Abstract
We herein report the case of a 65-year-old woman diagnosed with myasthenia gravis (MG) after complaining of double vision. The patient had anti-low-density lipoprotein receptor-related protein 4 (LRP4) antibody in her serum, although antibodies against the acetylcholine receptor and muscle-specific tyrosine kinase were not detected. Chest computed tomography showed an anterior mediastinal tumor with a high uptake on fluorodeoxyglucose-positron emission tomography. Endoscopic thymectomy successfully ameliorated her ocular symptoms and showed the lesion to be thymoma. The present case revealed that anti-LRP4 antibody-associated MG can be associated with thymoma, which has been regarded as a rare complication of this disease thus far.
Keywords: myasthenia gravis (MG), anti-LRP4 antibody, thymoma, endoscopic thymectomy
Introduction
Myasthenia gravis (MG) is an autoimmune syndrome that affects the neuromuscular junction, causing fluctuating muscle weakness and easy fatigability. Approximately 85% of all MG patients have an autoantibody against the nicotinic acetylcholine receptor (AChR) in their serum (1). A subset of the remaining MG patients, referred to as anti-AChR antibody-negative, have an autoantibody against muscle-specific tyrosine kinase (MuSK) (2). In addition to AChR and MuSK, the low-density lipoprotein receptor-related protein 4 (LRP4) has been identified as another autoantigen causing MG (3). Anti-LRP4 antibodies were detected in approximately 2-46% of MG patients who were negative for both AChR and MuSK antibodies (3-7). The thymus plays an important role in the pathogenesis of MG. Approximately 15% of all MG patients have a thymoma, and almost all of them have anti-AChR antibodies (8,9). Thymectomy has a beneficial therapeutic effect for MG patients by reducing the chance of disease progression or recurrence. Up to 70% of the remaining MG patients have thymic hyperplasia (8). An international, randomized, and controlled trial involving 126 adult MG patients with anti-AChR antibody showed a distinct benefit of thymectomy in patients <50 years old at onset compared with those ≥50 years old (10). It has been previously reported that anti-LRP4 antibody-positive MG patients rarely develop thymomas or thymic abnormalities (6).
We herein report a case in which anti-LRP4 antibody-positive MG was complicated by a thymoma, and surgical resection of this thymoma successfully ameliorated the patient's symptoms.
Case Report
A 65-year-old woman was referred to the Department of Neurology at Gunma University Hospital as she had reported double vision. Approximately seven months before her first visit to our hospital, she began to complain of double vision when looking to her right. The symptom lasted for a month and then spontaneously disappeared. On an examination during her first visit, limited ocular movement or blepharoptosis was not confirmed with certainty. However, two months after this first evaluation, her double vision reappeared. She was therefore admitted to our hospital for further investigation.
Her blood pressure was 128/64 mmHg and pulse rate was 77 beats per minute with a regular rhythm. Her mood, memory, and language function were normal. The primary position of her eyes was almost normal. Although her right, downward gaze was mildly impaired, the oculomotor disturbance in the other direction fluctuated. Hess chart records localized this disturbance to the right inferior rectus muscle (Fig. 1A). She constantly experienced double vision when gazing to the right and occasionally in other directions. The diameter of her pupils was 3.5 mm in both eyes, her direct and indirect light reflexes were prompt, and no blepharoptosis was confirmed. Investigations into other cranial nerves, the motor and sensory systems, tendon reflexes, and gait all produced normal results. She had no habit of smoking or drinking and reported no use of illicit drugs. She had a history of diabetes, dyslipidemia, hypertension, and palmoplantar pustulosis, although there was no family history of neuromuscular diseases.
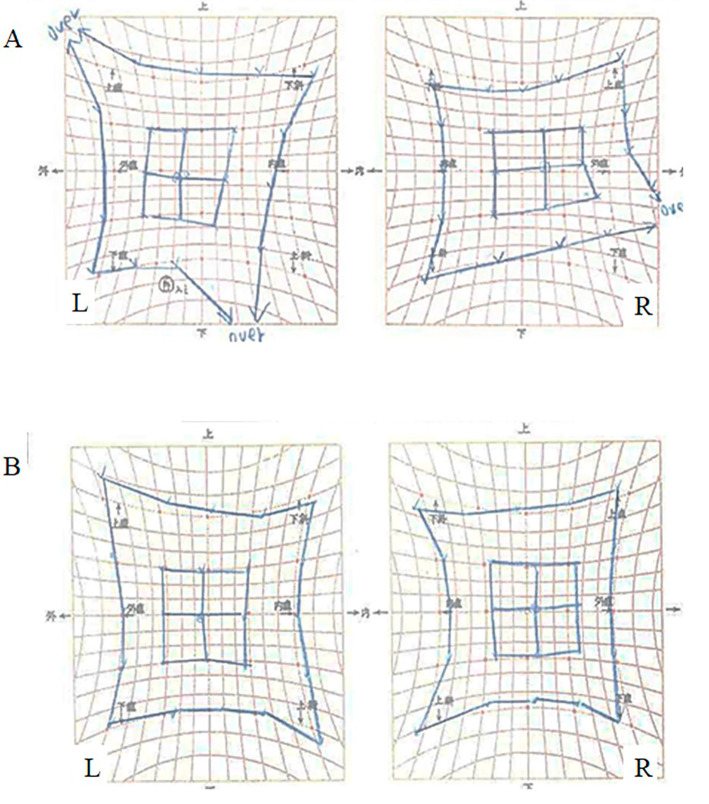
Figure 1.
Hess chart records depicting ocular movement before and after thymectomy. (A) Hess chart records before thymectomy illustrating that the oculomotor disturbance is localized at the right inferior rectus muscle. (B) Hess chart records after thymectomy displaying an improvement in ocular movements.
Laboratory tests confirmed a mild elevation of hemoglobin A1c (6.7%), and other routine tests, including blood cell counts, chemistry panel, and the thyroid function, showed normal values. Serum anti-AChR and anti-MuSK antibodies were not detected. The edrophonium test revealed that there was no improvement in the double vision or oculomotor disturbance. Repetitive nerve stimulation tests were performed in the bilateral abductor digiti minimi muscles at a rate of 1, 3, 10, 20, or 50 Hz and the left trapezius and left orbicularis oculi muscles at a rate of 3 Hz. The results showed no decremental or incremental responses of the compound muscle action potentials.
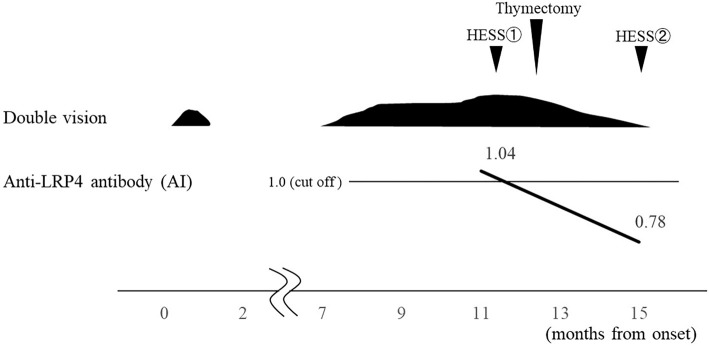
Cranial magnetic resonance imaging (MRI) revealed no abnormal signal intensities or aneurysms. However, chest computed tomography (CT) showed an anterior mediastinal tumor without contrast enhancement (Fig. 2A, B). In addition to this, 18F-fluorodeoxyglucose-positron emission tomography displayed the significant and homogenous uptake of the marker by the tumor [maximum standardized uptake value (SUVmax) 6.87] (Fig. 2C). Presence of anti-LRP4 antibody was subsequently confirmed in her serum by luciferase immunoprecipitation system [antibody index (AI): 1.04]. Extended endoscopic thymectomy was performed, and the tumor pathology was investigated, leading to the diagnosis of a thymoma with mixed types B1 and B2 (Fig. 2D, E). Using the Masaoka system, it was classified as stage II. Endoscopic thymectomy successfully ameliorated the presenting oculomotor disturbances and double vision without the need for medication, which in such cases is typically oral pyridostigmine or prednisolone. Hess chart records following thymectomy showed improvement in the patient's ocular movements (Fig. 1B). The serum anti-LRP4 antibody evaluation at 3 months after thymectomy showed a value (AI: 0.78) under the cut-off, and she has remained intact for 3 years without medication. The clinical course and transition of the serum anti-LRP4 antibody values are shown in Fig. 3.
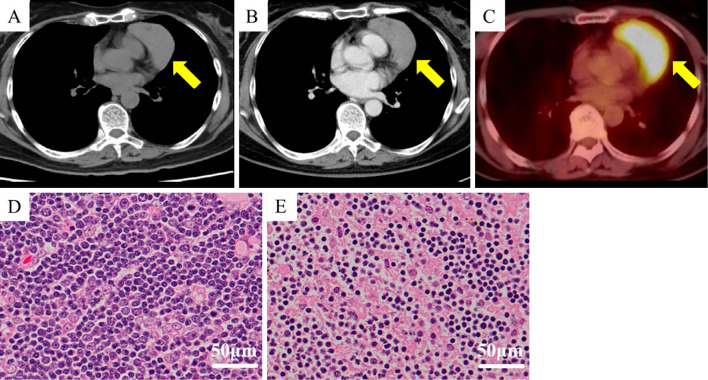
Figure 2.
Radiological and pathological findings of the thymoma. (A) Thoracic plain computed tomography (CT) showed a well-defined mass lesion (64×40 mm2) at the anterior mediastinum (arrow). (B) Contrast-enhanced CT showed that the tumor was not enhanced. (C) 18F-fluorodeoxyglucose-positron emission tomography demonstrated a significant and homogenous uptake by the tumor (SUVmax=6.87). (D) The histological investigation of the tumor revealed a combination of lymphocytic accumulation and scattered neoplastic epithelial components, so it was considered type B1 thymoma. (E) The neoplastic epithelial components here are more identifiable among immature lymphocytes, so this section was considered type B2 thymoma. Based on the observations that the B1 component was identified in a larger area than the B2 component, “combined thymic epithelial tumors” of stage II Masaoka classification were pathologically diagnosed.
Figure 3.
The clinical course and transition of the antibody index (AI) of the serum low-density lipoprotein receptor-related protein 4 (LRP4) antibody before and after thymectomy are shown. The AI was calculated by dividing the measurement value of the patient’s serum by the cut-off value.
Discussion
LRP4 is a member of the low-density lipoprotein receptor family and is a transmembrane protein expressed at the postsynaptic muscle membrane. Here, it acts as a receptor for agrin and an activator of MuSK, which is necessary to maintain the AChR function (11). In 2011, an autoantibody against the extracellular domain of LRP4 was first reported in the sera of anti-AChR antibody-negative MG patients (3). In previous reports, this anti-LRP4 antibody has been detected in approximately 2-46% of MG patients who were seronegative for both anti-AChR and anti-MuSK antibodies (3-7). MG patients with anti-LRP4 antibodies were mainly female, and their symptoms were relatively mild with predominant ocular muscle involvement. In contrast, anti-LRP4 antibody-positive MG patients simultaneously possessing the anti-MuSK antibody showed more severe myasthenic symptoms. Because the anti-LRP4 antibody belongs to the IgG1 subclass (3,6), complement activation is suspected to be involved in the disease pathogenesis, similar to anti-AChR antibody-positive MG.
The present MG case with anti-LRP4 antibodies exhibited mild ocular symptoms, as shown in the previous reports (6), and very interestingly, she was complicated by a thymoma, a rare occurrence that has been associated with anti-LRP4 antibody-positive MG before. Previous studies have reported that thymic hyperplasia is observed in 31% of anti-LRP4 antibody-positive MG patients (6); however, thymomas were very rarely observed (6,7,12). Zisimopoulou et al. reported 42 MG cases with anti-LRP4 antibody that did not have thymomas. They also reported that 33% possessed a normal thymus, 31% displayed thymic hyperplasia, 29% had an involuted thymus which is in the process of atrophy, and 7% possessed an atrophied thymus (6). Only two MG cases with anti-LRP4 antibody that were complicated by thymomas were reported independently (7,12); however, the pathological findings of their thymomas and the therapeutic effect of thymectomy were not described. Our patient represents the first MG case that was positive for anti-LRP4 antibody showing clinical improvement after pathology-proven thymectomy.
The relationship between anti-LRP4 antibody-positive MG and thymic abnormality has been very poorly elucidated. Since the present anti-LRP4 antibody-positive MG case was successfully treated by thymectomy, thymic abnormalities may be involved in the pathogenesis, as in MG cases with anti-AChR antibodies. Further investigations are required to clarify this point and to understand the association between thymic abnormalities and the pathogenesis of anti-LRP4 antibodies.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was supported in part by Grants-in-Aid for Scientific Research (C) (19K07813 to Y.I.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun 48-49: 143-148, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve 44: 36-40, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 69: 418-422, 2011. [DOI] [PubMed] [Google Scholar]
- 4. Pevzner A, Schoser B, Peters K, et al. . Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol 259: 427-435, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Zhang B, Tzartos JS, Belimezi M, et al. . Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol 69: 445-451, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Zisimopoulou P, Evangelakou P, Tzartos J, et al. . A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 52: 139-145, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Zhang Y, Cai G, et al. . Anti-LRP4 autoantibodies in Chinese patients with myasthenia gravis. Muscle Nerve 56: 938-942, 2017. [DOI] [PubMed] [Google Scholar]
- 8. Drachman DB. Myasthenia gravis. N Engl J Med 330: 1797-1810, 1994. [DOI] [PubMed] [Google Scholar]
- 9. Marx A, Pfister F, Schalke B, et al. . The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 12: 875-884, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe GI, Kaminski HJ, Aban IB, et al. . Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 375: 511-522, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 133: 4993-5000, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Marino M, Scuderi F, Samengo D, et al. . Flow cytofluorimetric analysis of anti-LRP4 (LDL receptor-related protein 4) autoantibodies in Italian patients with myasthenia gravis. PLoS One 10: e0135378, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]