conclusions
A risk score can be implemented to detect who may be at high risk of serious adverse outcomes, but all patients on OPAT may require monitoring to prevent or detect adverse events.
Keywords: antibiotic complication, catheter complications, home infusion, OPAT
Patients receiving home-based outpatient parenteral antimicrobial therapy (OPAT) are at risk of complications of the venous catheter, adverse drug events, infection relapse, and hospital readmission. Recent OPAT guidelines recommend integrated multidisciplinary care for all OPAT patients to prevent many of these complications [1, 2]. These patients may be at particular risk of developing complications within the first 2 weeks postdischarge [3], and early follow-up by infectious diseases (ID) physicians has been associated with improved outcomes [4].
However, in the United States, the majority of ID physicians in a national survey described barriers to appropriate monitoring of these complicated patients, such as lack of time, support staff, and reimbursement [5]. It is possible that certain groups of patients may be at higher risk of OPAT complications, and therefore require even more attention and resources (eg, more frequent ID clinic appointments, phone calls from clinic staff, visits by home health nurses, etc.).
We used a prospective cohort to create a risk score to predict the development of OPAT complications (serious catheter complications, serious adverse drug events [ADEs], infection relapse, or 30-day hospital readmission) to identify which patients might be at particularly high risk of poor outcomes and benefit from more intensive follow-up.
METHODS
Patient Population and Setting
We analyzed a prospective cohort of patients receiving home infusion therapy, expanding a previously described cohort [3,6–8]. Eligible patients were ≥18 years of age and discharged from 2 tertiary care academic medical centers in Baltimore, Maryland, March 2015–December 2018, to home with assistance from home infusion agencies. Patients were required to have peripherally inserted central catheters (PICCs), tunneled central venous catheters (tCVCs), or midline catheters for OPAT. Patients were ineligible if they were in hospice care. As patients orally consented for the study and completed a telephone survey, we excluded patients who did not speak English or could not verbally consent. Eligible patients were contacted 2 weeks after hospital discharge. Three attempts were made to contact each patient during business hours. Patients could have used any home infusion agency for antimicrobial agents and supplies or home nursing agency for training and support in catheter care.
Instrument
Consenting patients underwent a 10-minute telephone survey focusing on OPAT complications (Appendix). The survey instrument was piloted among 10 patients before the study, with changes made based on feedback, focusing on OPAT outcomes [9].
The electronic health record (EHR) was abstracted for demographic information, CVC characteristics, OPAT indication, clinical data, readmissions, catheter complications, and ADEs through 1 month after OPAT completion, and infection relapse and mortality through 6 months after hospital discharge.
Development of Derivation and Validation Cohorts
As the hospitals implemented interventions to improve the quality of OPAT care over time, we elected to use random sampling via a random number generator to divide the total cohort into equally sized derivation and validation cohorts.
Variables
Age was dichotomized at 65 years, allowing an assessment of the population of Medicare-eligible patients [10]. As few enrolled patients were Asian American, Hispanic, or other racial/ethnic groups, racial/ethnic group was categorized as white non-Hispanic, black non-Hispanic, or other. Insurer was characterized as Medicare, Medicaid, private or Veterans Affairs insurance, or uninsured. Catheter type was categorized as PICC, tCVC, or midline. Charlson comorbidity index (CCI) [11] scores were calculated and dichotomized at 2 based on their distribution with the outcome. OPAT days were calculated as the number of days between hospital discharge and documented OPAT completion. Gender was categorized as male or female. Use of the catheter for chemotherapy or parenteral nutrition was documented. Location of infection, pathogen(s), and type and number of antimicrobial agents were documented. We counted each day of OPAT, no matter the number of antimicrobials received, as 1 day of OPAT, and divided this course into 1–13, 14–27, and ≥28 days.
Indications for OPAT were characterized by infection site and causative organism; patients could have had >1 indication and >1 causative organism. Only parenteral agents were recorded.
We had 2 potential sources of data about adverse OPAT outcomes: patient surveys and the EHR. If a patient listed an adverse OPAT outcome that was not noted in the EHR, information was sought from other sources (eg, admission or clinic visit notes from other regional health systems that can be pulled into the local health system EHR through a collaborative) to verify the adverse OPAT outcome. Only those adverse OPAT outcomes documented in the medical record were included in this analysis.
The primary outcome was a serious adverse OPAT outcome. This could have been any of a serious adverse drug event (ADE), serious catheter complication, or readmission within 1 month after completion of OPAT, or death or infection relapse within 6 months of hospital discharge. Serious ADEs included ADEs documented in the EHR by a physician, physician assistant, or nurse practitioner, considered by the reviewers to be likely caused by the antimicrobial agent, that resulted in (1) a hospital admission, (2) change in antimicrobial agent, (3) early termination of antimicrobial therapy, (4) requiring injected filgastrim, or (5) Clostridiodies difficile infection. Serious catheter complications included having a deep vein thrombosis (DVT) or pulmonary embolus (PE), central line–associated bloodstream infection (CLABSI), or bloodstream infection (BSI) that did not meet CLABSI criteria and was not related to the OPAT indication. Infection relapse within 6 months included a need for further surgical or interventional radiology intervention including debridement, drainage, or amputation during or after the OPAT course, new positive blood cultures or cultures at the site of infection with the same organism, or imaging suggesting worsening of infection.
As a secondary analysis, we used the outcome of any adverse OPAT outcome. This could have been any ADE, any catheter complication, readmission, or emergency department (ED) visit within 1 month after OPAT completion, or death or infection relapse within 6 months of hospital discharge. ADEs were any ADEs documented in the EHR by a physician, physician assistant, or nurse practitioner and considered by the reviewers to be likely caused by the parenteral antimicrobial agent. Catheter complications included the serious catheter complications above, catheter occlusion requiring tissue plasminogen activator, and the catheter being inadvertently removed without an order from a clinician.
Data Analysis
Descriptive statistics were used for demographic, clinical, and outcome data for the derivation and validation cohorts (Stata, version 14.0; College Station, TX, USA). Predictors included demographic and clinical variables.
Multivariate logistic regression models were built to estimate predictors of the presence of (1) a serious adverse OPAT outcome and (2) any adverse OPAT outcome in the derivation cohort. Covariates were considered if the association with the outcome was P ≤ .20 (2-sided) and were removed in a stepwise fashion if the association with the outcome was P > .20 (2-sided). Once a model was developed in the derivation cohort, an integer scoring system was used to generate a score for serious adverse OPAT outcomes based on the regression coefficient–based Beta/Schneeweiss scoring system, as it has lower Akaike information criteria and a higher McFadden’s adjusted R2 than risk ratio–based models and classifies more people into the correct strata than risk ratio–based models [12, 13].
Next, the validation cohort was analyzed. Multivariate logistic regression was performed using the same models for serious adverse OPAT outcome and any adverse OPAT outcome as developed in the derivation cohort. We believed that it was most important to ensure that those at high risk for adverse outcomes were captured by the risk score. Therefore we evaluated the sensitivity of the risk score. The ability of the multivariate logistic regression model to predict sensitivity was compared with the model using the integer scoring system. The integer scoring system was used to divide the cohort into those at lower risk and higher risk for serious adverse OPAT events.
A total of 660 patients in the combined derivation and validation cohort were needed to estimate the sensitivity of the prediction model at 90% with a precision of 3.3%.
The study was approved as expedited with oral consent by the Johns Hopkins University School of Medicine Institutional Review Board.
RESULTS
A total of 664 patients were enrolled in the study—332 in the derivation cohort and 332 in the validation cohort—of 1272 eligible patients (52.2%) (Appendix). Patients enrolled in the study were similar to those not enrolled in the study on race/ethnicity, gender, and 30-day hospital readmission. More patients were on OPAT for ≥28 days in the derivation cohort than the validation cohort (Table 1). More patients in the derivation cohort than the validation cohort had methicillin-resistant Staphylococcus aureus (MRSA) infections. Otherwise, the 2 cohorts were similar in demographic characteristics and characteristics of the infection.
Table 1.
Demographics and Clinical Characteristics of Patients in the Validation and Derivation Cohorts at Time of Discharge
| Characteristic | Derivation Cohort, No. (% of 332) | Validation Cohort, No. (% of 332) | Total, No. (% of 664) | P difference |
|---|---|---|---|---|
| Aged ≥65 y | 84 (25.3) | 74 (22.3) | 158 (23.8) | .068 |
| Race/ethnicity: white | 226 (68.1) | 242 (72.9) | 468 (70.5) | .36 |
| Black | 83 (25.0) | 72 (21.7) | 155 (23.3) | .38 |
| Hispanic or other | 22 (6.6) | 19 (5.7) | 41 (6.2) | .38 |
| Gender: female | 146 (44.0) | 169 (50.9) | 315 (47.4) | .074 |
| Insurance: private, Veterans Administration, or military | 201 (60.5) | 194 (58.4) | 395 (59.5) | .93 |
| Medicaid | 40 (12.1) | 42 (12.7) | 82 (12.4) | — |
| Medicare | 89 (26.8) | 93 (28.0) | 182 (27.4) | — |
| None or self-pay | 2 (0.6) | 3 (0.9) | 5 (0.75) | — |
| Type of catheter: PICC | 226 (68.1) | 243 (73.2) | 469 (70.6) | .34 |
| Tunneled central venous catheter | 89 (26.8) | 76 (22.9) | 165 (24.9) | — |
| Midline catheter | 17 (5.1) | 13 (3.9) | 30 (4.5) | — |
| Time on OPAT <14 d | 87 (26.2) | 79 (23.8) | 166 (25.0) | .014 |
| 14–27 d | 64 (19.3) | 96 (28.9) | 160 (24.1) | — |
| ≥28 d | 181 (54.5) | 157 (47.3) | 338 (50.9) | — |
| Using catheter for chemotherapy | 31 (9.3) | 29 (8.7) | 60 (9.0) | .79 |
| Using catheter for parenteral nutrition | 9 (2.7) | 13 (3.9) | 22 (3.3) | .39 |
| Solid organ transplant | 31 (9.6) | 33 (9.6) | 64 (9.6) | .79 |
| Charlson comorbidity index ≥2 | 214 (64.5) | 191 (57.5) | 405 (61.0) | .067 |
| Bacteremia | 71 (21.4) | 66 (19.9) | 137 (20.6) | .63 |
| Meningitis | 32 (9.6) | 34 (10.2) | 66 (9.9) | .80 |
| Osteomyelitis | 102 (30.7) | 102 (30.7) | 204 (30.7) | 1.00 |
| Septic arthritis | 31 (9.3) | 26 (7.8) | 57 (8.6) | .49 |
| Hardware infection | 60 (18.1) | 53 (16.0) | 113 (17.0) | .47 |
| Pneumonia, empyema, or lung abscess | 38 (11.5) | 49 (14.5) | 87 (13.1) | .21 |
| Abdominal infection | 30 (9.0) | 30 (9.0) | 60 (9.0) | 1.00 |
| Cellulitis | 22 (6.6) | 17 (5.1) | 39 (5.9) | .41 |
| Endovascular infection or endocarditis | 28 (8.4) | 29 (8.7) | 57 (8.6) | .89 |
| Discitis or epidural abscess | 19 (5.7) | 24 (7.2) | 43 (6.5) | .43 |
| Vancomycin | 101 (30.4) | 80 (24.1) | 181 (27.3) | .067 |
| Any beta-lactama | 248 (74.7) | 255 (76.8) | 503 (75.6) | .53 |
| Aminoglycosidesb | 16 (4.8) | 27 (8.1) | 43 (6.5) | .083 |
| Daptomycin | 7 (2.1) | 6 (1.8) | 13 (2.0) | .78 |
| Antifungalc | 10 (3.9) | 12 (3.6) | 22 (3.3) | .67 |
| Antiviral (ganciclovir = 19, acyclovir = 12) | 14 (4.2) | 17 (5.1) | 31 (4.7) | .58 |
| >1 OPAT agent | 67 (20.2) | 68 (20.5) | 135 (20.3) | .92 |
| Methicillin-resistant Staphylococcus aureus | 39 (11.8) | 23 (6.9) | 62 (9.3) | .033 |
| Methicillin-sensitive Staphylococcus aureus | 47 (14.2) | 44 (13.3) | 91 (13.7) | .74 |
| Coagulase-negative Staphylococcus | 39 (11.8) | 38 (11.5) | 77 (11.6) | .90 |
| Enterococcus | 23 (6.9) | 22 (6.6) | 45 (6.8) | .88 |
| Borrelia | 11 (3.3) | 12 (3.6) | 23 (3.5) | .83 |
| Anaerobe | 11 (3.3) | 11 (3.3) | 22 (3.3) | 1.00 |
| Gram-negative bacilli | 80 (24.1) | 89 (26.8) | 169 (25.5) | .42 |
| Fungi (Candida = 14, Cryptococcus = 1, other = 12) | 14 (4.2) | 12 (3.6) | 26 (3.9) | .69 |
| Viruses (HSV = 7, VZV = 4, CMV = 18) | 13 (3.9) | 16 (4.8) | 29 (4.3) | .57 |
| Empiric | 65 (19.6) | 63 (19.0) | 128 (19.3) | .84 |
| Polymicrobial infection | 77 (23.2) | 79 (23.8) | 156 (23.5) | .86 |
Abbreviations: CMV, Cytomegalovirus; HSV, Herpes simplex virus; OPAT, outpatient parenteral antimicrobial therapy; PICC, peripherally inserted central catheter; VZV, Varicella zoster virus.
aAny beta-lactam: oxacillin = 52, nafcillin = 4, piperacillin-tazobactam = 50, penicillin = 37, ampicillin = 14, ampicillin-sulbactam = 41, cefazolin = 27, ceftriaxone = 96, cefepime = 53, ceftaroline = 3, ceftazidime = 28, ceftolozane-tazobactam = 1, meropenem = 35, imipenem = 7, ertapenem = 59.
bAminoglycosides: gentamicin = 5, tobramycin = 30, amikacin = 8.
cAntifungals: micafungin = 15, fluconazole = 1, liposomal amphotericin = 6.
Poor outcomes were common among enrolled participants (Table 2; Appendix Table 2). Half of participants experienced a severe adverse OPAT outcome: 16.0% experienced a serious ADE, 7.5% experienced a serious catheter complication, 24.3% experienced an infection relapse within 6 months of initiating OPAT, 32.4% were readmitted while on OPAT (74.9% of these were related to OPAT), and 1.4% were deceased within 6 months of initiating OPAT. Meanwhile, 60.2% of patients experienced any adverse OPAT outcome: 17.0% had any ADE, 20.9% had any catheter complication, and 28.2% had an ED visit while on OPAT.
Table 2.
Outcomes
| Outcome | Derivation Cohort, No. (% of 332) | Validation Cohort, No. (% of 332) | Total, No. (% of 664) | P |
|---|---|---|---|---|
| Serious adverse outcomes | 167 (50.3) | 165 (49.7) | 332 (50.0) | .88 |
| Any serious adverse drug event | 50 (15.1) | 56 (16.9) | 106 (16.0) | .53 |
| Serious catheter complication: thromboembolic event | 14 (4.2) | 7 (2.1) | 21 (3.2) | .12 |
| Central line–associated bloodstream infection | 11 (3.3) | 9 (2.7) | 20 (3.0) | .65 |
| Bloodstream infection | 7 (2.1) | 4 (1.2) | 11 (1.7) | .36 |
| Any serious catheter complication | 30 (9.0) | 20 (6.0) | 50 (7.5) | .14 |
| Infection relapse within 6 mo of hospital discharge | 72 (21.7) | 89 (26.8) | 161 (24.3) | .12 |
| Readmission while on OPATa | 110 (33.1) | 105 (31.6) | 215 (32.4) | .68 |
| Readmission within 30 d of hospital discharge | 68 (20.5) | 63 (19.0) | 131 (19.7) | .63 |
| Death while on OPAT | 4 (1.2) | 5 (1.5) | 9 (1.4) | .74 |
| Any adverse outcome | 205 (61.8) | 195 (58.7) | 400 (60.2) | .43 |
| Any adverse drug event | 59 (17.8) | 54 (16.3) | 113 (17.0) | .61 |
| Catheter complication: occlusion requiring alteplase | 43 (13.0) | 39 (11.8) | 82 (12.4) | .64 |
| Inadvertent removal of catheter | 9 (2.7) | 10 (3.0) | 19 (2.9) | .81 |
| Any catheter complication | 74 (22.3) | 65 (19.6) | 139 (20.9) | .39 |
| Emergency department visit while on OPAT | 95 (28.6) | 92 (27.7) | 187 (28.2) | .80 |
| Emergency department visit within 30 d of hospital discharge | 61 (18.4) | 763 (19.0) | 124 (18.7) | .84 |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy.
aOne hundred sixty-one readmissions were related to OPAT.
We first developed a multivariate logistic regression model for any serious outcome using the derivation cohort (Table 3). In the adjusted model, having a midline catheter was associated with an increased odds of a serious outcome when compared with having a PICC (adjusted odds ratio [aOR], 4.55; 95% confidence interval [CI], 1.47–14.04). Use of the catheter for chemotherapy was also associated with increased odds of a serious outcome (aOR, 2.83; 95% CI, 1.17–6.89), as was receiving vancomycin (aOR, 2.33; 95% CI, 1.34–3.94), being treated for Enterococcus (aOR, 3.47; 95% CI, 1.27–9.52), or being treated for a fungal infection (aOR, 5.80; 95% CI, 1.22–27.47). We did not include MRSA in the model due to collinearity with receipt of vancomycin and as the model was more predictive of the outcome with vancomycin receipt included.
Table 3.
Development of a Model Using the Derivation Cohort (n = 332); for Serious Outcome
| Characteristic | OR for Serious Outcome (95% CI) | Adjusted OR for Serious Outcome (95% CI) | OR for Any Outcome (95% CI) | Adjusted OR for Any Outcome (95% CI) |
|---|---|---|---|---|
| Aged ≥65 y | 0.87 (0.53–1.42) | Not included | 0.63 (0.38–1.05) | 0.55 (0.32–0.96) |
| Race/ethnicity: white | — | Not included | — | Not included |
| Black | 0.85 (0.51–1.41) | Not included | 1.01 (0.60–1.70) | Not included |
| Hispanic or other | 0.59 (0.24–1.41) | Not included | 0.66 (0.28–1.56) | Not included |
| Gender: female | 1.13 (0.73–1.75) | Not included | 0.99 (0.64–1.55) | Not included |
| Type of catheter: PICC | — | — | — | Not included |
| Tunneled central venous catheter | 1.36 (0.83–2.23) | 1.34 (0.78–2.30) | 1.08 (0.65–1.80) | 1.18 (0.66–2.11) |
| Midline catheter | 2.04 (0.73–5.70) | 4.55 (1.47–14.0) | 1.17 (0.42–3.27) | 3.44 (1.10–10.8) |
| Time on OPAT <14 d | — | — | — | — |
| 14–27 d | 1.56 (0.81–2.98) | 1.99 (0.98–4.04) | 1.43 (0.74–2.74) | 1.73 (0.85–3.53) |
| ≥28 d | 1.39 (0.83–2.33) | 1.59 (0.90–2.84) | 2.07 (1.23–3.50) | 2.27 (1.26–4.09) |
| Also receiving chemotherapy | 2.23 (1.02–4.89) | 2.83 (1.17–6.89) | 1.88 (0.81–4.34) | 3.25 (0.97–6.60) |
| Also receiving home parenteral nutrition | 2.01 (0.49–8.18) | Not included | 2.21 (0.45–10.8) | Not included |
| Charlson comorbidity score ≥2 | 1.26 (0.80–1.97) | Not included | 1.17 (0.74–1.86) | Not included |
| Osteomyelitis | 1.09 (0.69–1.72) | Not included | 0.99 (0.62–1.58) | Not included |
| Septic arthritis | 1.64 (0.77–3.49) | 1.76 (0.79–3.93) | 0.98 (0.46–2.09) | Not included |
| Hardware infection | 0.99 (0.56–1.72) | Not included | 0.84 (0.48–1.48) | Not included |
| Endovascular infection or endocarditis | 0.72 (0.33–1.58) | Not included | 0.81 (0.37–1.78) | Not included |
| Discitis or epidural abscess | 0.56 (0.21–1.45) | Not included | 0.54 (0.21–1.36) | 0.46 (0.17–1.23) |
| Vancomycin | 1.91 (1.18–3.07) | 2.33 (1.34–3.94) | 2.99 (1.75–5.13) | 3.41 (1.90–6.14) |
| Any beta-lactam | 0.57 (0.34–0.94) | Not included | 0.52 (0.30–0.90) | Not included |
| Aminoglycosides | 1.29 (0.47–3.54) | Not included | 1.03 (0.37–2.92) | Not included |
| >1 OPAT agent | 1.38 (0.81–2.37) | Not included | 1.46 (0.82–2.59) | Not included |
| Methicillin-resistant Staphylococcus aureus | 2.16 (1.07–4.36) | Not included | 4.85 (1.85–12.8) | Not included |
| Methicillin-sensitive Staphylococcus aureus | 1.04 (0.56–1.92) | Not included | 1.00 (0.53–1.88) | Not included |
| Coagulase-negative Staphylococcus | 1.68 (0.85–3.33) | Not included | 1.67 (0.80–3.48) | Not included |
| Enterococcus | 3.00 (1.15–7.82) | 3.47 (1.27–9.52) | 1.82 (0.70–4.75) | 2.01 (0.70–5.72) |
| Other gram-positive cocci | 0.61 (0.19–1.89) | Not included | 0.52 (0.17–1.57) | Not included |
| Anaerobe | 4.64 (0.99–21.82) | Not included | 6.46 (0.82–51.1) | Not included |
| Fungi | 7.17 (0.87–58.98) | 5.80 (1.22–27.5) | 3.89 (0.86–17.7) | 3.71 (0.78–17.7) |
| Viruses | 0.61 (0.19–1.89) | Not included | 0.37 (0.12–1.16) | 0.43 (0.13–1.47) |
| Empiric | 0.81 (0.47–1.40) | 0.63 (0.34–1.17) | 0.78 (0.45–1.35) | 0.52 (0.27–0.98) |
| Polymicrobial infection | 1.64 (0.98–2.76) | Not included | 1.62 (0.93–2.80) | Not included |
| Gram-negative bacilli | 1.20 (0.72–1.99) | Not included | 1.20 (0.71–2.03) | Not included |
Abbreviations: CI, confidence interval; OPAT, outpatient parenteral antimicrobial therapy; OR, odds ratio; PICC, peripherally inserted central catheter.
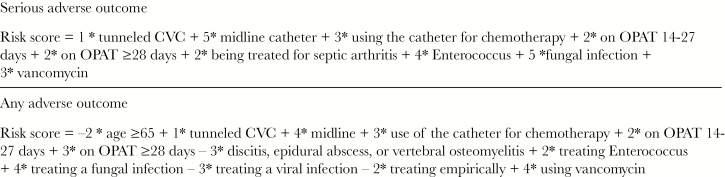
We then transformed the coefficients associated with the regression model for a serious adverse OPAT outcome to a risk score (Table 4, Figure 1). In the derivation cohort, the AUC for the risk score equation was 0.74, and a serious adverse OPAT outcome risk score ≥2 provided a sensitivity of 94.0% and a specificity of 15.2% for predicting a serious adverse OPAT outcome. We then applied the logistic regression model and risk score equation to the validation cohort. In the validation cohort, the risk score equation AUC was 0.74, and a serious adverse OPAT outcome score ≥2 had a sensitivity of 90.9% and a specificity of 18.0% for predicting a serious adverse outcome. Those in the low-risk group (<2) had a 28.6% risk of a serious adverse outcome, while those in the high-risk group (≥2) had a 52.9% risk of a serious adverse outcome.
Table 4.
Sensitivity and Specificity of Risk Score Equation in Predicting Outcomes
| Risk Score Equation for Outcome | Derivation Cohort: Sensitivity (95% CI), % | Derivation Cohort: Specificity (95% CI), % | Validation Cohort: Sensitivity (95% CI), % | Validation Cohort: Specificity (95% CI), % |
|---|---|---|---|---|
| Any adverse outcome ≥0 | 94.6 (90.6–97.3) | 12.6 (7.4–19.7) | 94.4 (90.1–97.2) | 13.9 (7.4–19.1) |
| Serious adverse outcome score ≥2 | 94.0 (89.3–97.1) | 15.2 (10.1–21.5) | 90.9 (85.4–94.8) | 18.0 (13.0–25.3) |
Abbreviation: CI, confidence interval.
Figure 1.
Risk score equations for an outpatient parenteral antimicrobial therapy (OPAT)–related serious adverse outcome and any OPAT-related adverse outcome, respectively.
We also investigated predictors for any adverse OPAT outcome using the derivation cohort (Table 3). Patients aged ≥65 years were less likely to have any adverse outcome (aOR, 0.55; 95% CI, 0.32–0.96). Patients with a midline catheter had increased odds of any adverse outcome when compared with those with a PICC (aOR, 3.44; 95% CI, 1.10–10.83). Patients on OPAT ≥28 days also had higher odds of any adverse outcome than patients on OPAT <14 days (aOR, 2.27; 95% CI, 1.26–4.09). In addition, patients on vancomycin had increased odds of any adverse outcome (aOR, 3.41; 95% CI, 1.90–6.14). Patients being treated empirically had decreased odds of any adverse outcome (aOR, 0.52; 95% CI, 0.27–0.98). We did not include MRSA in the model due to collinearity with receipt of vancomycin and as the model was more predictive of the outcome with vancomycin included.
We then transitioned the coefficients associated with the regression model for any adverse OPAT outcome to a risk score (Table 4). In the derivation cohort, an any adverse OPAT outcome score ≥0 provided a sensitivity of 94.6% and a specificity of 12.6%, and the AUC for the risk score equation was 0.71. When we applied the same process to the validation cohort, an any adverse OPAT outcome score ≥0 had a sensitivity of 94.4% and a specificity of 13.9%, and the risk score equation produced an AUC of 0.62. Those in the low-risk (risk score < 0) and high-risk (≥0) groups had 40.7% and 63.6% risk of any adverse OPAT outcome, respectively.
Discussion
We developed a risk score to determine who may be at higher risk for serious outcomes among patients receiving OPAT in the home after hospital discharge. We found that a model including age <65 years, type of catheter, using the catheter for chemotherapy, length of time on OPAT, treatment for septic arthritis, Enterococcus infection, fungal infection, and treatment with vancomycin could be used to predict the risk of a serious outcome such as readmission, severe ADE, severe catheter complication, death, or infection relapse. Clinicians could use this risk score, containing variables available before hospital discharge, to evaluate who might benefit from closer follow-up.
Half of patients had a serious adverse OPAT outcome, and more than half had any adverse OPAT outcome. These values are higher than in other studies that have focused solely on readmissions, ADEs, infection relapse, or catheter complications [4, 6, 14, 15]. Our data suggest that studying these adverse OPAT outcomes in isolation do not present the true picture of serious events among patients receiving OPAT. Readmissions included any readmissions within 30 days of OPAT completion (which often exceeded the standard 30-day readmission measures) as we wished to capture all treatment complications. Readmissions also included all hospital readmissions, even those not necessarily associated with OPAT, as readmissions for non-OPAT reasons may also indicate patients who may need closer monitoring.
With half of this patient population experiencing serious adverse OPAT outcomes, it is clear that close monitoring of patients on OPAT is needed. Early ID physician evaluation (within 1 to 2 weeks of hospital discharge) of OPAT patients reduces readmissions [4, 16, 17]. In addition, home health agencies and home infusion companies may use these data in determining who may need more frequent evaluations, that is, more than weekly.
The highest-risk group of patients had a 52.9% risk of a serious adverse OPAT outcome and may benefit the most from close follow-up. With such a high risk of serious adverse outcomes, preventing all events may not be possible, so we should also focus our efforts on mitigating the impact of serious adverse outcomes. For example, perhaps this group of patients may benefit from more frequent laboratory testing to detect and mitigate ADEs before they result in a hospital readmission, that is, more than weekly. Alternatively, this group may benefit from close attention to choice of less toxic antimicrobial agents [18]. This group may also benefit from enhanced training when they are first learning how to access the catheter and perform infusions [19, 20].
It is important to note that this population did not include patients receiving skilled nursing facility (SNF)–based OPAT. Patients receiving OPAT at SNFs may have higher readmission rates [14, 21, 22]; may perform more poorly on process measures including receipt of laboratory test results, follow-up, and receiving the appropriate antibiotic [5, 21]; and may report lower satisfaction or increased safety concerns [22]. The implications of high-risk patients receiving OPAT at SNFs deserves further study.
Interestingly, in this study, older patients were less likely to have a poor outcome, adding to other studies demonstrating that providing OPAT to older adults is safe [1, 23–25]. It is possible that younger patients may be less compliant with therapy [26], such as the complicated steps required for OPAT, or have less support at home. In addition, those who received empiric therapy were less likely to have a poor outcome, possibly as these patients may have had a lower burden of infectious organisms that resulted in their cultures not being revealing, and as they may have been given lower doses of antibiotics in the presence of not having revealing cultures.
Patients with PICCs were at lower risk of poor outcomes than patients with tCVCs. Other studies have pointed to PICCs as having poor outcomes when compared with tCVCs [27]. Several factors may lead to this discrepancy. In our hospitals, choosing a tCVC may be a marker of the team being concerned about the patient needing a longer length of therapy, which may indicate a sicker patient. Alternatively, in our setting, patients with tCVCs require a separate visit to an interventional radiology suite for removal, while home health nurses or clinic staff remove PICCs. With the additional need for a scheduled interventional radiology visit, the tCVC may remain in place longer than needed, increasing the risk of complications.
Patients with septic arthritis had more serious adverse outcomes than other groups. In our population of patients with septic arthritis, most were treated for prosthetic joint infections. It is possible that these patients have a higher risk of infection relapse [28] than other groups. The vast majority (86%, data not shown) of patients with septic arthritis were treated for an organism recovered in culture, so it is unlikely that empiric treatment caused the higher risk of serious adverse outcomes in this group. In fact, those being treated empirically had a decreased risk of adverse outcomes, perhaps related to a decreased burden of organisms or decreased antimicrobial agent doses.
Patients being treated for Enterococcus or fungal infections were at higher risk for serious adverse outcomes. Patients with serious Enterococcus or fungal infections may be more ill than those with other infections. In addition, fungal infections or Enterococcus infections may be more likely to relapse [29], and Enterococcus infections may be more likely to be treated with >1 antibiotic, increasing the likelihood of ADEs [30].
Vancomycin receipt was associated with a higher risk of a serious adverse OPAT outcome. Studies have pointed to the higher rates of ADEs among patients on vancomycin for OPAT [15, 31–33]. In addition, our practice had been to monitor vancomycin troughs rather than AUC, and many of the vancomycin ADEs were related to nephrotoxicity. Our data support recent guidelines recommending close laboratory monitoring of patients receiving vancomycin for OPAT [1] and suggest judicious use of vancomycin in OPAT. In addition, it is possible that with recent guidelines recommending vancomycin therapeutic drug monitoring with AUC instead of troughs, ADEs related to vancomycin may decrease [34].
We used a large prospective cohort with complete records of patient follow-up to identify risk factors for inclusion in the risk score. The data points used are those available at the time of hospital discharge to aid in risk score calculation. However, our study had several limitations. Patients had to consent to enroll in the cohort. It is possible that patients who were sicker were less likely to consent, so the number of patients with adverse outcomes may be even higher. The study was performed at 2 medical centers in 1 American city and would need to be repeated in other settings. However, challenges experienced by the 2 hospitals, such as perceived need for more resources and improved care coordination, are common throughout the United States [5]. We used chart reviews and were able to capture readmissions and most clinic visits throughout the state and local region, but we may have underestimated poor outcomes that occurred outside of the region. We also focused on documented adverse OPAT outcomes as we felt these would be more accurate, but there were other outcomes that were only reported by patients [3]. Therefore, our rates of adverse OPAT outcomes may be an underestimation. We did not use laboratory definitions or signs or symptoms in defining ADEs, but felt that the increased capture of ADEs possible in our approach outweighed any loss in accuracy of our definition. In addition, the OPAT clinic structures at the 2 hospitals evolved over time. We attempted to address these changes by having random assignment of the patients to the cohorts. We were able to identify the date of OPAT completion for all patients, but it is possible that patients after this period may have had adverse outcomes (especially infection relapse) not noted in the medical record. Although we believe that the majority of these patients would have presented for management of these adverse outcomes, missing data could have also led to an underestimation of adverse OPAT outcomes. Finally, our risk scores had a relatively low specificity. However, we felt that it was more important to capture patients at high risk of adverse outcomes to mitigate these outcomes, even at the cost of also capturing some patients at lower risk.
We have developed a risk score for poor outcomes in patients with OPAT. Half of the patients experienced a serious OPAT adverse outcome, emphasizing the need for close follow-up of all OPAT patients. Those with the highest risk scores should be considered for even closer monitoring, with more frequent nursing visits and more frequent physician visits. Further work should focus on how to improve quality of care and mitigate these poor outcomes in this rapidly growing area of health care.
Supplementary Material
Acknowledgments
We acknowledge the assistance of the Johns Hopkins Home Care Group staff in identifying patients for enrollment. We acknowledge the assistance of Mayo Levering, BS, for assistance in enrolling patients.
Author contributions. Dr. Keller designed the study and all study tools, collected data, analyzed data, interpreted data, and wrote the manuscript. Dr. Wang assisted with study design, data analysis, and data interpretation and reviewed the manuscript. Ms. Salinas assisted with study design and data collection and reviewed the manuscript. Dr. Townsend assisted with data interpretation and manuscript review. Dr. Cosgrove assisted with study design, data analysis, data interpretation, and manuscript review.
Financial support. This work was supported by the Agency for Healthcare Research and Quality (K08HS025782 to S.C.K.) and the National Center for Advancing Translational Sciences/Johns Hopkins Institute for Clinical and Translational Research (KL2TR001077 to S.C.K.). This work was supported by the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Award.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68:e1–e35. [DOI] [PubMed] [Google Scholar]
- 2. Berrevoets MAH, Oerlemans AJM, Tromp M, et al. Quality of outpatient parenteral antimicrobial therapy (OPAT) care from the patient’s perspective: a qualitative study. BMJ Open 2018; 8:e024564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keller SC, Williams D, Gavgani M, et al. Rates of and risk factors for adverse drug events in outpatient parenteral antimicrobial therapy. Clinical Infect Dis 2018; 66:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saini E, Ali M, Du P, et al. Early infectious disease outpatient follow-up of outpatient parenteral antimicrobial therapy patients reduces 30-day readmission. Clin Infect Dis 2019; 69:865–8. [DOI] [PubMed] [Google Scholar]
- 5. Hamad Y, Lane MA, Beekmann SE, Polgreen PM, Keller SC. Perspectives of United States-based infectious diseases physicians on outpatient parenteral antimicrobial therapy practice. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keller SC, Dzintars K, Gorski LA, et al. Antimicrobial agents and catheter complications in outpatient parenteral antimicrobial therapy. Pharmacotherapy 2018; 38:476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keller SC, Williams D, Gavgani M, et al. Environmental exposures and the risk of central venous catheter complications and readmissions in home infusion therapy patients. Infect Control Hosp Epidemiol 2017; 38:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keller SC, Williams D, Levering M, Cosgrove SE. Health-related quality of life in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keller SC, Williams D, Gavgani M, et al. Environmental exposures and the risk of central venous catheter complications and readmissions in home infusion therapy patients. Infect Control Hosp Epidemiol 2017; 38:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keller S, Pronovost P, Cosgrove S. What medicare is missing. Clin Infect Dis 2015; 61:1890–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993; 32:382–7. [PubMed] [Google Scholar]
- 12. Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res 2003; 38:1103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mehta HB, Mehta V, Girman CJ, et al. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol 2016; 79:22–8. [DOI] [PubMed] [Google Scholar]
- 14. Bhavan KP, Brown LS, Haley RW. Self-Administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med 2015; 12:e1001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schrank GM, Wright SB, Branch-Elliman W, LaSalvia MT. A retrospective analysis of adverse events among patients receiving daptomycin versus vancomycin during outpatient parenteral antimicrobial therapy. Infect Control Hosp Epidemiol 2018; 39:947–54. [DOI] [PubMed] [Google Scholar]
- 16. Palms DL, Jacob JT. Close patient follow-up among patients receiving outpatient parenteral antimicrobial therapy. Clin Infect Dis 2020; 70:67–74. [DOI] [PubMed] [Google Scholar]
- 17. Shah A, Petrak R, Fliegelman R, et al. Infectious diseases specialty intervention is associated with better outcomes among privately insured individuals receiving outpatient parenteral antimicrobial therapy. Clin Infect Dis 2019; 68:1160–5. [DOI] [PubMed] [Google Scholar]
- 18. Karino S, Kaye KS, Navalkele B, et al. Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother 2016; 60:3743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keller SC, Cosgrove SE, Arbaje AI, et al. It’s complicated: patient and informal caregiver performance of outpatient parenteral antimicrobial therapy-related tasks. Am J Med Qual 2020; 35:133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keller SC, Cosgrove SE, Arbaje AI, et al. Roles and role ambiguity in patient- and caregiver-performed outpatient parenteral antimicrobial therapy. Jt Comm J Qual Patient Saf 2019; 45:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keller SC, Ciuffetelli D, Bilker W, et al. The impact of an infectious diseases transition service on the care of outpatients on parenteral antimicrobial therapy. J Pharm Technol 2013; 29:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansour O, Arbaje AI, Townsend JL. Patient experiences with outpatient parenteral antibiotic therapy: results of a patient survey comparing skilled nursing facilities and home infusion. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cox AM, Malani PN, Wiseman SW, Kauffman CA. Home intravenous antimicrobial infusion therapy: a viable option in older adults. J Am Geriatr Soc 2007; 55:645–50. [DOI] [PubMed] [Google Scholar]
- 24. Barr DA, Semple L, Seaton RA. Self-administration of outpatient parenteral antibiotic therapy and risk of catheter-related adverse events: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2012; 31:2611–9. [DOI] [PubMed] [Google Scholar]
- 25. Pérez-López J, San José Laporte A, Pardos-Gea J, et al. Safety and efficacy of home intravenous antimicrobial infusion therapy in older patients: a comparative study with younger patients. Int J Clin Pract 2008; 62:1188–92. [DOI] [PubMed] [Google Scholar]
- 26. Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res 2013; 11:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chopra V, O’Horo JC, Rogers MA, et al. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2013; 34:908–18. [DOI] [PubMed] [Google Scholar]
- 28. Osmon DR, Berbari EF, Berendt AR, et al. ; Infectious Diseases Society of America Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 29. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baddour LM, Wilson WR, Bayer AS, et al. ; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–434. [DOI] [PubMed] [Google Scholar]
- 31. Shrestha NK, Mason P, Gordon SM, et al. Adverse events, healthcare interventions and healthcare utilization during home infusion therapy with daptomycin and vancomycin: a propensity score-matched cohort study. J Antimicrob Chemother 2014; 69:1407–15. [DOI] [PubMed] [Google Scholar]
- 32. Ingram PR, Lye DC, Tambyah PA, et al. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother 2008; 62:168–71. [DOI] [PubMed] [Google Scholar]
- 33. Norton K, Ingram PR, Heath CH, Manning L. Risk factors for nephrotoxicity in patients receiving outpatient continuous infusions of vancomycin in an Australian tertiary hospital. J Antimicrob Chemother 2014; 69:805–8. [DOI] [PubMed] [Google Scholar]
- 34. Ryback MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharmacy 2020; 77:835–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.