Highlights
-
•
Characteristics of COVID-19 in imported and non-imported patients were analyzed.
-
•
Fever was the most common symptom at the onset of illness.
-
•
Half of the patients had a low-grade temperature, with duration of fever <7 days.
-
•
Viral load was undetectable on day 14 in all patients in the tertiary group.
-
•
The virus was detectable on day 14 in one third of the imported and secondary patients.
Keywords: COVID-19, SARS-CoV-2, Ct value, Imported
Abstract
Objectives
To compare the clinical characteristics and the dynamics of viral load between imported and non-imported patients with COVID-19.
Design and methods
Data from 51 laboratory-confirmed patients were retrospectively analyzed.
Results
The incubation period in the tertiary group was longer than that in the imported and secondary groups (both p < 0.05). Fever was the most common symptom at the onset of illness (73.33%, 58.82%, and 68.42%, respectively), and half of the patients had a low-grade temperature (<38.0 °C) with a short duration of fever (<7 days). CT scans showed that most patients in the three groups had bilateral pneumonia (80.00%, 76.47%, and 73.68%, respectively). Ct values detected in the tertiary patients were similar to those for the imported and secondary groups at the time of admission (both p > 0.05). For the tertiary group, the viral load was undetectable in half of the patients (52.63%) on day 7, and in all patients on day 14. For one third of the patients in the imported and secondary groups, the viral load remained positive on day 14 after the admission.
Conclusions
COVID-19 can present as pneumonia with a low onset of symptoms, and the infectivity of SARS-CoV2 may gradually decrease in tertiary patients.
Introduction
The 2019 novel coronavirus (nCoV-2019, now known as SARS-CoV-2) infection, which can cause respiratory failure, poses a global threat to public health (Chan et al., 2020a, Chen et al., 2020a). The coming weeks are crucial in monitoring the status of community transmission (Heymann and Shindo, 2020, Hui et al., 2020).
The imported patients with COVID-19 in this study were those who had visited or originated from Wuhan city, and were detected at the end of January 2020; subsequently, secondary and tertiary transmissions occurred nationwide (Lipsitch et al., 2020). Person-to-person transmission of SARS-CoV-2 in hospital and family settings was confirmed in other geographical regions (Chan et al., 2020b). Although the speed of transmission declined in China, concerns about the outbreak of pneumonia in other countries are constantly rising (Hui et al., 2020, Boldog et al., 2020, Mahase, 2020). To date, the differences between cases of imported, secondary, and tertiary transmission remain largely unknown.
For this study we analyzed the characteristics of COVID-19 in Changzhou city, Jiangsu province. The clinical, laboratory, and radiological characteristics, as well as the dynamics of viral load, were compared between the imported, secondary, and tertiary patients.
Methods
Patients
A cohort of 51 patients in Changzhou was confirmed with COVID-19, according to Chinese guidelines for the diagnosis and treatment of COVID-19 (Anon., 2020), and admitted to the Third Hospital of Changzhou, a designated hospital, between January 23 and February 18, 2020. The outcomes were followed up until February 27, 2020. Epidemiological history and symptom data were confirmed by two doctors in a negative-pressure ward. Viral RNA was detected in throat swabs obtained from the patients, and a computed tomography (CT) scan was acquired immediately after symptom onset.
The 51 patients were divided into three groups — imported (15 cases), secondary (17 cases), and tertiary (19 cases) — according to their epidemiological history. The imported patients with COVID-19 were those who had visited or originated from Wuhan city, Hubei province. Secondary patients were defined as those who had not visited or come from Wuhan, but who had been in close contacted with the imported patients. The tertiary cases were those had not visited Wuhan or come into contact with the imported patients, but had acquired infection through contact with the secondary cases (Huang et al., 2019, Lim et al., 2020, Park et al., 2016).
All data for the included individuals were reported to the Chinese Center for Disease Control and Prevention (CDC).
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay
COVID-19 was confirmed according to the cycle threshold (Ct) values for Orf1ab and N genes ascertained by RT-PCR assay. The assay was performed by Changzhou CDC and the Clinical Laboratory of The Third People’s Hospital of Changzhou using a commercial kit (Biogerm Medical Biotechnology Co., Shanghai, China). Ct values were inversely related to viral RNA copy numbers (Zou et al., 2020), with a Ct value <40 being considered positive.
Statistical analysis
Continuous variables were expressed as median (IQR) or mean ± standard deviation and compared using the Kruskal–Wallis test or one-way ANOVA, followed by post-hoc tests to compare the differences between the three groups. Categorical values were expressed as frequencies, and the differences were analyzed using Fisher’s exact test. All analyses were performed using SPSS 23.0 software (Chicago, IL, USA). A two-sided p < 0.05 was considered statistically significant.
Results
Demographics and clinical characteristics of patients with COVID-19
The demographic and clinical characteristics of the patients are shown in Table 1 . Twelve family clusters were found in this study. Six patients without any symptoms were diagnosed according to the chest CT scans and the Ct values detected in throat swabs. 10/15 (66.67%) patients in the imported group were males, while almost half of the patients in the secondary and tertiary groups were females. The patients in the tertiary group were significantly older than those in the secondary group (p = 0.03) and had more comorbidities than those in the imported group (χ2 = 8.259, p < 0.01).
Table 1.
Demographic and clinical characteristics of patients with COVID-19.
| Variables | Imported (n = 15) | Secondary (n = 17) | Tertiary (n = 19) | Z or χ2 | p-value |
|---|---|---|---|---|---|
| Age, years | 35.0 (29.0–51.0) | 37.0 (24.0–47.5) | 53.0 (35.0–65.0)* | 5.614 | 0.06 |
| Male, n (%) | 10 (66.7) | 7 (41.2) | 8 (42.1) | 2.601 | 0.28 |
| Comorbidity, n (%) | 0 (0) | 4 (23.5) | 8 (42.1)** | 8.766 | 0.01 |
| Pulmonary disease | 0 (0) | 0 (0) | 1 (5.3) | 1.648 | 0.99 |
| Cardiovascular disease | 0 (0) | 2 (11.8) | 3 (15.8) | 2.383 | 0.36 |
| Diabetes | 0 (0) | 1 (5.9) | 3 (15.8) | 2.463 | 0.37 |
| Chronic liver disease | 0 (0) | 0 (0) | 1 (5.3) | 1.648 | 0.99 |
| Chronic kidney disease | 0 (0) | 1 (5.9) | 0 (0) | 1.871 | 0.63 |
| Incubation period, days | 8.0 (4.0–10.0) | 8.0 (4.0–11.0) | 12.0 (9.0–14.0)*, ** | 10.943 | <0.01 |
| Fever, N (%) | 11 (73.3) | 10 (58.8) | 13 (68.4) | 0.820 | 0.75 |
| Highest temperature <38℃, n (n/N, %) | 5 (45.5) | 5 (50.0) | 8 (61.5) | 0.742 | 0.76 |
| Duration, days | 2.0 (0–3.0) | 2.0 (0–4.0) | 2.0 (0–5.0) | 0.573 | 0.75 |
| Duration <7 days, n (n/N, %) | 11 (100.0) | 8 (80.0) | 11 (84.6) | 2.278 | 0.43 |
| Cough, n (%) | 5 (33.3) | 9 (52.9) | 9 (47.4) | 1.309 | 0.53 |
| Sputum production, n (%) | 3 (20.0) | 6 (35.3) | 4 (21.1) | 1.258 | 0.56 |
| Pharyngalgia, n (%) | 1 (6.7) | 0 (0) | 2 (10.5) | 1.746 | 0.50 |
| Fatigue, n (%) | 1 (6.7) | 1 (5.9) | 0 (0) | 1.547 | 0.52 |
| Myalgia, n (%) | 4 (26.7) | 2 (11.8) | 2 (10.5) | 1.814 | 0.44 |
| Dyspnea, n (%) | 2 (13.3) | 1 (5.9) | 1 (5.3) | 0.982 | 0.67 |
| Diarrhea, n (%) | 2 (13.3) | 1 (5.9) | 2 (10.5) | 0.623 | 0.86 |
Data are expressed as median (IQR) and n (%). Comparisons were conducted using the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical values.
Tertiary vs secondary, p < 0.05.
Tertiary vs imported, p < 0.05.
Since none of the patients developed severe pneumonia or ARDS, all patients survived, and no mechanical ventilation was given in this study. Interestingly, the incubation period for the tertiary group was longer than that for the imported and secondary groups (p < 0.01 and p = 0.03, respectively).
Fever was the most common symptom at the onset of illness (73.33%, 58.82%, and 68.42%, respectively), with about half of the patients (45.45%, 50.00%, and 61.54%, respectively) having a low-grade temperature (<38.0 °C) with a short duration of fever (<7 days). However, no significant differences were detected in the development of fever and other symptoms, including cough, sputum production, pharyngalgia, fatigue, myalgia, dyspnea, and diarrhea, between the three groups (all p > 0.05).
Laboratory and radiology findings for patients with COVID-19
Four patients in the tertiary group showed an elevated level (<85 U/L) of alanine transaminase (ALT) and aspartate aminotransferase (AST) at admission. The levels of D-dimer, troponina, and creatine kinase were normal in all patients.
Leucopenia (white blood cell count <4 × 109/L) was more common in the tertiary group than in the imported patients (χ2 = 8.295, p = 0.01) (Table 2 ). Moreover, B lymphocyte counts were lower in the tertiary patients compared with those in the imported group (p < 0.01).
Table 2.
Laboratory and radiology findings of patients with COVID-19.
| Variables | Imported (n = 15) | Secondary (n = 17) | Tertiary (n = 19) | Z or χ2 | p-value |
|---|---|---|---|---|---|
| Laboratory findings | |||||
| ALT, U/L | 22.9 (16.6–31.0) | 13.5 (11.6–21.1) | 25.2 (11.7–36.2)* | 7.785 | 0.02 |
| AST, U/L | 19.0 (17.0–31.0) | 17.0 (12.5–19.5) | 22.0 (17.0–34.0)* | 9.382 | <0.01 |
| C-reactive protein, mg/L | 4.4 (1.3–11.2) | 2.2 (1.0–16.2) | 7.0 (0.8–20.0) | 0.930 | 0.63 |
| WBC, E + 09/L | 5.2 (4.6–6.3) | 4.8 (3.4–6.2) | 3.9 (3.1–5.7)** | 5.747 | 0.06 |
| WBC < 4 E + 09/L, n (%) | 1 (6.67) | 6 (35.29) | 10 (52.63)** | 8.295 | 0.01 |
| Lymphocytes, E + 09/L | 1.5 (0.9–2.1) | 1.1 (1.0–1.9) | 1.3 (0.9–1.6) | 1.077 | 0.58 |
| CD3+ T lymphocyte, % | 69.9 (62.9–78.2) | 65.9 (60.0–72.9) | 72.2 (65.8–76.1) | 2.297 | 0.32 |
| CD4+ T lymphocyte, % | 39.9 (38.2–45.7) | 37.5 (32.2–43.9) | 37.4 (32.6–40.4) | 3.266 | 0.20 |
| CD8+ T lymphocyte, % | 28.7 (22.7–33.9) | 26.7 (20.8–31.9) | 30.3 (26.0–34.0) | 1.592 | 0.45 |
| B lymphocyte, % | 13.9 (9.8–18.1) | 11.4 (8.4–16.1) | 9.1 (7.5–10.9)** | 7.425 | 0.02 |
| CT findings | |||||
| Unilateral pneumonia | 2 (13.3) | 3 (17.7) | 3 (15.8) | 0.256 | 0.99 |
| Bilateral pneumonia | 12 (80.0) | 13 (76.5) | 14 (73.7) | 0.269 | 0.99 |
| Multiple mottling and ground-glass opacity | 1 (6.7) | 1 (5.9) | 2 (10.5) | 0.517 | 0.99 |
| Ct value on admission | |||||
| ORF1ab gene | 28.0 (26.0–30.0) | 30.0 (28.0–31.5) | 30.0 (22.0–34.0) | 1.301 | 0.52 |
| N gene | 30.0 (26.0–32.0) | 30.0 (27.5–32.0) | 32.0 (26.0–34.0) | 0.778 | 0.68 |
| Ct < 40 on day 7, n (%) | |||||
| ORF1ab gene | 9 (60.0) | 15 (88.2) | 9 (47.4)* | 6.767 | 0.03 |
| N gene | 7 (46.7) | 14 (82.4)*** | 7 (36.8)* | 8.088 | 0.02 |
| Ct < 40 on day 14, n (%) | |||||
| ORF1ab gene | 5 (33.3) | 6 (35.3) | 0 (0)*, ** | 8.345 | 0.02 |
| N gene | 4 (26.7) | 5 (29.4) | 0(0)*, ** | 6.530 | 0.04 |
Data are expressed as median (IQR) and n (%). Comparisons were conducted using the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical values.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; WBC, white blood cell counts; CT, computed tomography; Ct, cycle threshold.
Tertiary vs secondary, p < 0.05.
Tertiary vs imported, p < 0.05.
Imported vs secondary, p < 0.05.
CT scans showed that most patients in the three groups had bilateral pneumonia (80.00%, 76.47%, and 73.68%, respectively).
Dynamic changes of Ct value in patients with COVID-19
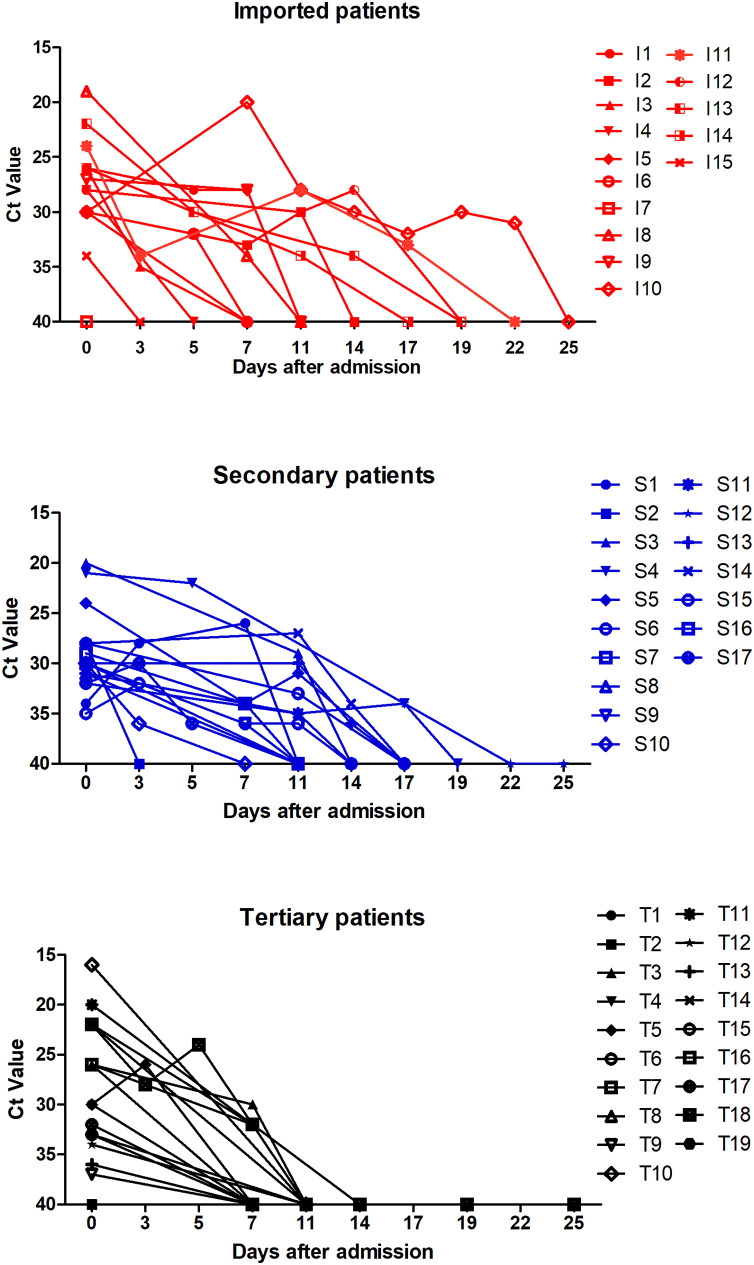
Of the 51 patients, 49 (96.08%) showed positive RNA tests in the throat swabs, while one patient had a positive RNA test in the bronchoalveolar lavage fluid, and another tested positive in the anal swab. As shown in Figure 1 , the viral load in throat swabs with respect to days was analyzed after admission.
Figure 1.
Dynamic changes in cycle threshold (Ct) values in patients with imported, secondary, and tertiary COVID-19.
Ct values detected in the tertiary patients were similar to those for the imported and secondary groups on admission (both p > 0.05). For the tertiary group, the viral load was undetectable in half of the patients (52.63%) on day 7 and in all patients on day 14, but remained positive in one-third of the patients in the imported and secondary groups on day 14 after admission.
Discussion
For this study we analyzed the clinical, laboratory, and radiological characteristics, as well as the dynamics of viral load, in patients with COVID-19. Half of the patients had low-grade (<38.0 ℃) fever of short duration (<7 days). The incubation period in the tertiary group was longer than that in the imported and secondary groups, with the viral load undetectable in the early stages.
Compared with SARS-CoV infection, COVID-19 shows less onset of symptoms (Sun et al., 2020, Wu and McGoogan, 2020). Gao et al. (Huang et al., 2020) reported that the majority of the patients in Wuhan presented a moderate or high temperature (>38.0 ℃), while low-grade fever with or without mild respiratory symptoms was common in our study because no patients with severe pneumonia were included. Moreover, SARS-CoV-2 causes lower respiratory tract lesions even in patients without any clinical symptoms (Chen et al., 2020b, Wang et al., 2020). Interestingly, six patients in our study had radiological evidence of pneumonia, but with no fever or cough during infection. These findings were in agreement with those of previous studies (Chen et al., 2020b, Huang et al., 2020). Thus, chest CT scans need to be monitored routinely in clinical practice.
Although none of the patients developed a severe infection in this study, comorbidities were common in the tertiary patients, demanding a focus on elderly patients. Moreover, low B lymphocyte counts in the tertiary patients may be related to these comorbidities, thereby necessitating additional studies to elucidate the immunological mechanisms during COVID-19 infection (Li et al., 2020).
To date, the epidemiological characteristics of COVID-19 are unclear. Notably, the long incubation period suggests a potential transmission route in tertiary, even asymptomatic, patients. Although lopinavir/ritonavir and arbidol have been administered in clinical practice, their effectiveness in inhibiting SARS-CoV-2 replication is not yet confirmed (Anon., 2020). Interestingly, the viral load could not be detected early in tertiary patients; thus, it could be speculated that the infectivity of SARS-CoV-2 may gradually decrease.
This study has several limitations. First, the incubation period may have been short for several of the patients who lived in Wuhan because they were infected several days before coming to Changzhou. Second, the number of patients was limited, and therefore a multicenter study with a large sample size would be essential to further substantiate our findings.
In conclusion, COVID-19 can present as pneumonia with the onset of few symptoms, and the infectivity of SARS-CoV-2 may gradually decrease in tertiary patients.
Authors’ contributions
Study design: Yuan Xue and Hong Dai. Data collection: Zhen Zhu, Manman Cui, Chunhua Chen, Cong Chen, and Yuan Xue. Data analysis: Yuan Xue, Tianmin Xu, and Cong Chen. Writing: Yuan Xue, Tianmin Xu, and Cong Chen. All authors read and approved the final manuscript. Tianmin Xu and Cong Chen contributed equally to this work.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The study was anonymous, and the protocol was approved by the Ethics Committee of The Third People’s Hospital of Changzhou, according to the Declaration of Helsinki, 2013. Written informed consent was obtained from all participants.
Acknowledgment
This work was supported by the Natural Science Foundation of Jiangsu province [BK20180183] and the Science and Technology Project of Changzhou [CJ20179030].
Contributor Information
Hong Dai, Email: czsgbyjs@163.com.
Yuan Xue, Email: xueyuan80908@163.com.
References
- Anon [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. doi: 10.3760/cma.j.issn.1001-0939.2020.0019. [DOI] [PubMed] [Google Scholar]
- Boldog P., Tekeli T., Vizi Z., Dénes A., Bartha F.A., Röst G. Risk assessment of novel coronavirus COVID-19 outbreaks outside China. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10233):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Shindo N. COVID-19: what is next for public health? Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.I., Tai M.C., Wu K.B., Chen W.C., Huang A.E., Cheng W.Y. Measles transmission at an international airport — Taiwan, March–April 2018. Int J Infect Dis. 2019;86:188–190. doi: 10.1016/j.ijid.2019.07.038. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of Lopinavir/Ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- Mahase E. Coronavirus: UK screens direct flights from Wuhan after US case. BMJ. 2020;368:m265. doi: 10.1136/bmj.m265. [DOI] [PubMed] [Google Scholar]
- Park S.H., Kim Y.S., Jung Y., Choi S.Y., Cho N.H., Jeong H.W. Outbreaks of Middle East respiratory syndrome in two hospitals initiated by a single patient in Daejeon, South Korea. Infect Chemother. 2016;48(2):99–107. doi: 10.3947/ic.2016.48.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kang H., Liu X., Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. 2020 doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]