Abstract
Myositis generally affects the proximal muscles. However, we herein report a case of a 48-year-old woman with intractable myositis affecting the intrinsic muscles of the hands. Her myositis, which developed in childhood, was refractory to treatment with steroids and several immunosuppressants, causing walking disability. After experiencing pain and swelling in the hands for six months, she was diagnosed with myositis of the intrinsic muscles of the hands and tested positive for the anti-signal recognition particle antibody. Intravenous immunoglobulin therapy improved the myositis of the hands. This case suggests that inflammation caused by intractable myositis can extend to the hands.
Keywords: inflammatory myopathy, hand myositis, anti-SRP antibody, intravenous immunoglobulin therapy
Introduction
Myositis is clinically characterized by symptoms such as weakness and pain, which reflect inflammation in the proximal muscles. Another clinical symptom is joint pain without bone destruction. The presence of different types of myositis-specific autoantibodies reflects to some extent the diversity in symptoms associated with the disease (1). However, myositis affecting the intrinsic muscles of the hands has never been reported.
We herein report a case of a woman with myositis who complained of hand pain and swelling. The cause of the symptoms was not found to be arthritis, but myositis, which was improved following intravenous immunoglobulin therapy.
Case Report
A 48-year-old woman complained of pain and swelling in her hands and lower legs. She had a history of myositis from childhood. At 7 years old, she had developed difficulties in climbing the stairs and received steroid treatment. She had no family history of myopathy. At 15 years of age, in the early 1980s, her myositis recurred. A muscle biopsy showed diffuse mild granulocytic infiltration of the interstitium. Inflammatory cells were not observed in the endomysium, around non-necrotic fibers, or around blood vessels. Some muscle fibers were atrophied with mild fibrosis. Mild necrosis and regenerated fibers were noted. She was pathologically diagnosed with myositis, and increased steroid doses improved her strength and creatine kinase (CK) level. Azathioprine, methotrexate, cyclosporine, or tacrolimus were used in combination with steroids, but the recurrence of myositis due to a decrease in the steroid dose could not be suppressed. Her muscle atrophy and weakness progressed, and at 43 years old, she was living in a wheelchair.
On a physical examination at admission, the muscle weakness and atrophy, observed mainly in the trunk and proximal muscles, were not significantly different from that observed during prior visits (Fig. 1). Impaired right eye movement with limited abduction and elevation had been present since her childhood, but myasthenia gravis had been excluded after repetitive nerve stimulation and edrophonium test examinations. Most notably, she had pain and swelling in her hands and lower legs, including the soles. There was no swelling or redness in the finger joints. The fingers were not sclerotic, and there was no restriction in the range of motion. Raynaud's phenomenon and rashes were not observed.
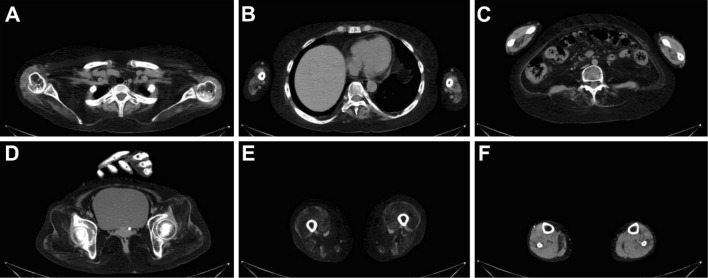
Figure 1.
Computed tomography performed when the patient was experiencing hand and foot symptoms. The muscle groups of the scapula (A), paravertebral spine (B, C), hip (D), and thigh (E) have marked atrophy and are replaced by fat. The muscles in the forearm and lower limbs are relatively undamaged (C, F).
A blood test showed that the CK level had increased to 80 IU/L, compared to approximately 30 IU/L in her stable phase, and her C-reactive protein level was elevated at 8.93 mg/dL. Her thyroid function was normal. Anti-nuclear antibody, anti-DNA antibody, anti-Sm antibody, anti-SS-A antibody, anti-SS-B antibody, anti-RNP antibody, anti-Scl-70 antibody, anti-centromere antibody, anti-cyclic citrullinated peptide antibody, and rheumatoid factor tests were negative. The matrix metalloproteinase-3 (MMP-3) level was 323.6 ng/mL. X-ray images of the hand were normal. Ultrasonography and computed tomography examinations excluded lower extremity phlebitis and lower extremity venous thrombosis. Empirical antibiotic administration did not improve her symptoms.
The pain and mild serological inflammatory parameters persisted. Magnetic resonance imaging (MRI) of the hand taken six months after the hand symptoms developed showed active inflammation in the hand muscles (Fig. 2A-D). Her serologic examination showed weak positivity for the anti-signal recognition particle (SRP) antibody and positivity for the anti-Ro-52 antibody (Table). We diagnosed her symptoms as indicative of active myositis in the distal muscles, including the hands and lower limbs.
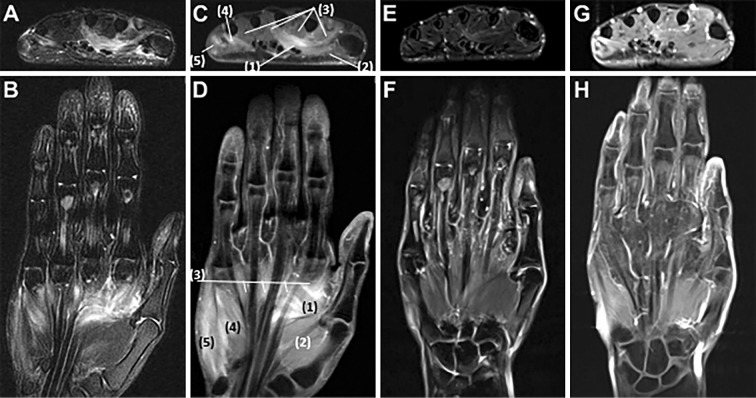
Figure 2.
Magnetic resonance imaging of the left hand. Axial and coronary fat suppression T2-weighted images show edematous changes of the intrinsic muscles of the hand such as the abductor pollicis, abductor digiti minimi, and interosseous muscles (A, B). Axial and coronal contrast T1-weighted images show contrast enhancement of these muscles (C, D). Fat-suppressed T2-weighted images (E, F) and contrast T1-weighted images (G, H) taken 13 days after IVIg treatment show improvement in these inflammatory changes. (1) adductor pollicis, (2) abductor pollicis brevis, (3) lumbricals, (4) flexor digiti minimi brevis, (5) abductor digiti minimi.
Table.
Myositis-specific Antibodies and Myositis-associated Antibodies in This Case.
| myositis-specific antibodies | ||
| anti-ARS antibody | ||
| anti-Jo-1 antibody | (-) | |
| anti-PL-7 antibody | (-) | |
| anti-PL-12 antibody | (-) | |
| anti-OJ antibody | (-) | |
| anti-EJ antibody | (-) | |
| anti-SRP antibody | (+-) | |
| anti-Mi-2 antibody | (-) | |
| anti-MDA5 antibody | (-) | |
| anti-TIF1-γ antibody | (-) | |
| myositis-associated antibodies | ||
| anti-Ro-52 antibody | (1+) | |
| anti-Ku antibody | (-) | |
| anti-PM-Scl100 antibody | (-) | |
| anti-PM-Scl75 antibody | (-) |
ARS: aminoacyl-tRNA synthetase, SRP: signal recognition particle, MDA5: melanoma differentiation-associated gene 5, TIF1-γ: transcriptional intermediary factor 1-γ, PM-Scl: polymyositis-scleroderma
She was worried about the loss of the ability to use her hands, such as eating with chopsticks and writing letters. However, she was hesitant to increase the steroid dose because she had steroid-induced osteoporosis. Therefore, intravenous immunoglobulin therapy (IVIg) was performed, resulting in the pain and swelling in her hands and lower limbs being cured and her serological parameters, including the CK levels, being improved. The inflammatory findings in the hand muscles on MRI were also improved (Fig. 2E-H). After the first IVIg administration, the symptoms re-emerged several times but always improved after subsequent IVIg administration (Fig. 3).
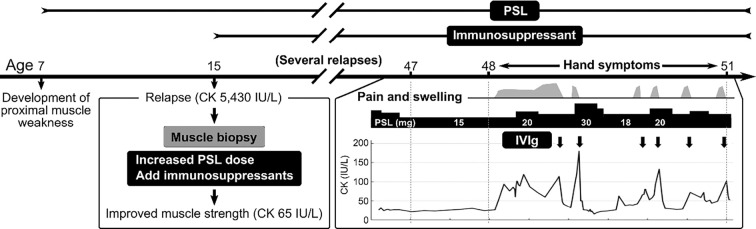
Figure 3.
Clinical course of this case. The patient suffered from myositis predominantly in the proximal muscles from childhood to 47 years of age. The myositis improved with increasing doses of prednisolone (PSL) but relapsed many times due to decreased dose of PSL despite the combined use of immunosuppressive drugs. At the age of 48, pain and swelling of the hands and lower legs appeared. These symptoms and creatine kinase (CK) levels improved with repeated intravenous immunoglobulin therapy (IVIg). CK: creatine kinase, IVIg: intravenous immunoglobulin treatment, PSL: prednisolone
Discussion
This case suggests that myositis can affect even the hand muscles. The proximal muscles in our patient had been replaced by fat following relapses of myositis over the course of approximately 40 years. Repeated IVIg administration was effective against inflammation of the hand muscles. To our knowledge, no cases of myositis in which inflammation has progressed to the hand muscles have ever been reported.
We had to carefully determine whether or not the hand symptoms in the present case could be attributed to a disease other than the myositis that had been present from childhood in our patient. First, collagen diseases, including rheumatoid arthritis, were ruled out based on a physical examination and X-ray and serological findings. High levels of MMP-3 are consistent with steroidal increases seen in patients with non-rheumatic inflammation (2). In addition, there was no doubt that the inflammation was in the muscles of the hand based on the physical and MRI findings. Second, soft tissue inflammatory pseudotumors, such as localized myositis and osteomyositis, can damage the hand muscles (3). However, this case was not considered to be one of such diseases because no tumorous lesions were observed and the symptoms were in both hands and both lower legs. Third, inclusion body myositis is characterized by the involvement of the forearm flexors (4), but there have been no reports of hand muscle inflammation, and IVIg has little effect on this disease. Taken together, the symptoms of the hand in the present case were considered to be indicative of myositis, which had been predominant in the proximal muscles, spreading to the distal muscles.
Myositis in the present case was diagnosed based on a muscle biopsy at 15 years old, performed in the early 1980s. The recorded pathological findings and remaining HE-stained specimens did not fit a diagnosis of polymyositis or inclusion body myositis according to the criteria of the Muscle Research Group (MSG)/European Neuromuscular Center Mediator (ENMC) published in 2004 (5). Immune-mediated necrotic myopathy, which is positive for anti-SRP antibodies, may develop at a young age and have a chronic progressive course. In the present case, the infiltration of inflammatory cells was mild, and necrosis and regeneration were observed to a slight degree, which is consistent with the pathological findings of immune-mediated necrotic myopathy (IMNM). However, the pathological record in this case was insufficient to diagnose IMNM. Hereditary myopathies, including muscular dystrophy, should also be differentiated. However, the course in this case differed from that of muscular dystrophy in that increasing steroid administration improved the muscle symptoms and CK levels, and IVIg was clearly effective against hand muscle symptoms (6). Given the clinical course in which IVIg was effective, re-performance of a muscle biopsy for Western blotting and immunostaining and genetic testing to rule out hereditary myopathy were discouraged. We attempted to reexamine the stored muscle tissue, but evaluation was difficult due to time-related deterioration. These are the limitations of this report.
Anti-SRP antibody is a myositis-specific autoantibody detected in 6% of all inflammatory myopathy cases (7). Myopathy positive for anti-SRP antibodies is classified as IMNM, and patients with inflammatory myopathy positive for this antibody tend to be resistant to steroid therapy (8). The onset of the chronic form of this myopathy occurs early, at an average of 15.4 years of age (9). Most of the inflammatory myopathies positive for the anti-SRP antibody are proximal muscle-dominant (10). However, 3% of these myopathies are reportedly distal muscle-dominant (9). Anti-Ro-52 antibody has been reported to be positive in 43% of patients with anti-SRP antibody-positive myositis (11). However, since this antibody is also detected in other muscle-specific antibody-positive myositis cases, its significance is unknown. Whether or not anti-SRP antibody or anti-Ro-52 antibody is involved in hand symptoms needs to be assessed in future case reports. Alternatively, it may be worth investigating whether or not these antibody-positive cases have hand symptoms.
In clinical practice, myositis in which the inflammation spreads to the muscles of the hands may be extremely rare. Therefore, it may be difficult to notice that the hand symptoms are caused by myositis. Nevertheless, a diagnosis of hand myositis should be considered, as proper treatment such as with IVIg may improve the patient's functional prognosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Betteridge Z, Tansley S, Shaddick G, et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun 101: 48-55, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribbens C, Martin y Porras M, Franchimont N, et al. Increased matrix metalloproteinase-3 serum levels in rheumatic diseases: relationship with synovitis and steroid treatment. Ann Rheum Dis 61: 161-166, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maguire JK Jr, Milford LW, Pitcock JA. Focal myositis in the hand. J Hand Surg Am 13: 140-142, 1988. [DOI] [PubMed] [Google Scholar]
- 4. Cox FM, Reijnierse M, van Rijswijk CS, et al. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology 50: 1153-1161, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, the Netherlands. Neuromuscul Disord 14: 337-345, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Moxley RT 3rd, Pandya S, Ciafaloni E, et al. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol 25: 1116-1129, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Kao AH, Lacomis D, Lucas M, et al. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum 50: 209-215, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki S, Nishikawa A, Kuwana M, et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis 10: 61, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki S, Hayashi YK, Kuwana M, et al. Myopathy associated with antibodies to signal recognition particle: disease progression and neurological outcome. Arch Neurol 69: 728-732, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Miller T, Al-Lozi MT, Lopate G, et al. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry 73: 420-428, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frank MB, McCubbin V, Trieu E, et al. The association of anti-Ro52 autoantibodies with myositis and scleroderma autoantibodies. J Autoimmun 12: 137-142, 1999. [DOI] [PubMed] [Google Scholar]