Abstract
Introduction
About one-third of critically ill patients with acute kidney injury (AKI) develop persistently decreased kidney function, known as acute kidney disease (AKD), which may progress to chronic kidney disease (CKD). Although sepsis is the most common cause of AKI, little is known about sepsis-associated AKD.
Methods
Using data from a large randomized trial including 1341 patients with septic shock, we studied patients with stage 2 or 3 AKI on day 1 of hospitalization. We defined AKD as a persistently reduced glomerular filtration rate for >7 days. In addition to clinical data, we measured several urinary biomarkers (tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7 [TIMP-2∗IGFBP7], neutrophil gelatinase-associated lipocalin [NGAL], kidney injury molecule-1 [KIM-1], liver-type fatty acid binding protein, and type 4 collagen) at 0, 6, and 24 hours, to predict AKD.
Results
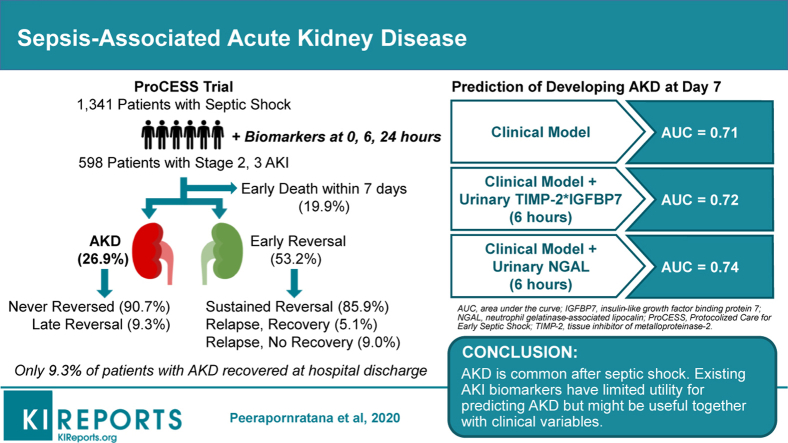
Of 598 patients, 119 (19.9%) died within 7 days, 318 (53.2%) had early reversal of AKI within the first 7 days, whereas 161 (26.9%) developed AKD. In patients with early reversal, 45 (14.2%) had relapsed AKI after early reversal, and only about one-third of these recovered. Among patients developing AKD, only 15 (9.3%) recovered renal function prior to discharge. Male sex, African American race, and underlying CKD were more predominant in patients developing AKD. None of the biomarkers tested performed well for prediction of AKD, although NGAL modestly increased the performance of a clinical model.
Conclusions
AKD is common in patients with septic shock, especially among African American males and those with underlying CKD. Existing AKI biomarkers have limited utility for predicting AKD but might be useful together with clinical variables. Novel predictive biomarkers for renal recovery are needed.
Keywords: acute kidney disease, acute kidney injury, biomarker, predict, recovery, sepsis
Graphical abstract
Globally, more than half of all patients in the intensive care unit (ICU) suffer from AKI, and sepsis is the most common etiology.1,2 Evidence from epidemiologic studies has shown that AKI is associated not only with short-term adverse effects but also important long-term consequences.3, 4, 5 Despite apparent recovery from AKI, patients are still at risk for recurrent kidney injury, which can worsen clinical outcomes.6,7
Both AKI severity and duration are associated with progression to CKD and other adverse clinical outcomes.8 Persistent reduction in glomerular filtration rate beyond 7 days but for less than 90 days is considered AKD.9,10 Data on the epidemiology and course of AKD are still limited, and few studies have focused on sepsis-associated AKD. However, several observational studies have shown that the shorter the duration of AKI, the better the outcome. About 70% to 90% of hospitalized patients with AKI experience recovery of kidney function within 3 to 4 days of AKI onset.11,12 In several studies, complete and sustained reversal of AKI within 48 to 72 hours was associated with better outcomes than was AKI of longer duration.11,13, 14, 15, 16, 17 However, when compared to those with no AKI, patients with AKI with early reversal still experienced a higher risk of in-hospital mortality and developing de novo CKD.11,12 We recently reported distinct recovery phenotypes following AKI among nearly 17,000 critically ill patients.7 About 64% of patients with stage 2 or 3 AKI had normal kidney function within 7 days after AKI onset, which was associated with the most-favorable long-term outcomes. Interestingly, the remaining 36% had persistent AKI beyond 7 days, compatible with AKD, and this was associated with poorer outcomes. In the setting of sepsis, early reversible AKI within 24 hours was associated with improved survival.18 Another study in patients with sepsis-associated AKI found that persistent AKI for more than 7 days was associated with increased ICU length of stay, ventilator use, and cardiovascular failure, but not in-hospital mortality by 30 days.19 A recent meta-analysis showed that duration of AKI was independently associated with long-term mortality, cardiovascular events, and development of incident CKD of stage 3 or greater.20 This accumulating evidence emphasizes the clinical impact of persistently reduced kidney function following AKI. This group of patients may need more-intensive monitoring and follow-up care.9 Thus, we sought to examine AKD and associated outcomes in patients with sepsis. Given that early recognition of patients at risk of developing AKD would provide clinicians with a better chance of early implementation of interventions to facilitate recovery, we also examined whether clinical variables either alone or together with existing AKI biomarkers could predict AKD in these patients.
Methods
Study Design
We performed secondary analyses using data from the ProCESS (Protocolized Care for Early Septic Shock) trial. Full details of the ProCESS trial have been published elsewhere,21 and additional details related to AKI in this cohort have also been published.22 In brief, ProCESS was a multicenter randomized controlled trial comparing alternative resuscitation strategies for patients with septic shock in the US. Patients who had either confirmed or suspected infection with hypotension were enrolled and randomized to be resuscitated by early goal-directed therapy,23 protocol-based standard care, or usual care during the initial 6 hours of hospital admission. In this analysis, we focused on patients with stage 2 or 3 AKI and stratified them by different AKI trajectories using the definitions developed in our previous study7 (see below). We analyzed the performance of urinary biomarkers collected at 0, 6, and 24 hours after hospital admission in order to differentiate patients with AKD and early reversal of AKI. The in-hospital and long-term survival, up to 1 year, associated with different trajectories of AKI were also determined.
Patients
The ProCESS trial was approved by the University of Pittsburgh Institutional Review Board. Our cohort included those patients with stage 2 or 3 AKI according to Kidney Disease: Improving Global Outcomes criteria10 that either were present at the time of enrollment or developed within the first 24 hours of hospitalization. Patients with end-stage kidney disease or prior kidney transplant, with known baseline creatinine >4 mg/dl, or with missing enrollment serum creatinine values, and those who withdrew from the trial, were excluded. From a total of 1341 patients in the ProCESS trial, our analysis cohort was 598 patients.
Definitions of Recovery Status
We classified patients by AKI definition and staging according to the Kidney Disease: Improving Global Outcomes criteria, using both serum creatinine and urine output. AKI stages were determined each day until ICU discharge or at least 72 hours, based on maximum severity by either creatinine or urine output criteria. We determined baseline serum creatinine level within 12 months prior to hospital admission, taking a median value if multiple measurements were available. Reference serum creatinine level was determined by the lower of baseline and admission serum creatinine level as previously described.15,24,25 For patients with missing baseline or admission serum creatinine level (without history of CKD), we calculated26 a serum creatinine level by solving the Modification of Diet in Renal Disease equation as recommended in the Kidney Disease: Improving Global Outcomes AKI guideline, assuming a glomerular filtration rate of 75 ml/min per 1.73 m2. Missing urine output was not imputed and did not contribute to staging for patients.
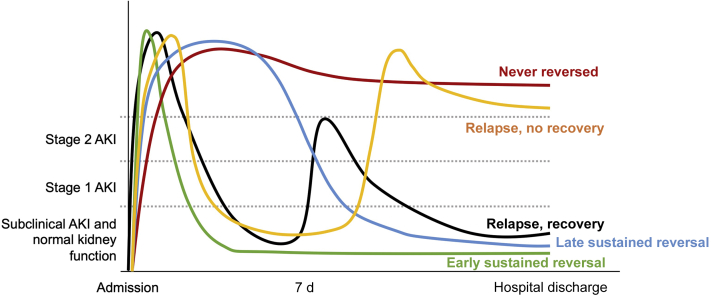
Figure 1 illustrates the definition of trajectories after AKI onset used in our study. Early reversal was defined as the absence of any stage of AKI (even stage 1) by either serum creatinine or urine output criteria for at least a 24-hour period on any day within 7 days of the first documented onset of AKI. Patients with stable kidney function through hospital discharge after early reversal were considered to have sustained reversal of AKI. Those who had persistent AKI of stage 1 or greater for more than 7 days up to 90 days after AKI onset were classified as having AKD, according to consensus criteria for AKD following AKI.9 Those who appeared to have normalized their kidney function after day 7 were classified as having late reversal. Subsequent AKI of any stage after initial early reversal was considered a relapse. In cases with multiple subsequent AKI episodes, we considered only the first relapse episode for further analyses. After 7 days, we determined recovery status again only at hospital discharge. We defined recovery to mean alive, free of renal replacement therapy, and with an absence of AKI by both criteria. Death before hospital discharge was considered to be nonrecovery. AKI trajectories were adjudicated by computer and verified by a nephrologist.
Figure 1.
Trajectories after acute kidney injury (AKI).
Clinical Data Collection and Biomarker Measurement
In the ProCESS trial, clinical data for participants were collected from the time of enrollment until hospital discharge, including demographics, prior health history, hourly urine output during ICU stay, severity of illness, and reference serum creatinine level. Blood and urine samples were obtained at 0, 6, and 24 hours for chemistry and biomarker testing. Serum creatinine level was determined daily for at least the first 7 days and according to clinical need thereafter. We determined the stage of AKI each day, based on maximum severity by either creatinine or urine output criteria.
Urine samples for biomarker testing were collected at the time of enrollment (0 hour), at the end of 6-hour resuscitation, and at 24 hours, by standard methods. The centrifuged samples were stored at –80 °C and thawed immediately prior to analysis. We analyzed biomarker levels at each time point in relation to clinical AKI trajectories. We investigated several urinary biomarkers, including markers of kidney stress—TIMP-2 and IGFBP7, measured using a clinical immunoassay (Astute Medical, San Diego, CA); markers of tubular injury—NGAL using enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN); KIM-1 (Bioassay Works, Ijamsville, Maryland); liver-type fatty acid binding protein (CMIC Holdings Co., Ltd., Tokyo, Japan); and a marker of glomerular injury—type IV collagen (Stanbio Laboratory, Boerne, Texas).
Outcomes
Our primary outcome was AKD at 7 days following AKI stage 2 to 3 (first day). Secondary outcomes included ICU and hospital length of stay, in-hospital mortality, and mortality at 90 days and 1 year. Vital status was determined from the National Center for Health Statistics National Death Index database.
Statistical Analyses
Statistical comparisons for categorical and continuous data were performed using χ2/Fisher exact test and Mann-Whitney U test/Kruskal-Wallis test, respectively. Data are reported as counts (percentages) for categorical data, and median with interquartile range for continuous data. No imputation was performed for missing data. The predictive value for biomarkers was analyzed using receiver operating curve (ROC) analysis, and the area under the ROC curve (AUC) was calculated. Previously, validated risk factors for each outcome (such as AKD, early reversal) were analyzed and transformed as appropriate, followed by multivariable logistic regression. Odds ratios with 95% confidence intervals (CIs) of estimated odds ratios were reported. For AKD prediction, in order to provide more statistical accuracy, bootstrapping was performed with 50 bootstrap samples.
Survival analyses were performed using Cox proportional hazard models adjusted by age, and adjusted hazard ratios were reported. The log rank test was used to compare survival curves. All statistical analyses were performed using Stata, version 15.1 (StataCorp, College Station, TX), R version 3.5.2 (The R Foundation for Statistical Computing) and EZR version 1.38 (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan), which is a graphical user interface for R.27 A P value of less than 0.05 was considered to indicate statistical significance, for all tests performed.
Results
AKI Trajectories and Baseline Characteristics
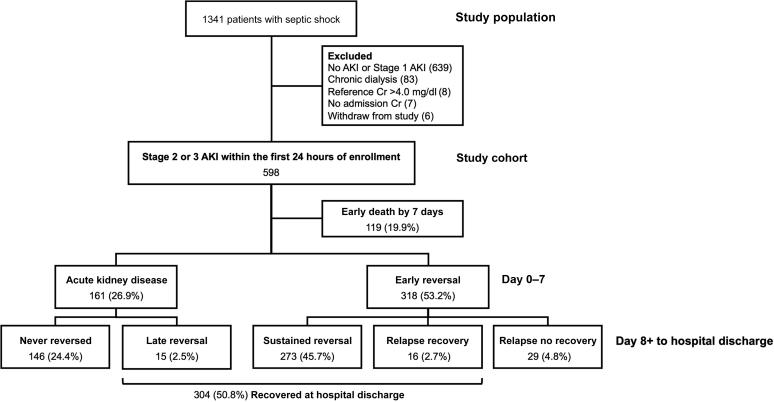
From the study population of 1341 patients, 598 (44.6%) patients had moderate to severe AKI (stage 2 or 3) within the first 24 hours of hospital admission (372 [63.2%] had stage 2 AKI, and 226 [36.8%] had stage 3 AKI). Of these, 119 (19.9%) patients died within 7 days after enrollment and were not included in our primary analysis (Figure 2). At 7 days, 318 (53.2%) patients had early recovery of kidney function, whereas 161 (26.9%) patients had persistent AKI compatible with AKD. The majority of patients (273 [45.7%]) who had early recovery had sustained normal kidney function throughout the remaining hospital course and were thus categorized as early sustained reversal, whereas the remaining patients (45 [7.5%]) had one or more relapse episodes of AKI, and about two-thirds of these did not ultimately recover by hospital discharge. On the other hand, 90% of patients with AKD never reversed AKI. Only 15 (2.5%) patients experienced reversal later after 7 days. Overall, 304 patients (50.8%) achieved complete recovery of kidney function at hospital discharge.
Figure 2.
Study flow. AKI, acute kidney injury; Cr, creatinine.
Table 1 summarizes the main baseline patient characteristics by clinical status at 7 days. The median age was comparable between the 2 groups. Male sex and African American race were more predominant in the AKD group. Patients with AKD also had significantly higher median body weight (86.1 kg vs. 79.3 kg; P = 0.03). As expected, the AKD group had a significantly higher rate of preexisting CKD than the early reversal group (20.5% vs. 12.9%; P = 0.03). The AKD group also had a higher baseline sequential organ failure assessment score (9 vs. 7; P < 0.001). Pneumonia and urinary tract infection were the 2 most common sources of sepsis. However, urosepsis was more common in the AKD group (31.1% vs. 22.0%; P = 0.03) and was the only site of infection that was significantly associated with developing AKD (odds ratio, 1.60; 95% CI, 1.04–2.44; P = 0.031; Supplementary Table S1). Serum creatinine level at hospital admission in the AKD group was significantly higher than that in the early reversal group (3.20 mg/dl vs. 2.20 mg/dl; P < 0.001), despite a slightly lower reference serum creatinine value for patients who developed AKD (0.94 mg/dl vs. 1.03 mg/dl; P = 0.004).
Table 1.
Baseline characteristics
| Characteristic | Early death (n = 119) | AKD (n = 161) | Early reversal (n = 318) |
P value 3 groups |
P value 2 groupsa |
|---|---|---|---|---|---|
| Age, yr | 67 (52–76) | 60 (51–73) | 62 (51–75) | 0.14 | 0.37 |
| Male sex | 60 (50.84) | 115 (71.4) | 165 (51.9) | <0.001 | <0.001 |
| Race | |||||
| White | 82 (68.9) | 93 (57.8) | 223 (70.1) | 0.02 | 0.02 |
| African American | 27 (22.7) | 60 (37.3) | 79 (24.8) | 0.01 | 0.01 |
| Others | 10 (8.4) | 8 (5.0) | 16 (5.0) | 0.36 | 0.98 |
| Body weight, kg | 74.9 (63.9–94.0) | 86.1 (70.0–103.4) | 79.3 (67.1–96.6) | 0.02 | 0.03 |
| Comorbidities | |||||
| Hypertension | 80 (67.2) | 90 (55.9) | 205 (64.5) | 0.10 | 0.07 |
| Diabetes mellitus | 50 (42.0) | 59 (36.6) | 116 (36.5) | 0.54 | 1.00 |
| Chronic lung disease | 20 (16.8) | 27 (16.8) | 82 (25.8) | 0.03 | 0.03 |
| Chronic kidney disease | 24 (20.2) | 33 (20.5) | 41 (12.9) | 0.04 | 0.03 |
| Cancer | 32 (26.9) | 23 (14.4) | 47 (14.8) | 0.01 | 1.00 |
| Cerebral vascular disease | 13 (10.9) | 16 (9.9) | 43 (13.5) | 0.51 | 0.30 |
| Congestive heart failure | 16 (13.4) | 14 (8.7) | 38 (11.9) | 0.42 | 0.35 |
| History of myocardial infarction | 11 (9.2) | 19 (11.8) | 30 (9.4) | 0.68 | 0.43 |
| Cirrhosis | 15 (12.6) | 14 (8.7) | 21 (6.6) | 0.13 | 0.46 |
| Peripheral vascular disease | 18 (15.1) | 9 (5.6) | 20 (6.3) | 0.01 | 0.84 |
| Dementia | 9 (7.6) | 12 (7.5) | 28 (8.8) | 0.90 | 0.73 |
| AIDS | 4 (3.4) | 4 (2.5) | 9 (2.8) | 0.89 | 1.00 |
| Charlson comorbidity score | 3 (1–6) | 2 (1–4) | 2 (1–3) | <0.001 | 0.66 |
| APACHE III score | 73 (59–94) | 62 (49–81) | 60 (48–74) | <0.001 | 0.24 |
| Total SOFA score | 10 (7–13) | 9 (6–11) | 7 (5–10) | <0.001 | <0.001 |
| Source of Infection | |||||
| Pneumonia | 33 (27.7) | 38 (23.6) | 101 (31.8) | 0.17 | 0.06 |
| Urosepsis | 27 (22.7) | 50 (31.1) | 70 (22.0) | 0.08 | 0.03 |
| Intra-abdominal | 25 (21.0) | 18 (11.2) | 42 (13.2) | 0.05 | 0.53 |
| Skin and soft tissue | 6 (5.0) | 11 (6.8) | 21 (6.6) | 0.80 | 0.93 |
| Catheter-related | 0 (0) | 4 (2.5) | 6 (1.9) | 0.25 | 0.74 |
| Central nervous system | 1 (0.8) | 2 (1.2) | 2 (0.6) | 0.79 | 0.61 |
| Endocarditis | 0 (0) | 2 (1.2) | 2 (0.6) | 0.45 | 0.61 |
| Unknown | 17 (14.3) | 19 (11.8) | 45 (14.2) | 0.75 | 0.57 |
| Other | 8 (6.7) | 14 (8.7) | 20 (6.3) | 0.62 | 0.33 |
| Positive blood culture | 47 (39.5) | 67 (41.6) | 106 (33.3) | 0.16 | 0.08 |
| Initial lactate, mg/dl | 5.9 (4.4–9.0) | 4.8 (3.0–7.0) | 4.6 (2.8–6.2) | <0.001 | 0.15 |
| Creatinine, mg/dl | |||||
| Hospital admission | 2.31 (1.59–3.20) | 3.20 (2.21–4.48) | 2.20 (1.58–2.98) | <0.001 | <0.001 |
| Reference value | 1.01 (0.80–1.36) | 0.94 (0.80–1.07) | 1.03 (0.81–1.30) | 0.01 | 0.004 |
| Max AKI stage in first 24 h of admission | |||||
| Stage 2 | 74 (62.2) | 71 (44.1) | 227 (71.4) | <0.001 | <0.001 |
| Stage 3 | 45 (37.8) | 90 (55.9) | 91 (28.6) | <0.001 | <0.001 |
| Resuscitation strategies in the first 6 h | |||||
| EGDT | 42 (35.3) | 47 (29.2) | 109 (34.3) | 0.46 | 0.26 |
| PSC | 32 (26.9) | 58 (36.0) | 100 (31.4) | 0.26 | 0.31 |
| UC | 45 (37.8) | 56 (34.8) | 109 (34.3) | 0.78 | 0.91 |
| Refractory hypotension | 59 (49.6) | 78 (48.4) | 180 (56.6) | 0.17 | 0.09 |
| Pre-randomization fluid volume, L | 2.00 (1.00–3.00) | 2.00 (1.00–3.00) | 2.00 (1.01–3.00) | 0.60 | 0.56 |
| 6-h fluid volume, L | 3.00 (1.69–4.50) | 2.60 (1.53–4.15) | 2.84 (1.74–4.15) | 0.80 | 0.92 |
| Received antibiotics by intervention | 86 (72.9) | 121 (75.2) | 258 (81.1) | 0.11 | 0.13 |
AKD, acute kidney disease; AKI, acute kidney injury; APACHE III, Acute Physiology and Chronic Health Evaluation III; EGDT, early goal-directed therapy; PSC, protocol-based standard care; SOFA, Sequential Organ Failure Assessment; UC, usual care.
Data shown as n (%) or median (interquartile range).
P value for AKD compared with early-reversal groups.
Clinical Model for Prediction of AKD
We validated a clinical model that had already been developed for predicting nonrecovery AKI from our previous study in 15,266 critically ill patients with an AUC-ROC of 0.65.7 Male sex, African American race, and high acute physiology score III significantly increased the odds of developing AKD (Table 2). By contrast, high urine output and the use of mechanical ventilation significantly favored early reversal of AKI. In the current study, this clinical model provided an AUC-ROC of 0.71 (95% CI, 0.66–0.76).
Table 2.
Validation of multivariable clinical model for predicting AKD
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Age, by unit of 5 yr | 1.00 | 1.00–1.00 | 0.83 |
| Male | 2.56 | 1.79–3.67 | <0.001 |
| Race | |||
| African American vs. White | 1.89 | 1.12–3.19 | 0.02 |
| Others vs. White | 1.32 | 0.49–3.60 | 0.59 |
| Hypertension | 0.68 | 0.43–1.09 | 0.11 |
| Cardiac disease | 0.76 | 0.39–1.48 | 0.42 |
| APS-III score,a by units of 10 | 1.18 | 1.09–1.29 | <0.001 |
| Weight-adjusted urine output,a,b by 100 ml | 0.98 | 0.97–0.99 | <0.001 |
| Vasopressorc | 1.01 | 0.54–1.88 | 0.98 |
| Mechanical ventilationc | 0.44 | 0.25–0.75 | 0.003 |
AKD, acute kidney disease; aOR, adjusted odds ratio; APS-III, Acute Physiology Score III; CI, confidence interval.
N = 476; area under the receiver operating characteristic curve = 0.71 (95% CI, 0.66–0.76); goodness-of-fit P value = 0.49.
Square-root transformation was used for selected variables in the model.
The available urine volume in the 24 hours after intensive-care unit admission was summed and divided by the weight.
Captured in 6 hours after randomization.
Biomarkers for Prediction of AKD
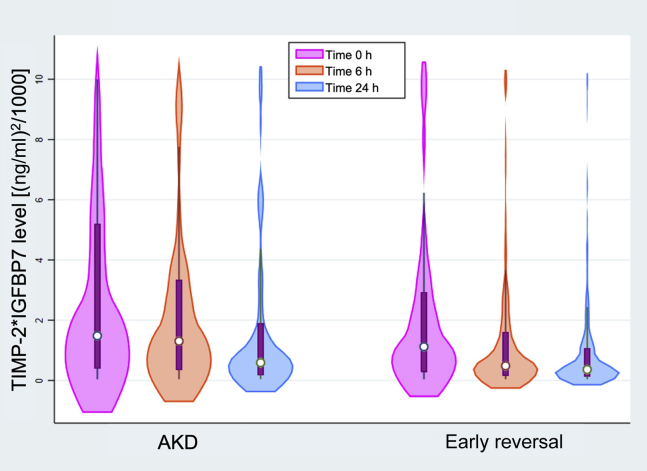
The median levels of urinary biomarkers at 0, 6, and 24 hours were compared between the AKD and the early-reversal groups (Table 3). The baseline biomarker levels at enrollment did not differ between these 2 groups. Baseline urinary TIMP-2∗IGFBP7 levels in both groups were higher than the diagnostic cutoff level of 0.3 (ng/ml)2/1000, but they were not statistically different. However, patients developing AKD had significantly higher urinary TIMP-2∗IGFBP7 levels at both 6 hours (1.31 vs. 0.49 (ng/ml)2/1000; P < 0.001) and 24 hours (0.59 vs. 0.36 (ng/ml)2/1000; P = 0.003) compared to patients with early reversal (Figure 3). The median changes of TIMP-2∗IGFBP7 level from baseline to 6 and 24 hours were not different between the 2 groups (Supplementary Table S2). The remaining biomarkers did not differ between the 2 groups at any time point.
Table 3.
Biomarker levels at 0, 6, and 24 hours
| Urinary biomarker | Time measured (h) | Early death | AKD | Early reversal |
P value 3 groups |
P value 2 groupsa |
|---|---|---|---|---|---|---|
| TIMP-2∗IGFBP7, (ng/ml)2/1000 | 0 | 1.32 (0.30–3.91) n = 78 | 1.48 (0.39–5.28) n = 108 | 1.12 (0.29–2.93) n = 239 | 0.23 | 0.11 |
| 6 | 1.69 (0.30–4.33) n = 79 | 1.31 (0.34–3.38) n = 126 | 0.49 (0.17–1.60) n = 263 | <0.001 | <0.001 | |
| 24 | 1.63 (0.48–5.01) n = 62 | 0.59 (0.17–1.92) n = 129 | 0.36 (0.13–1.08) n = 264 | <0.001 | 0.003 | |
| NGAL, ng/ml | 0 | 331.7 (117.3–577.6) n = 12 | 465.2 (14.7–629.5) n = 11 | 332.9 (38.4–629.5) n = 42 | 0.95 | 0.93 |
| 6 | 897.9 (130.6–2098.4) n = 21 | 1907.4 (391.6–2098.4) n = 47 | 714.4 (161.6–2098.4) n = 82 | 0.04 | 0.01 | |
| 24 | 1168.0 (414.9–2098.4) n = 21 | 1244.3 (365.8–2098.4) n = 47 | 613.6 (117.7–2098.4) n = 82 | 0.11 | 0.09 | |
| KIM-1, pg/ml | 0 | 1.50 (0.76–4.84) n = 36 | 1.30 (0.70–3.70) n = 47 | 1.30 (0.40–2.80) n = 123 | 0.21 | 0.16 |
| 6 | 1.60 (0.90–4.77) n = 34 | 1.30 (0.58–3.54) n = 59 | 1.00 (0.50–3.61) n = 130 | 0.08 | 0.63 | |
| 24 | 1.82 (0.78–5.36) n = 30 | 1.33 (0.40–3.46) n = 59 | 1.20 (0.50–3.09) n = 125 | 0.30 | 0.99 | |
| L-FABP, pg/ml | 0 | 73.6 (44.2–1779.0) n = 16 | 49.8 (16.9–155.4) n = 27 | 89.4 (15.9–247.8) n = 61 | 0.39 | 0.65 |
| 6 | 187.3 (92.90–2121.6) n = 11 | 54.1 (16.9–145.3) n = 29 | 77.3 (19.8–165.8) n = 66 | 0.04 | 0.64 | |
| 24 | 52.5 (25.6–4552.8) n = 10 | 45.4 (20.5–138.9) n = 29 | 56.8 (13.8–117.6) n = 66 | 0.72 | 0.84 | |
| Type IV collagen, mcg/L | 0 | 13.02 (7.31–36.85) n = 33 | 23.94 (5.11–69.68) n = 44 | 13.93 (5.30–27.05) n = 116 | 0.34 | 0.14 |
| 6 | 22.27 (12.65–58.90) n = 32 | 22.55 (9.23–82.59) n = 56 | 14.10 (6.10–41.6) n = 122 | 0.048 | 0.13 | |
| 24 | 26.93 (15.87–71.84) n = 28 | 23.89 (8.09–71.58) n = 56 | 21.72 (9.17–40.94) n = 117 | 0.49 | 0.45 |
AKD, acute kidney disease; IGFBP7, insulin-like growth factor-binding protein 7; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.
Data shown as median (interquartile range).
P value for AKD compared with early-reversal groups.
Figure 3.
Distribution of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7 (TIMP-2∗IGFBP7) level at baseline, and 6 and 24 hours by clinical status at 7 days. AKD, acute kidney disease.
Urinary biomarkers measured at each time point were used for predicting AKD (Table 4). None of the 5 biomarkers at baseline were predictive for AKD at 7 days (AUC-ROC < 0.6). TIMP-2∗IGFBP7 at 6 hours had an AUC-ROC of 0.63 (95% CI, 0.57–0.69; P < 0.001) for predicting AKD. NGAL at 6 hours also performed similarly, with an AUC-ROC of 0.63 (95% CI, 0.53–0.73; P = 0.02). The remaining biomarkers (KIM-1, liver-type fatty acid binding protein, and type IV collagen) were not predictive for AKD (AUC-ROCs < 0.60). The changes between two timepoints of TIMP-2∗IGFBP7 levels during the first 24 hours also demonstrated an AUC-ROC of <0.60 for predicting AKD (Supplementary Table S2 ).
Table 4.
Urinary biomarkers for predicting AKD
| Urinary biomarker | Time measured (h) | N | AUC-ROC for prediction of AKD | 95% CI | P value |
|---|---|---|---|---|---|
| TIMP-2∗IGFBP7 | 0 | 347 | 0.55 | 0.49–0.62 | 0.11 |
| 6 | 389 | 0.63 | 0.57–0.69 | <0.001 | |
| 24 | 393 | 0.59 | 0.53–0.65 | 0.003 | |
| NGAL | 0 | 53 | 0.51 | 0.31–0.71 | 0.93 |
| 6 | 129 | 0.63 | 0.53–0.73 | 0.02 | |
| 24 | 129 | 0.59 | 0.49–0.69 | 0.09 | |
| KIM-1 | 0 | 170 | 0.57 | 0.48–0.67 | 0.16 |
| 6 | 189 | 0.52 | 0.43–0.61 | 0.63 | |
| 24 | 184 | 0.50 | 0.41–0.60 | 0.99 | |
| L-FABP | 0 | 88 | 0.47 | 0.35–0.59 | 0.65 |
| 6 | 95 | 0.47 | 0.35–0.59 | 0.64 | |
| 24 | 95 | 0.49 | 0.36–0.61 | 0.84 | |
| Type IV collagen | 0 | 160 | 0.58 | 0.47–0.69 | 0.14 |
| 6 | 178 | 0.57 | 0.48–0.66 | 0.13 | |
| 24 | 173 | 0.54 | 0.44–0.63 | 0.45 |
AKD, acute kidney disease; AUC-ROC, area under the receiver operating characteristic curve; CI, confidence interval; IGFBP7, insulin-like growth factor-binding protein 7; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.
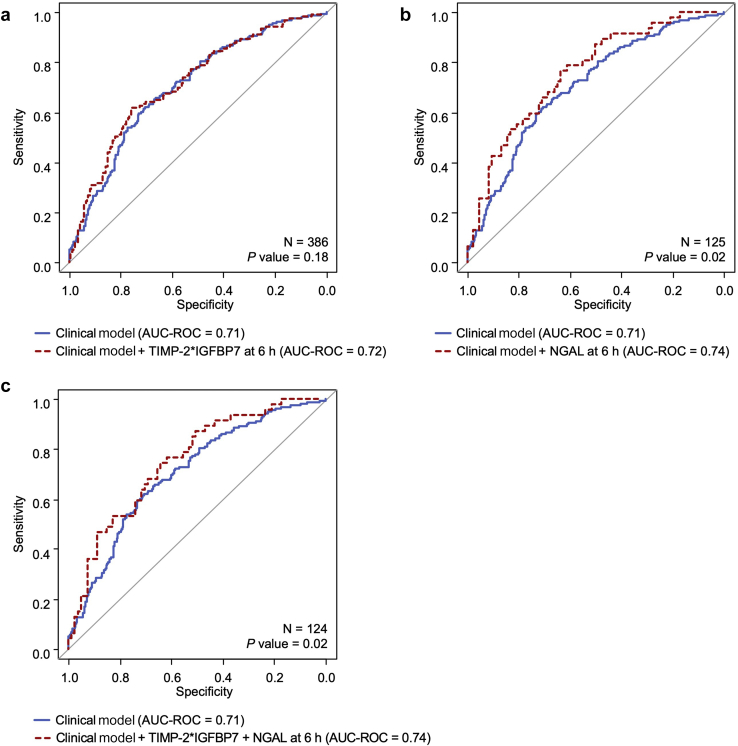
We further explored the prediction performance for AKD by adding urinary TIMP-2∗IGFBP7 and NGAL into the clinical model (Table 5 and Supplementary Table S3). When we added TIMP-2∗IGFBP7 at 6 hours into the clinical model, the AUC-ROC was slightly increased to 0.72 but was not statistically significantly different when compared to that for the clinical model alone (P = 0.18; Figure 4a). However, NGAL at 6 hours significantly increased the AUC-ROC to 0.74 (95% CI, 0.65–0.83) when the marker was added to the clinical model (P = 0.02; Figure 4b). The clinical model plus both TIMP-2∗IGFBP7 and NGAL at 6 hours provided a similar AUC-ROC of 0.74 (95% CI, 0.65–0.83; P = 0.02; Figure 4c).
Table 5.
Comparison of prediction models for AKD
| Model | N | AUC-ROC | 95% CI | P value |
|---|---|---|---|---|
| A: clinical model (reference) | 476 | 0.71 | 0.66–0.76 | — |
| B: clinical model + TIMP-2∗IGFBP7a at 6 h | 386 | 0.72 | 0.66–0.77 | 0.18 |
| D: clinical model + NGALb at 6 h | 125 | 0.74 | 0.65–0.83 | 0.02 |
| F: clinical model + TIMP-2∗IGFBP7a + NGALb at 6 h | 124 | 0.74 | 0.65–0.83 | 0.02 |
AKD, acute kidney disease; AUC-ROC, area under the receiver operating characteristic curve; CI, confidence interval; IGFBP7, insulin-like growth factor-binding protein 7; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.
See Supplementary Table S2 for full models.
Variable was transformed on log scale (natural logarithm).
Variable was transformed on square-root scale.
Figure 4.
(a–c) Comparing areas under the receiver operating characteristic curve (AUC-ROCs) for prediction of acute kidney disease. NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2∗IGFBP7, tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7.
Clinical Outcomes Related to AKI Trajectories
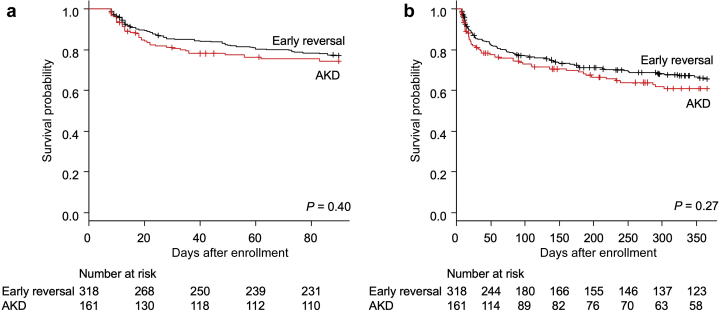
More patients in the AKD group received renal replacement therapy by the first week (11.7% vs. 1.3%; P < 0.001). The crude mortality up to 1 year was not different between patients with AKD and those with early reversal (Table 6). The observed unadjusted median hospital length of stay in our cohort was significantly different between these 2 groups (11 vs. 10 days; P = 0.045), but the ICU length of stay was not. Survival analyses using Cox proportional hazard model adjusted by age (Figure 5) showed no significant difference in mortality (between patients developing AKD and those with early reversal) by 90 days (adjusted hazard ratio, 1.17; 95% CI, 0.81–1.71; P = 0.40) and 1 year (adjusted hazard ratio, 1.20; 95% CI, 0.87–1.65; P = 0.27).
Table 6.
Clinical outcomes
| Outcome | AKD (n = 161) | Early reversal (n = 318) | P value |
|---|---|---|---|
| Mortality | |||
| In-hospital death by 60 d | 23 (14.3) | 33 (10.4) | 0.21 |
| Death by 90 d | 43 (26.7) | 76 (23.9) | 0.50 |
| Death by 1 yr | 59 (36.6) | 101 (31.8) | 0.28 |
| ICU length of stay,a d | 4 (3–8) | 4 (3–7) | 0.99 |
| Hospital length of stay, d | 11 (7–20) | 10 (7–15) | 0.045 |
AKD, acute kidney disease; ICU, intensive care unit.
Data shown as n (%) or median (interquartile range).
Number of patients admitted in ICU = 453 (AKD = 151; early reversal = 302).
Figure 5.
Patient survival comparing acute kidney disease (AKD) and early-reversal groups (a) by 90 days and (b) by 1 year.
Sensitivity Analyses
Because death and AKD are competing risks, we included 119 patients who died early by 7 days in sensitivity analyses (Figure 2). These patients were incorporated into the never-reversed group. Thus, a total of 294 (49.2%) patients were considered to be nonrecovery at hospital discharge, including those who had never-reversed AKI and those with relapsed AKI and no recovery, whereas 304 (50.8%) patients had recovered (early reversal, late sustained reversal, and relapse with recovery).
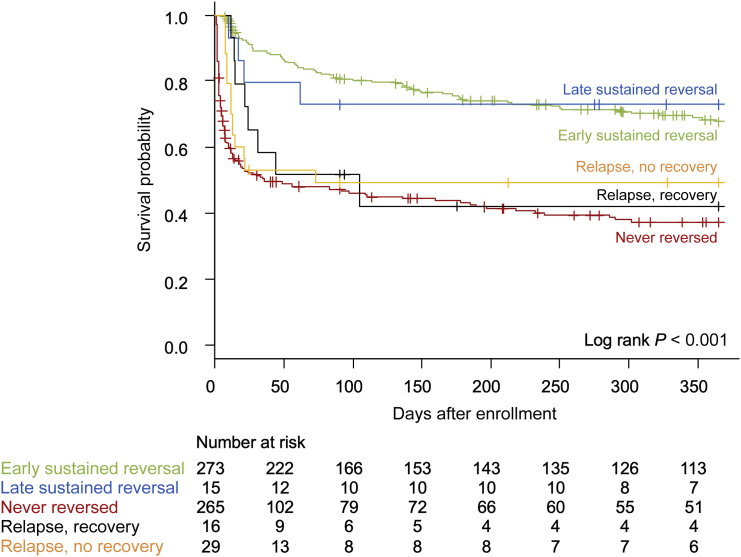
Long-term survival was related to recovery patterns at hospital discharge (Figure 6), not AKD status at day 7. Nearly 10% of patients developing AKD (15 of 161) eventually recovered, and nearly 10% of those with early reversal (29 of 318) had an AKI relapse from which they did not recover.
Figure 6.
Patient survival by 1 year stratified by recovery trajectories of acute kidney injury at hospital discharge.
We validated the same clinical model to discriminate between recovery and nonrecovery AKI (Supplementary Table S4). The clinical model performed less well to predict nonrecovery AKI (AUC-ROC of 0.68; 95% CI, 0.64–0.73). None of the candidate biomarkers were predictive for nonrecovery AKI at hospital discharge (Supplementary Tables S5 and S6).
Discussion
This study is the first to examine clinical risk for AKD in patients with sepsis, and one of the first studies to demonstrate various patterns of renal recovery after sepsis-associated AKI. When we compared the clinical outcomes between patients with AKD and those who had early reversal of AKI, we found no significant difference in mortality rates up to 1 year. This finding appeared to be due to 3 factors. First, about 20% of patients died before day 7 and thus were never classified as having AKD. Second, about 10% of patients with AKD experienced late recovery, and their outcomes mirrored those of patients with early sustained reversal. Third, about 10% of patients with early sustained reversal experienced an AKI relapse from which they did not recover, and these patients had outcomes similar to patients with AKD. Said differently, kidney function at hospital discharge was more predictive of long-term survival than AKD status at 7 days. Federspiel et al. also showed no difference in 30-day mortality between persistent AKI beyond 7 days and AKI with shorter duration.19
Overall, our results suggest that the recovery trajectories after AKI in patients with septic shock are similar to those of other critically ill patients, as previously reported (Figures 2 and 5).7 Either early or late reversal of AKI was associated with high likelihood of survival by 1 year. However, relapsed AKI with or without recovery and never-reversed AKI were associated with a significantly worse prognosis compared to the reversal groups. The rate of AKD at 7 days in this study was similar to that in 2 previous reports. We recently reported a rate of 36.2% for nonreversal AKI by 7 days in critically ill patients.7 A post hoc analysis of a randomized controlled trial in patients with sepsis and acute respiratory distress syndrome found that the rate of persistent AKI beyond 7 days was 32.4%.19 In our primary analyses, which excluded patients with early death, 90.7% of patients with AKD at 7 days ultimately experienced nonrecovery at hospital discharge. These findings highlight the importance of early recognition of patients at risk for AKD. On the other hand, for patients experiencing early reversal, 85.8% experience no further AKI episodes and had recovery at hospital discharge. However, the remaining 14.2% of this group experienced one or more relapse episodes of AKI during hospitalization, which was related to an adverse outcome as well. The rate of relapse of AKI in patients with severe sepsis was also reported by Rodrigo et al. at about 20%, and they also found an association with increased risk of death.28 Interestingly, in patients with preexisting CKD, it probably does make sense that some patients have “AKI on CKD” that resolves slowly and includes a period of AKD. The term “AKD on CKD” is a little awkward, but current definitions still allow for it. With this definition, we could use the term “stable CKD” or “worsening CKD” to specify the aftermath of AKI and AKD in CKD patients.
AKD is thought to be part of an AKI-to-CKD transition in which maladaptive repair plays a major role. Some animal studies showed a promising role of tubular injury markers for predicting fibrosis after AKI. Albuminuria and urinary NGAL, KIM-1, and liver-type fatty acid binding protein, 1 day after ischemia-reperfusion injury, were correlated with the degree of tubulointerstitial fibrosis at 40 days.29 Persistent expression of NGAL or KIM-1 in kidney tissue correlated with fibrosis after AKI.30,31 However, in humans, only a few small studies have used AKI biomarkers for predicting reversal of AKI, and these have demonstrated only fair to good performance. de Geus et al. found that urinary NGAL measured on ICU admission had an AUC-ROC of 0.77 (95% CI, 0.64–0.90) for discrimination of sustained AKI (>24 hours; n = 37) and transient AKI (n = 17).32 Dewitte et al. also reported the performance of multiple biomarkers for prediction of early renal recovery within 48 hours after AKI onset in 57 critically ill patients including urinary TIMP-2∗IGFBP7 and plasma NGAL at 0 and 24 hours, which had AUC-ROCs ranging from 0.70 to 0.78.33 Aregger et al. showed the predictive value of urinary IGFBP7 and NGAL measured on day 1 in 52 critically ill patients for AKI recovery within 7 days with AUC-ROCs of 0.74 and 0.70, respectively.34 We validated a clinical model that provided only fair performance for prediction of AKD (AUC-ROC, 0.71). Furthermore, none of the 5 biomarkers of AKI measured in this study were predictive for AKD at 7 days. Only urinary NGAL measured at 6 hours significantly improved the prediction of AKD when added to the clinical model (AUC-ROC = 0.74). Thus, new markers to predict AKD need to be developed.
Timing of biomarker measurement is an important factor because each marker has different kinetics, and knowing the exact onset of renal recovery is nearly impossible. Findings from our previous report indicated that the median time to reversal of AKI ranged from 30 to 47 hours after its onset.7 For this reason, we only analyzed biomarkers within the first 24 hours of recognized AKI.
To our knowledge, this is the first study specifically examining the epidemiology and outcome of AKD and also reporting the performance of existing AKI biomarkers for prediction of AKD at 7 days. Our analyses were based on data from a multicenter randomized controlled trial, which provided access to a large set of clinical data, timely sample collection, and standardized definition of AKI and clinical outcomes. We also used the current definition of AKD.9 Nevertheless, there are some limitations. First, although sepsis is presumed to be the insult occurring prior to admission, in some cases, the etiology of AKI may be multifactorial and not solely sepsis-associated. However, data for other insults of AKI were not collected in the original ProCESS trial. Second, some AKI trajectories used in our study have no uniform consensus definition. Our definition of relapsed AKI is pragmatic. A new rise in serum creatinine level and/or fall in urine output after early reversal of AKI could be either a new episode of AKI resulting from new insults or a stuttering course from the previous AKI episode. Third, as our data are derived from patients with sepsis shock who developed AKI within 24 hours of hospital admission, which might be considered to be community-acquired, generalizability of these results needs to be validated in other AKI phenotypes and in hospital-acquired settings. Fourth, we did not, as a rule, adjust for multiple comparisons when looking at individual biomarkers, because our goal was to identify whether any AKI markers might be used to predict AKD. Last, we have a limited number of patients in some recovery patterns (relapsed AKI and late-reversal AKI), the results related to these subgroups should be interpreted with caution.
In conclusion, AKD is common in patients with septic shock. The existing biomarkers of kidney injury are clearly not useful for prediction of AKD. Urinary NGAL might be useful when added to a clinical model. The discovery and development of novel biomarkers for AKD are still needed. Long-term survival of patients with sepsis-associated AKI is strongly related to recovery status at hospital discharge.
Disclosure
JAK discloses grant support and/or consulting fees paid by Astute Medical, BioMerieux, and Bioporto; LSC discloses consulting fees paid by Astute Medical; and DCA discloses consulting fees paid by Ferring Pharmaceuticals Inc, Bayer AG, BMS, and Beckman Coulter. All the other authors declared no competing interests.
Acknowledgments
This work was supported by grants ProCESS: National Institutes of Health (NIH)/National Institute of General Medical Sciences (P50 GM076659) and ProGReSS AKI: NIH/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK083961).
Footnotes
Table S1. Site of infection and risk of developing AKD.
Table S2. TIMP-2∗IGFBP7 level changes between 2 time points and predictive value for AKD at 7 days.
Table S3. Clinical models with urinary biomarkers for predicting AKD.
Table S4. Validation of clinical model for predicting nonrecovery AKI at hospital discharge.
Table S5. Urinary biomarkers for predicting nonrecovery AKI at hospital discharge.
Table S6. Clinical model with urinary biomarkers for predicting nonrecovery AKI at hospital discharge.
Contributor Information
John A. Kellum, Email: kellum@pitt.edu.
ProCESS and ProGReSS-AKI Investigators:
Derek C. Angus, Lakhmir S. Chawla, David T. Huang, Christopher Keener, John A. Kellum, Nicole Lucko, Paul M. Palevsky, Francis Pike, Kai Singbartl, Ali Smith, Donald M. Yealy, Sachin Yende, Amber E. Barnato, Tammy L. Eaton, Elizabeth Gimbel, Kyle Landis, Diana K. Stapleton, Lisa A. Weissfeld, Michael Willochell, Kourtney A. Wofford, Erik Kulstad, Hannah Watts, Arvind Venkatv, Peter C. Hou, Anthony Massaro, Siddharth Parmar, Alexander T. Limkakeng, Jr., Kori Brewer, Theodore R. Delbridge, Allison Mainhart, James R. Miner, Todd L. Allen, Colin K. Grissom, Stuart Swadron, Steven A. Conrad, Richard Carlson, Frank LoVecchio, Ednan K. Bajwa, Michael R. Filbin, Blair A. Parry, Timothy J. Ellender, Andrew E. Sama, Jonathan Fine, Soheil Nafeei, Thomas Terndrup, Margaret Wojnar, Ronald G. Pearl, Scott T. Wilber, Richard Sinert, David J. Orban, Jason W. Wilson, Jacob W. Ufberg, Timothy Albertson, Edward A. Panacek, Sohan Parekh, Scott R. Gunn, Jon S. Rittenberger, Richard J. Wadas, Andrew R. Edwards, Matthew Kelly, Henry E. Wang, Talmage M. Holmes, Michael T. McCurdy, Craig Weinert, Estelle S. Harris, Wesley H. Self, Diane Dubinski, Carolyn A. Phillips, and Ronald M. Migues
Appendix
ProGReSS AKI Investigators and Staff
Derek C. Angus, Lakhmir S. Chawla, David T. Huang, Christopher Keener, John A. Kellum, Nicole Lucko, Paul M. Palevsky, Francis Pike, Kai Singbartl, Ali Smith, Donald M. Yealy, and Sachin Yende
ProCESS Coordinating Center
Derek C. Angus, Amber E. Barnato, Tammy L. Eaton, Elizabeth Gimbel, David T. Huang, Christopher Keener, John A. Kellum, Kyle Landis, Francis Pike, Diana K. Stapleton, Lisa A. Weissfeld, Michael Willochell, Kourtney A. Wofford, and Donald M. Yealy
Recruiting Centers
Site principal investigators are listed in italics. Advocate Christ Medical Center, Oak Lawn, IL: Erik Kulstad and Hannah Watts. Allegheny General Hospital, Pittsburgh, PA: Arvind Venkat. Brigham and Women’s Hospital, Boston, MA: Peter C. Hou, Anthony Massaro, and Siddharth Parmar. Duke University Medical Center, Durham, NC: Alexander T. Limkakeng, Jr. East Carolina University, Greenville, NC: Kori Brewer, Theodore R. Delbridge, and Allison Mainhart. George Washington University Medical Center, Washington, DC: Lakhmir S. Chawla. Hennepin County Medical Center, Minneapolis, MN: James R. Miner. Intermountain Medical Center, Murray, UT: Todd L. Allen and Colin K. Grissom. Los Angeles County + USC Medical Center, Los Angeles, CA: Stuart Swadron. Louisiana State University Health Sciences Center, Shreveport, LA: Steven A. Conrad. Maricopa Medical Center, Phoenix, AZ: Richard Carlson and Frank LoVecchio. Massachusetts General Hospital, Boston, MA: Ednan K. Bajwa and Michael R. Filbin. Blair A. Parry. Methodist Research Institute, Indianapolis, IN: Timothy J. Ellender. North Shore University Hospital, Manhasset, NY: Andrew E. Sama. Norwalk Hospital, Norwalk, CT: Jonathan Fine. Penn State Hershey College of Medicine, Hershey, PA: Soheil Nafeei, Thomas Terndrup, and Margaret Wojnar. Stanford University School of Medicine, Stanford, CA: Ronald G. Pearl. Summa Health System, Akron, OH: Scott T. Wilber. SUNY Downstate Medical Center, Brooklyn, NY: Richard Sinert. Tampa General Hospital, Tampa, FL: David J. Orban and Jason W. Wilson. Temple University Hospital, Philadelphia, PA: Jacob W. Ufberg. UC Davis Medical Center, Sacramento, CA: Timothy Albertson and Edward A. Panacek. University Medical Center Brackenridge, Austin, TX: Sohan Parekh. UPMC Presbyterian/Shadyside, Pittsburgh, PA: Scott R. Gunn, Jon S. Rittenberger, and Richard J. Wadas. University of Alabama at Birmingham, Birmingham, AL: Andrew R. Edwards, Matthew Kelly, and Henry E. Wang. University of Arkansas for Medical Sciences, Little Rock, AR: Talmage M. Holmes. University of Maryland at Baltimore, Baltimore, MD: Michael T. McCurdy. University of Minnesota Medical Center, Fairview, MN: Craig Weinert. University of Utah Health Sciences Center, Salt Lake City, UT: Estelle S. Harris. Vanderbilt University Medical Center, Nashville, TN: Wesley H. Self and Diane Dubinski. Washington Hospital Center, Washington, DC: Carolyn A. Phillips and Ronald M. Migues.
Supplementary Material
References
- 1.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard J., Acharya A., Cerda J. A Prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta R.L., Bouchard J., Soroko S.B. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37:241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.See E.J., Jayasinghe K., Glassford N. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–172. doi: 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Linder A., Fjell C., Levin A. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med. 2014;189:1075–1081. doi: 10.1164/rccm.201311-2097OC. [DOI] [PubMed] [Google Scholar]
- 6.Harris D.G., Koo G., McCrone M.P. Recurrent kidney injury in critically ill surgical patients is common and associated with worse outcomes. J Trauma Acute Care Surg. 2014;76:1397–1401. doi: 10.1097/TA.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 7.Kellum J.A., Sileanu F.E., Bihorac A. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195:784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla L.S., Eggers P.W., Star R.A. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 11.Uchino S., Bellomo R., Bagshaw S.M. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–1839. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 12.Bucaloiu I.D., Kirchner H.L., Norfolk E.R. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–485. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 13.Brown J.R., Kramer R.S., Coca S.G. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90:1142–1148. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca S.G., King J.T., Jr., Rosenthal R.A. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellum J.A., Sileanu F.E., Murugan R. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perinel S., Vincent F., Lautrette A. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43:e269–e275. doi: 10.1097/CCM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 17.Roman-Pognuz E., Elmer J., Rittenberger J.C. Markers of cardiogenic shock predict persistent acute kidney injury after out of hospital cardiac arrest. Heart Lung. 2019;48:126–130. doi: 10.1016/j.hrtlng.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sood M.M., Shafer L.A., Ho J. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care. 2014;29:711–717. doi: 10.1016/j.jcrc.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Federspiel C.K., Itenov T.S., Mehta K. Duration of acute kidney injury in critically ill patients. Ann Intensive Care. 2018;8:30. doi: 10.1186/s13613-018-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta S., Chauhan K., Patel A. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018;19:91. doi: 10.1186/s12882-018-0876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yealy D.M., Kellum J.A., Huang D.T. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellum J.A., Chawla L.S., Keener C. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivers E., Nguyen B., Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 24.Hoste E.A., Clermont G., Kersten A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zavada J., Hoste E., Cartin-Ceba R. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 26.Bellomo R., Ronco C., Kellum J.A. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigo E., Suberviola B., Santibanez M. Association between recurrence of acute kidney injury and mortality in intensive care unit patients with severe sepsis. J Intensive Care. 2017;5:28. doi: 10.1186/s40560-017-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hisamichi M., Kamijo-Ikemori A., Sugaya T. Increase in urinary markers during the acute phase reflects the degree of chronic tubulointerstitial injury after ischemia-reperfusion renal injury. Biomarkers. 2017;22:5–13. doi: 10.3109/1354750X.2016.1153723. [DOI] [PubMed] [Google Scholar]
- 30.Saito H., Tanaka T., Tanaka S. Persistent expression of neutrophil gelatinase-associated lipocalin and M2 macrophage markers and chronic fibrosis after acute kidney injury. Physiol Rep. 2018;6:e13707. doi: 10.14814/phy2.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphreys B.D., Xu F., Sabbisetti V. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Geus H.R., Woo J.G., Wang Y. Urinary neutrophil gelatinase-associated lipocalin measured on admission to the intensive care unit accurately discriminates between sustained and transient acute kidney injury in adult critically illpatients. Nephron Extra. 2011;1:9–23. doi: 10.1159/000330428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewitte A., Joannes-Boyau O., Sidobre C. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol. 2015;10:1900–1910. doi: 10.2215/CJN.12651214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aregger F., Uehlinger D.E., Witowski J. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85:909–919. doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.