Abstract
Background
It is difficult to select an appropriate empirical antibiotic treatment regimen for patients with culture-negative pyogenic vertebral osteomyelitis (PVO). Having knowledge of the distribution of microorganisms according to patient characteristics can help clinicians make informed choices regarding empirical antibiotics. The aim of this study was to determine the microbial distribution among individuals with PVO according to their demographic and clinical characteristics.
Methods
We reviewed the medical records of patients admitted to our hospital with culture-confirmed PVO between January 2005 and December 2017 and collected data on demographics, underlying diseases, and radiographic and microbiological results. Statistical analysis was performed to identify associations between specific bacteria and specific patient characteristics.
Results
A total of 586 patients were included in the study. The prevalence of Staphylococcus aureus infections was higher in young patients than in old patients, while gram-negative bacterial infections and Enterococcus were more prevalent in older patients. Gram-negative bacterial infections were more common in women than in men (32.1% vs 16.4%; P < .05), in patients with cirrhosis than in those without (32.7% vs 21.1%; P < .05), and in patients with a solid tumor than in those without (31.0% vs 20.7%; P < .05). Methicillin-resistant S. aureus infections were more prevalent in patients with chronic renal disease than in those without (34.4% vs 14.7%; P < .05).
Conclusions
The microbial etiology of PVO varies according to patient characteristics. Patient characteristics should thus be considered when choosing empirical antibiotics in patients with culture-negative PVO.
Keywords: spondylitis, vertebral osteomyelitis, gram-negative bacteria, Staphylococcus aureus, chronic renal insufficiency
Pyogenic vertebral osteomyelitis (PVO) affects the vertebrae and the paravertebral structures. Because long-term antibiotic treatment is essential, it is important to identify the causative microorganism. As part of disease management, tissue and/or blood cultures are obtained from patients with PVO to identify the pathogen [1]. Culture positivity rates of tissue and/or blood samples generally range between 30% and 80%, and over 20% of patients are generally regarded as having culture-negative PVO [2, 3]. It is difficult to select the most appropriate empirical antibiotic treatment regimen without knowing the causative microorganism. Having a knowledge of the distribution of microorganisms according to patient characteristics such as age and sex, the spinal level of the lesion, and other underlying disease, can help clinicians make informed choices regarding empirical antibiotics for patients with culture-negative PVO. The causative bacterial agents of PVO have been reported several times [3–8], but their distribution according to basic patient characteristics has not previously been described in detail. The aim of this study was to determine the distribution of etiologic agents of PVO according to patient demographic and clinical characteristics.
METHODS
We conducted a retrospective study at 3 university-affiliated teaching hospitals in Korea from January 2005 to December 2017. All 3 hospitals were tertiary referral centers with 900–1,700 beds.
Patients with culture-confirmed PVO were included in the study. The criteria for a diagnosis of culture-confirmed PVO were compatible clinical signs or symptoms, radiologic evidence of vertebral infection, and isolation of the causative microorganism from spinal/paraspinal tissues and/or blood. Compatible signs or symptoms were defined as pain, fever, or neurologic manifestations. Characteristic radiographic changes included decreased signal intensity in the vertebral body and disk, loss of endplate on T1-weighted images, and increased signal intensity of the disk and vertebral body on T2-weighted images [9]. When common skin contaminants such as coagulase-negative Staphylococci were identified on culture, they were considered to be pathogenic only if the same microorganism grew in 2 or more culture bottles. We excluded patients who had remnant prosthesis at the time of diagnosis of PVO or who had undergone spinal surgery in the year preceding the diagnosis of PVO. Patients aged <18 years and those with vertebral osteomyelitis caused by Mycobacterium tuberculosis, Brucella, or fungi were also excluded.
We collected information about patient demographics, underlying diseases, and radiographic and microbiological data. We also collected information about epidural block or vertebroplasty that had been performed less than 6 months before the diagnosis of PVO. Patients with cirrhosis were defined as either those diagnosed by a radiological method and a gastroenterologist or those with definite evidence of portal hypertension without any other known cause [10]. Patients were defined as diabetic if they had a fasting glucose level of ≥126 mg/dL, a nonfasting glucose level of ≥200 mg/dL, a glycated hemoglobin (HbA1c) of ≥6.5%, or were using glucose-lowering drugs. Patients were defined as having chronic renal disease they had an estimated glomerular filtration rate of <60 mL/min/1.73 m2 that had been present for ≥3 months.
Chi-square tests were used to compare categorical variables in order to identify associations between specific bacteria and specific patient characteristics. If the expected frequency of a given outcome was ≤5, the Fisher exact test was used instead. Trends in ordinal data were evaluated using the linear-by-linear association test. Bonferroni correction was used to adjust for multiple comparisons. All P values were 2-tailed, and P values <.05 were considered statistically significant. Statistical analysis was performed using SPSS, version 20.0 (IBM Corp, Armonk, NY, USA). The institutional review boards of the 3 participating hospitals approved the study. A waiver of consent was granted, given the retrospective nature of the study.
RESULTS
A total of 586 patients with culture-proven PVO were included. The demographic and clinical characteristics of these patients are shown in Table 1. Their median age was 65.1 years, and 371 patients (63.3%) were males. The most common comorbidities were diabetes mellitus (n = 180, 30.7%), followed by solid tumors (n = 84, 14.3%), chronic renal disease (n = 61, 10.4%), and cirrhosis (n = 55, 9.4%). The most commonly affected region was the lumbar spine (n = 387, 66.0%), followed by the thoracic spine (n = 115, 19.6%). The most common pathogens were Staphylococcus aureus (n = 255, 43.5%) regardless of patient characteristics, followed by gram-negative bacteria (n = 130, 22.2%) and Streptococcus species (n = 118, 20.1%). Echocardiography for the evaluation of concomitant infective endocarditis had been conducted in 64.0% (375/586) of patients, and 42 (11.2%) out of 375 patients had infective endocarditis.
Table 1.
Demographic and Clinical Characteristics of 586 Patients With Pyogenic Vertebral Osteomyelitis
| Patient Characteristic | Patients |
|---|---|
| Age, mean ± SD, y | 65.1 ± 12.5 |
| Male | 371 (63.3) |
| Comorbidity | |
| Diabetes mellitus | 180 (30.7) |
| Solid tumora | 84 (14.3) |
| Chronic renal disease | 61 (10.4) |
| Cirrhosis | 55 (9.4) |
| Cerebrovascular disease | 39 (6.6) |
| Congestive heart failure | 29 (4.9) |
| Immunosuppressantb | 28 (4.8) |
| Hematologic malignancy | 14 (2.4) |
| Blunt trauma | 14 (2.4) |
| Spinal location | |
| Cervical spine | 40 (6.8) |
| Thoracic spine | 115 (19.6) |
| Lumbar spine | 387 (66.0) |
| Sacrum | 44 (7.5) |
| Predisposing condition | |
| Epidural block | 111 (18.9) |
| Vertebroplasty | 19 (3.2) |
| No. of vertebrae involved | |
| ≤2 | 471 (80.4) |
| ≥3 | 115 (19.6) |
| Concomitant infective endocarditis | 42 (7.2) |
| Etiology | |
| Staphylococcus aureus | 255 (43.5) |
| MSSA | 157 (26.8) |
| MRSA | 98 (16.7) |
| MSCNS | 12 (2.0) |
| MRCNS | 19 (3.2) |
| Enterococcus species | 22 (3.8) |
| Streptococcus species | 118 (20.1) |
| Gram-negative bacilli | 130 (22.2) |
| Anaerobes | 11 (1.9) |
| Polymicrobial | 9 (1.5) |
| Otherc | 10 (1.7) |
Data are presented as No. (%) of patients unless otherwise indicated.
Abbreviations: MRCNS, methicillin-resistant coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSCNS, methicillin-sensitive coagulase-negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus.
aActive localized or metastatic solid tumor.
bCorticosteroids (>10 mg of prednisolone daily within 4 weeks), cytoreductive agents, immunophilin binding agents, mycophenolate, anti–tumor necrosis factor agents, or other monoclonal antibodies.
cThe other bacteria (count) were Ralstonia mannitolilytica (1), Listeria monocytogenes (1), Stenotrophomonas maltophilia (1), Burkholderia cepacia (1), Neisseria species (2), Granulicatella adiacens (1), Lactococcus gravieae (2), and Erysipelothrix rhusiopathiae (1).
Distribution of Bacteria According to Age
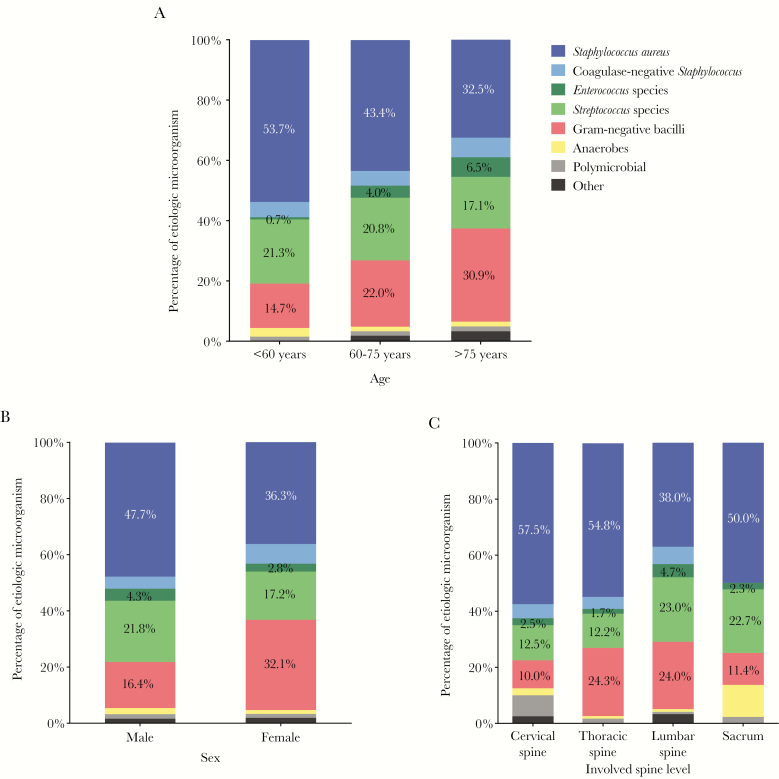
The distribution of bacteria according to age is summarized in Table 2 and Figure 1A. S. aureus was more common in patients aged <60 years (53.7%, vs 43.4% in patients aged 60–75 years and 32.5% in patients aged >75 years; linear-by-linear association, P < .05). Gram-negative bacteria were more common in patients aged 60 years or older (30.9%, vs 14.7% in patients aged <60 years and 22.0% in patients aged 60–75 years; linear-by-linear association, P < .05), as were Enterococcus species (6.5%, vs 0.7% in patients <60 years and 4.0% in patients aged 60–75 years; linear-by-linear association, P < .05).
Table 2.
Distribution of Etiologic Microorganisms in Patients With Pyogenic Vertebral Osteomyelitis According to Age, Sex, and Spinal Level
| Cervical Spine | Cervical Spine | Lumbar Spine | Sacrum | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Organism | Males (n = 33) | Females (n = 7) | Males (n = 77) | Females (n = 38) | Males (n = 236) | Females (n = 151) | Males (n = 25) | Females (n = 19) | Males (n = 371) | Females (n = 215) |
| <60 | Total | ||||||||||
| MSSA | 6 (50.0) | 1 (25.0) | 9 (52.9) | 5 (83.3) | 22 (43.1) | 8 (25.0) | 4 (36.4) | 2 (66.7) | 41 (45.1) | 16 (35.6) | |
| MRSA | 1 (8.3) | 1 (25.0) | 5 (29.4) | 0 (0) | 3 (5.9) | 4 (12.5) | 2 (18.2) | 0 (0) | 11 (12.1) | 5 (11.1) | |
| MSCNS | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.1) | 0 (0) | 0 (0) | 0 (0) | 1 (2.2) | |
| MRCNS | 0 (0) | 1 (25.0) | 0 (0) | 0 (0) | 3 (5.9) | 2 (6.3) | 0 (0) | 0 (0) | 3 (3.3) | 3 (6.7) | |
| Enterococcus species | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.1) | 0 (0) | |
| Streptococcus species | 2 (16.7) | 0 (0) | 1 (5.9) | 0 (0) | 16 (31.4) | 7 (21.9) | 3 (27.3) | 0 (0) | 22 (24.2) | 7 (15.6) | |
| Gram-negative bacilli | 0 (0) | 1 (25.0) | 2 (11.8) | 1 (16.7) | 5 (9.8) | 10 (31.3) | 0 (0) | 1 (33.3) | 7 (7.7) | 13 (28.9) | |
| Anaerobes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3.9) | 0 (0) | 2 (18.2) | 0 (0) | 4 (4.4) | 0 (0) | |
| Polymicrobial | 2 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2.2) | 0 (0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 60–75 | Total | ||||||||||
| MSSA | 7 (38.9) | 0 (0) | 14 (33.3) | 5 (25.0) | 28 (20.6) | 15 (18.1) | 4 (36.4) | 5 (33.3) | 53 (25.6) | 25 (20.8) | |
| MRSA | 5 (27.8) | 1 (50.0) | 10 (23.8) | 4 (20.0) | 32 (23.5) | 10 (12.0) | 0 (0) | 2 (13.3) | 47 (22.7) | 17 (14.2) | |
| MSCNS | 1 (5.6) | 0 | 2 (4.8) | 0 (0) | 3 (2.2) | 1 (1.2) | 0 (0) | 0 (0) | 6 (2.9) | 1 (0.8) | |
| MRCNS | 0 (0) | 0 (0) | 1 (2.4) | 0 (0) | 3 (2.2) | 5 (6.0) | 0 (0) | 0 (0) | 4 (1.9) | 5 (4.2) | |
| Enterococcus species | 0 (0) | 0 (0) | 0 (0) | 1 (5.0) | 8 (5.9) | 3 (3.6) | 1 (9.1) | 0 (0) | 9 (4.3) | 4 (3.3) | |
| Streptococcus species | 1 (5.6) | 0 (0) | 5 (11.9) | 2 (10.0) | 36 (26.5) | 17 (20.5) | 3 (27.3) | 4 (26.7) | 45 (21.7) | 23 (19.2) | |
| Gram-negative bacilli | 1 (5.6) | 1 (50.) | 9 (21.4) | 8 (40.0) | 23 (16.9) | 27 (32.5) | 2 (18.2) | 1 (6.7) | 35 (16.9) | 37 (30.8) | |
| Anaerobes | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 0 (0) | 1 (9.1) | 2 (13.3) | 3 (1.4) | 2 (1.7) | |
| Polymicrobial | 1 (5.6) | 0 (0) | 1 (2.4) | 0 (0) | 1 (0.7) | 1 (1.2) | 0 (0) | 1 (6.7) | 3 (1.4) | 2 (1.7) | |
| Other | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 4 (4.8) | 0 (0) | 0 (0) | 2 (1.0) | 4 (3.3) | |
| >75 | Total | ||||||||||
| MSSA | 1 (33.3) | 0 (0) | 6 (33.3) | 1 (8.3) | 9 (18.4) | 4 (11.1) | 0 (0) | 1 (100) | 16 (21.9) | 6 (12.0) | |
| MRSA | 0 (0) | 0 (0) | 2 (11.1) | 2 (16.7) | 5 (10.2) | 7 (19.4) | 2 (66.7) | 0 (0) | 9 (12.3) | 9 (18.0) | |
| MSCNS | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 3 (6.1) | 0 (0) | 0 (0) | 0 (0) | 3 (4.1) | 1 (2.0) | |
| MRCNS | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 3 (8.3) | 0 (0) | 0 (0) | 0 (0) | 4 (8.0) | |
| Enterococcus species | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 5 (10.2) | 2 (5.6) | 0 (0) | 0 (0) | 6 (8.2) | 2 (4.0) | |
| Streptococcus species | 2 (66.7) | 0 (0) | 5 (27.8) | 1 (8.3) | 7 (14.3) | 6 (16.7) | 0 (0) | 0 (0) | 14 (19.2) | 7 (14.0) | |
| Gram-negative bacilli | 0 (0) | 1 (100) | 3 (16.7) | 5 (41.7) | 15 (30.6) | 13 (36.1) | 1 (33.3) | 0 (0) | 19 (26.0) | 19 (38.0) | |
| Anaerobes | 0 (0) | 0 (0) | 1 (5.6) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) | 1 (1.4) | 1 (2.0) | |
| Polymicrobial | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 1 (2.0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.4) | 1 (2.0) | |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (8.2) | 0 (0) | 0 (0) | 0 (0) | 4 (5.5) | 0 (0) | |
Data are presented as No. (%) of patients unless otherwise indicated.
Abbreviations: MRCNS, methicillin-resistant coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSCNS, methicillin-sensitive coagulase-negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus.
Figure 1.
Microbial etiology of pyogenic vertebral osteomyelitis according to age (A), sex (B), and spinal level (C).
Distribution of Bacteria According to Sex
The distribution of bacteria according to sex is summarized in Table 2 and Figure 1B. The prevalence of gram-negative bacterial infection was higher in women than in men (32.1% vs 16.4%; P < .05). In contrast, S. aureus infection was more common in men than in women (47.7% vs 36.3%; P < .05).
Distribution of Bacteria According to Spinal Level
As shown in Table 2 and Figure 1C, S. aureus infection was less prevalent in the lumbar spine than in the cervical, thoracic, and sacral spine (38.0%, 57.5%, 54.8%, and 50.0%, respectively; P < .05). The prevalence of S. aureus was still significantly lower in the lumbar spine than in the thoracic spine after Bonferroni correction (38.0% vs 54.8%, respectively; P < .008). Streptococcus species were more common in PVO of the lumbar (23.0%) or sacral spine (22.7%) than in the cervical spine (12.5%) or thoracic spine (12.2%; P < .05). However, after Bonferroni correction, there was no significant difference in the occurrence of streptococcal infections according to spinal level.
Distribution of Bacteria According to Comorbid Conditions
The distribution of bacteria according to the presence of comorbidities is shown in Table 3. The distribution was similar between diabetic and nondiabetic patients. Patients with cirrhosis had a higher occurrence of gram-negative bacteria than those without (32.7% vs 21.1%; P < .05), and the proportion of patients with gram-negative infections increased as liver function deteriorated (3/11, 27.3%, in patients with Child-Pugh class A; 10/33, 30.3%, in patients with Child-Pugh class B; and 5/11, 45.5%, in patients with Child-Pugh class C). Methicillin-resistant S. aureus (MRSA) infection was more common in patients with chronic renal disease than in those without (34.4% vs 14.7%; P < .05). Patients with a solid tumor had a higher occurrence of PVO caused by gram-negative bacteria than those without a solid tumor (31.0% vs 20.7%; P < .05). There were no significant differences in the distribution of bacteria in the other comorbidities.
Table 3.
Distribution of Etiologic Microorganisms in Patients With Pyogenic Vertebral Osteomyelitis According to Comorbidities
| Patients With DM (n = 180) | Patients Without DM (n = 406) | P | Patients With LC (n = 55) | Patients Without LC (n = 531) | P | Patients With CRD (n = 61) | Patients Without CRD (n = 525) | P | Patients With Solid Tumor (n = 84) | Patients Without Solid Tumor (n = 502) | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | 84 (46.7) | 171 (42.1) | .306 | 14 (25.5) | 241 (45.4) | .005 | 32 (52.5) | 223 (42.5) | .14 | 27 (32.1) | 228 (45.4) | .023 |
| MSSA | 49 (27.2) | 108 (26.6) | .876 | 10 (18.2) | 147 (27.7) | .130 | 11 (18.0) | 146 (27.8) | .103 | 14 (16.7) | 143 (28.5) | .024 |
| MRSA | 35 (19.4) | 63 (15.5) | .240 | 4 (7.3) | 94 (17.7) | .048 | 21 (34.4) | 77 (14.7) | <.001 | 13 (15.5) | 85 (16.9) | .741 |
| MSCNS | 3 (1.7) | 9 (2.2) | .764 | 1 (1.8) | 11 (2.1) | 1.000 | 1 (1.6) | 11 (2.1) | >.99 | 3 (3.6) | 9 (1.8) | .394 |
| MRCNS | 5 (2.8) | 14 (3.4) | .672 | 2 (3.6) | 17 (3.2) | .696 | 1 (1.6) | 18 (3.4) | .709 | 1 (1.2) | 18 (3.6) | .500 |
| Enterococcus species | 10 (5.6) | 12 (3.0) | .127 | 0 (0) | 22 (4.1) | .252 | 5 (8.2) | 17 (3.2) | .068 | 1 (1.2) | 21 (4.2) | .346 |
| Streptococcus species | 34 (18.9) | 84 (20.7) | .616 | 16 (29.1) | 102 (19.2) | .082 | 10 (16.4) | 108 (20.6) | .441 | 23 (27.4) | 95 (18.9) | .074 |
| Gram-negative bacilli | 37 (20.6) | 93 (22.9) | .527 | 18 (32.7) | 112 (21.1) | .048 | 10 (16.4) | 120 (22.9) | .250 | 26 (31.0) | 104 (20.7) | .037 |
| Anaerobes | 3 (1.7) | 8 (2.0) | 1.000 | 3 (5.5) | 8 (1.5) | .075 | 0 (0) | 11 (2.1) | .615 | 0 (0) | 11 (2.2) | .379 |
| Polymicrobial | 2 (1.1) | 7 (1.7) | .729 | 1 (1.8) | 8 (1.5) | .591 | 2 (3.3) | 7 (1.3) | .239 | 2 (2.4) | 7 (1.4) | .624 |
| Other | 2 (1.1) | 8 (2.0) | .731 | 0 (0) | 10 (1.9) | .610 | 0 (0) | 10 (1.9) | .610 | 1 (1.2) | 9 (1.8) | >.99 |
Data are presented as No. (%) of patients unless otherwise indicated.
Abbreviations: CRD, chronic renal disease; DM, diabetes mellitus; LC, liver cirrhosis; MRCNS, methicillin-resistant coagulase-negative; MRSA, methicillin-resistant Staphylococcus aureus; MSCNS, methicillin-sensitive coagulase-negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus.
aActive localized or metastatic solid tumor.
Distribution of Bacteria According to Previous Epidural Block or Vertebroplasty
The distribution of bacteria according to previous epidural block or vertebroplasty is shown in Table 4. The distribution was similar in patients receiving previous spinal procedures or in patients who did not, except for methicillin-resistant coagulase-negative staphylococcus, which was more prevalent in patients who had received spinal procedures.
Table 4.
Causative Pathogens of Pyogenic Vertebral Osteomyelitis in Patients With or Without Spinal Procedures
| Organism | Patients With Previous Spinal Procedures (n = 128) | Patients Without Previous Spinal Procedures (n = 458) | P Value |
|---|---|---|---|
| S. aureus | 48 (37.5) | 207 (45.2) | .120 |
| MSSA | 33 (25.8) | 124 (27.1) | .770 |
| MRSA | 15 (11.7) | 83 (18.1) | .086 |
| MSCNS | 3 (2.3) | 9 (2.0) | .730 |
| MRCNS | 11 (8.6) | 8 (1.7) | .001 |
| Enterococcus spp. | 6 (4.7) | 16 (3.5) | .598 |
| Streptococcus spp. | 28 (21.9) | 90 (19.7) | .579 |
| G(-) bacilli | 23 (18.0) | 107 (23.4) | .194 |
| Anaerobes | 3 (2.3) | 8 (1.7) | .712 |
| Polymicrobial | 1 (0.8) | 8 (1.7) | .692 |
| Other | 5 (3.9) | 5 (1.1) | .045 |
Data are presented as No. (%) of patients unless otherwise indicated.
Abbreviations: MRCNS, methicillin-resistant coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus; MSCNS, methicillin-sensitive coagulase-negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus.
DISCUSSION
We investigated the distribution of 586 bacterial pathogens in patients with PVO over a 13-year period. Gram-negative infections were more common in older patients, women, and patients with cirrhosis or a solid tumor. Conversely, S. aureus infection was common in younger patients and men. MRSA was more common in patients with chronic renal disease. The selection of an empiric antimicrobial therapy for PVO should be based on the microorganism that is most likely to cause it. When choosing empirical antibiotics, we recommend including an effective agent against S. aureus regardless of demographic or clinical characteristics.
Compared with younger patients, older patients had a relatively low occurrence of S. aureus infections and a relatively high occurrence of gram-negative bacterial infections and enterococcal infections. These findings are consistent with previous studies [11–13]. The different distribution of pathogens according to age could be explained by the differences in the presumed sources of infection, with elderly patients having a higher percentage of intra-abdominal, urinary, and biliary tract infections and a lower percentage of skin and soft tissue infections than younger patients [11]. Unlike the current study, the predominance of gram-negative bacteria was not observed in a previous retrospective review, which is the largest study of PVO in elderly patients to date [14]. In that study, the proportion of patients with Enterobacteriaceae was 3.5% in those ≥75 years old and 3.8% in those <75 years old.
Gram-negative bacteria were more common in women. This result was consistent with the previous studies [4, 15, 16] and may be explained by a higher prevalence of urinary tract infections in women [4, 15].
There are only a few reports that have investigated the characteristics of PVO involving specific spine levels [17, 18]. It has been proposed that in patients with genitourinary infections, microorganisms can spread to the lumbar and sacral spine through vertebral valveless venous plexuses that anastomose with the pelvic venous plexus [19]. In recent years, some authors have suggested that the venous route in the pathogenesis of vertebral osteomyelitis is unproven and an arterial route of spread to the vertebrae appears more plausible, even in the cases with a genitourinary source of infection [20]. The radiologic finding in vertebral osteomyelitis displayed the characteristic anterior metaphyseal vertebral lesions, which are rich in arterial but not in venous supply. In this study, the proportion of gram-negative bacteria in patients with lumbar or sacral PVO did not differ from that of patients with cervical or thoracic PVO. Our results support the results of a previous study that found that vertebral venous plexuses do not have a role in the spread of infection to vertebrae [20]. In this study, the proportion of S. aureus in the lumbar spine was significantly low. The proportion of streptococcus in the lumbar spine was relatively high and contributed to a low proportion of S. aureus. We could not explain the reason why Streptococcus involved in the lumbar spine is more common. Of all streptococci involving the lumbar spine, viridians streptococcus was the most common, followed by S. agalactiae.
Diabetes mellitus was the most common underlying disease. In other studies, about 15%–40% of patients with PVO had diabetes [3, 4, 6, 8, 21, 22]. Although there has been a report that PVO due to gram-negative bacteria is associated with diabetes [16], we did not find any differences in the pattern of organisms in diabetic patients compared with nondiabetic patients [16]. In this study, the proportion of gram-negative bacteria was higher in cirrhotic patients. One previous study has investigated bacterial causes of PVO in patients with cirrhosis [23]. The most common microorganism was S. aureus, and 21% of the infections were caused by Enterobacteriaceae. Considering that the main route of bacteremia in cirrhotic patients is endogenous seeding from the gastrointestinal tract, it is not surprising that gram-negative enteric bacteria might be more common in cirrhotic patients with PVO [24]. MRSA was more common in patients with chronic renal disease regardless of being on dialysis treatment (data according to hemodialysis not shown). Hemodialysis is a well-known risk factor for MRSA colonization or infection [25, 26]. However, the risk of MRSA colonization or infection in patients with chronic kidney disease who were not treated with dialysis was not evaluated. We hypothesize that the risk of MRSA colonization or infection might also be high in these patients, and further studies are required. Our findings that the proportion of gram-negative bacteria was higher in patients with a solid tumor corresponded with data suggesting that the frequency of bacteremia caused by gram-negative bacteria increases in cancer patients [27–29].
Several studies have revealed that a substantial proportion of patients with vertebral osteomyelitis had concomitant infective endocarditis [30, 31]. In this study, echocardiography for the evaluation of concomitant infective endocarditis had been conducted in 64.0% (375/586) of patients. We suggest that the evaluation of concomitant infective endocarditis should be considered in all patients with vertebral osteomyelitis.
The present study has a few limitations. First, this study retrospectively reviewed medical records and may have inadvertently missed certain variables. Second, we classified patients with PVO by combining 2 vertebral levels, that is, cervical and thoracic, thoracic and lumbar, and lumbar and sacral, into 1 vertebral level that involved more. Nonetheless, similar results were obtained when we categorized vertebral levels more accurately (data not shown). Third, we excluded patients who had remnant prosthesis or had undergone spine operation within 1 year of the diagnosis of PVO, so our results cannot be applied to such patients. Finally, we could not analyze the association between previous asymptomatic bacteriuria and pathogens causing vertebral osteomyelitis because data regarding asymptomatic bacteriuria were not collected. Further studies are needed to clarify this correlation.
In patients with PVO, the distribution of bacteria differed according to the patients’ demographic and clinical characteristics. Our results indicate that an understanding of the distribution of bacteria in patients with PVO could be immensely helpful in informing the choice of empirical antibiotics in patients with culture-negative PVO. However, accurate microbiologic diagnosis is essential both for the optimization of antimicrobial therapy and to avoid unnecessary drug toxicity. Therefore, it cannot be overemphasized that a precise etiological diagnosis should be pursued in all cases, and empirical treatment should only be applied in selected cases.
Acknowledgments
Financial support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conceptualization: N. J. Kim, K. H. Park. Data collection: D. Y. Kim, U. J. Kim, Y. H. Yu, S. E. Kim, S. J. Kang, P. G. Choe, E. S. Kim, H. B. Kim, H. C. Jang, S. I. Jung. Data analysis and interpretation: D. Y. Kim, K. H. Song, C. K. Kang, K. I. Jun. Writing–original draft: D. Y. Kim, N. J. Kim. Writing–review & editing: N. J. Kim, K. H. Park, M. D. Oh. Authors N. J. Kim and K. H. Park contributed equally to this manuscript.
References
- 1. Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med 2010; 362:1022–9. [DOI] [PubMed] [Google Scholar]
- 2. Kim CJ, Song KH, Park WB, et al. . Microbiologically and clinically diagnosed vertebral osteomyelitis: impact of prior antibiotic exposure. Antimicrob Agents Chemother 2012; 56:2122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chong BSW, Brereton CJ, Gordon A, Davis JS. Epidemiology, microbiological diagnosis, and clinical outcomes in pyogenic vertebral osteomyelitis: a 10-year retrospective cohort study. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang WS, Ho MW, Lin PC, et al. . Clinical characteristics, treatments, and outcomes of hematogenous pyogenic vertebral osteomyelitis, 12-year experience from a tertiary hospital in central Taiwan. J Microbiol Immunol Infect 2018; 51:235–42. [DOI] [PubMed] [Google Scholar]
- 5. Kim UJ, Bae JY, Kim SE, et al. . Comparison of pyogenic postoperative and native vertebral osteomyelitis. Spine J 2019; 19:880–7. [DOI] [PubMed] [Google Scholar]
- 6. Lee YM, Cho OH, Park SY, et al. . Factors associated with sequelae after treatment of hematogenous pyogenic vertebral osteomyelitis. Diagn Microbiol Infect Dis 2019; 94:66–72. [DOI] [PubMed] [Google Scholar]
- 7. Gupta A, Kowalski TJ, Osmon DR, et al. . Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis 2014; 1:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernard L, Dinh A, Ghout I, et al. ; Duration of Treatment for Spondylodiscitis (DTS) study group Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385:875–82. [DOI] [PubMed] [Google Scholar]
- 9. Colmenero JD, Jiménez-Mejías ME, Sánchez-Lora FJ, et al. . Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 1997; 56:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oberti F, Valsesia E, Pilette C, et al. . Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology 1997; 113:1609–16. [DOI] [PubMed] [Google Scholar]
- 11. Aguilar-Company J, Pigrau C, Fernández-Hidalgo N, et al. . Native vertebral osteomyelitis in aged patients: distinctive features. An observational cohort study. Infection 2018; 46:679–86. [DOI] [PubMed] [Google Scholar]
- 12. Thompson D, Bannister P, Murphy P. Vertebral osteomyelitis in the elderly. Br Med J (Clin Res Ed) 1988; 296:1309–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belzunegui J, Intxausti JJ, De Dios JR, et al. . Haematogenous vertebral osteomyelitis in the elderly. Clin Rheumatol 2000; 19:344–7. [DOI] [PubMed] [Google Scholar]
- 14. Courjon J, Lemaignen A, Ghout I, et al. ; DTS (Duration of Treatment for Spondylodiscitis) study group Pyogenic vertebral osteomyelitis of the elderly: characteristics and outcomes. PLoS One 2017; 12:e0188470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang SJ, Jang HC, Jung SI, et al. . Clinical characteristics and risk factors of pyogenic spondylitis caused by gram-negative bacteria. PLoS One 2015; 10:e0127126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park KH, Cho OH, Jung M, et al. . Clinical characteristics and outcomes of hematogenous vertebral osteomyelitis caused by gram-negative bacteria. J Infect 2014; 69:42–50. [DOI] [PubMed] [Google Scholar]
- 17. Miyazaki M, Yoshiiwa T, Kodera R, Tsumura H. Clinical features of cervical pyogenic spondylitis and intraspinal abscess. J Spinal Disord Tech 2011; 24:E57–61. [DOI] [PubMed] [Google Scholar]
- 18. Urrutia J, Zamora T, Campos M. Cervical pyogenic spinal infections: are they more severe diseases than infections in other vertebral locations? Eur Spine J 2013; 22:2815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henriques CQ. Osteomyelitis as a complication in urology; with special reference to the paravertebral venous plexus. Br J Surg 1958; 46:19–28. [DOI] [PubMed] [Google Scholar]
- 20. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010; 65(Suppl 3):iii11–24. [DOI] [PubMed] [Google Scholar]
- 21. Kim CJ, Song KH, Jeon JH, et al. . A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2010; 35:E1096–100. [DOI] [PubMed] [Google Scholar]
- 22. Lora-Tamayo J, Euba G, Narváez JA, et al. . Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: the impact of cases with no microbiologic diagnosis. Semin Arthritis Rheum 2011; 41:247–55. [DOI] [PubMed] [Google Scholar]
- 23. Park SY, Park KH, Cho OH, et al. . Clinical characteristics and outcomes of pyogenic vertebral osteomyelitis in patients with cirrhosis. Hepatology 2016; 64:689. [DOI] [PubMed] [Google Scholar]
- 24. Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence 2016; 7:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuo G, Sun WC, Lu YA, et al. . Chronic dialysis patients with infectious spondylodiscitis have poorer outcomes than non-dialysis populations. Ther Clin Risk Manag 2018; 14:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control Prevention. Invasive methicillin-resistant Staphylococcus aureus infections among dialysis patients—United States, 2005. MMWR 2007; 56:197–9. [PubMed] [Google Scholar]
- 27. Anatoliotaki M, Valatas V, Mantadakis E, et al. . Bloodstream infections in patients with solid tumors: associated factors, microbial spectrum and outcome. Infection 2004; 32:65–71. [DOI] [PubMed] [Google Scholar]
- 28. Velasco E, Byington R, Martins CA, et al. . Comparative study of clinical characteristics of neutropenic and non-neutropenic adult cancer patients with bloodstream infections. Eur J Clin Microbiol Infect Dis 2006; 25:1–7. [DOI] [PubMed] [Google Scholar]
- 29. Mikulska M, Viscoli C, Orasch C, et al. ; Fourth European Conference on Infections in Leukemia Group (ECIL-4), a joint venture of EBMT, EORTC, ICHS, ELN and ESGICH/ESCMID Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect 2014; 68:321–31. [DOI] [PubMed] [Google Scholar]
- 30. Pigrau C, Almirante B, Flores X, et al. . Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med 2005; 118:1287. [DOI] [PubMed] [Google Scholar]
- 31. Park KH, Kim DY, Lee YM, et al. . Selection of an appropriate empiric antibiotic regimen in hematogenous vertebral osteomyelitis. PLoS One 2019; 14:e0211888. [DOI] [PMC free article] [PubMed] [Google Scholar]