This randomized clinical trial compares the albuminuria-lowering effects of Roux-en-Y gastric bypass surgery vs best medical treatment in patients with early-stage chronic kidney disease, type 2 diabetes, and obesity.
Key Points
Question
What are the effects of Roux-en-Y gastric bypass surgery (RYGB) vs best medical treatment on microalbuminuria in patients with type 2 diabetes, early-stage chronic kidney disease, and obesity?
Findings
In this randomized clinical trial of 100 patients randomly assigned to receive RYGB surgery vs best medical care, at 24-month follow-up, albuminuria remission occurred in 55% of patients after best medical treatment and 82% of patients after Roux-en-Y gastric bypass. The geometric mean urinary albumin-to-creatinine ratio was 55% lower in the Roux-en-Y gastric group, but there was no difference in serious adverse events.
Meaning
Roux-en-Y gastric bypass is a safe and more effective means of achieving remission of albuminuria and early-stage chronic kidney disease than best medical treatment in patients with type 2 diabetes, microalbuminuria, and obesity.
Abstract
Importance
Early-stage chronic kidney disease (CKD) characterized by microalbuminuria is associated with future cardiovascular events, progression toward end-stage renal disease, and early mortality in patients with type 2 diabetes.
Objective
To compare the albuminuria-lowering effects of Roux-en-Y gastric bypass (RYGB) surgery vs best medical treatment in patients with early-stage CKD, type 2 diabetes, and obesity.
Design, Setting, and Participants
For this randomized clinical trial, patients with established type 2 diabetes and microalbuminuria were recruited from a single center from April 1, 2013, through March 31, 2016, with a 5-year follow-up, including prespecified intermediate analysis at 24-month follow-up.
Intervention
A total of 100 patients with type 2 diabetes, obesity (body mass indexes of 30 to 35 [calculated as weight in kilograms divided by height in meters squared]), and stage G1 to G3 and A2 to A3 CKD (urinary albumin-creatinine ratio [uACR] >30 mg/g and estimated glomerular filtration rate >30 mL/min) were randomized 1:1 to receive best medical treatment (n = 49) or RYGB (n = 51).
Main Outcomes and Measures
The primary outcome was remission of albuminuria (uACR <30 mg/g). Secondary outcomes were CKD remission rate, absolute change in uACR, metabolic control, other microvascular complications, quality of life, and safety.
Results
A total of 100 patients (mean [SD] age, 51.4 [7.6] years; 55 [55%] male) were randomized: 51 to RYGB and 49 to best medical care. Remission of albuminuria occurred in 55% of patients (95% CI, 39%-70%) after best medical treatment and 82% of patients (95% CI, 72%-93%) after RYGB (P = .006), resulting in CKD remission rates of 48% (95% CI, 32%-64%) after best medical treatment and 82% (95% CI, 72%-92%) after RYGB (P = .002). The geometric mean uACRs were 55% lower after RYGB (10.7 mg/g of creatinine) than after best medical treatment (23.6 mg/g of creatinine) (P < .001). No difference in the rate of serious adverse events was observed.
Conclusions and Relevance
After 24 months, RYGB was more effective than best medical treatment for achieving remission of albuminuria and stage G1 to G3 and A2 to A3 CKD in patients with type 2 diabetes and obesity.
Trial Registration
ClinicalTrials.gov Identifier: NCT01821508
Introduction
Chronic kidney disease (CKD) is a major contributor to early mortality in patients with type 2 diabetes.1 Most of these patients have stage G1 to G3 and A2 to A3 CKD based on the presence of microalbuminuria (urinary albumin-to-creatinine ratio [uACR], 30-300 mg/g) or macroalbuminuria (uACR>300 mg/g) in the context of an estimated glomerular filtration rate (eGFR) above 30 mL/min/1.73 m2.
Contemporary advances in pharmacotherapy of type 2 diabetes show great promise in treating CKD.2,3,4,5 However, for many patients, CKD remains a chronic progressive disease despite best medical care.6 Obesity is an important independent risk factor for CKD. In an analysis7 of a large US cohort, a stepwise increase in the strength of the association between body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) and incident end-stage kidney disease (ESKD) at follow-up was found across classes of obesity and persisted after adjustment for those with class 2 and class 3 obesity. Observational studies8,9,10 showing that metabolic surgery is associated with reduced albuminuria and long-term incidence of ESKD support the premise that significant weight loss can play a critical role in effecting long-term reductions in kidney disease risk in patients with obesity and type 2 diabetes.
A previous study11 suggested that individuals with microalbuminuria and an eGFR of 60 mL/min/1.73 m2 or greater are at a significantly greater risk of progression to ESKD and doubling of serum creatinine levels compared with those with normal albumin excretion (uACR<30 mg/g). A previous large study12 identified that baseline albuminuria is independently associated with progression to ESKD. The variable degree of association of microalbuminuria with follow-up incidence of ESKD may in part be explained by oscillation around the diagnostic cutoff and spontaneous reversal in some patients13 as well as the effect of fatal cardiovascular events during follow-up in patients with progressive albuminuria. Even modest increases in albuminuria within the normal range (eg, uACR of 10-29.9 mg/g) have been associated with an augmented risk of myocardial infarction and all-cause mortality.14
Remission of microalbuminuria in response to multimodal treatment intensification in patients with type 2 diabetes is associated with preservation of kidney function at follow-up.15 Meta-regression analysis of medical therapy for CKD has demonstrated that the placebo-adjusted treatment effect on albuminuria correlates significantly with subsequent ESKD incidence, with risk of ESKD decreased by 23.7% for each 30% reduction in albuminuria.16
We report outcomes from the first 24 months of the Microvascular Outcomes after Metabolic Surgery (MOMS) trial.17 This trial was designed to test the hypothesis that RYGB would be more effective than best medical treatment as a means of achieving remission of microalbuminuria in patients with type 2 diabetes, obesity, and early-stage CKD at baseline, a cohort that has substantial residual risk of early morbidity and mortality.18,19
Methods
Study Design
For this randomized clinical trial, patients with established type 2 diabetes and microalbuminuria were recruited from a single center from April 1, 2013, through March 31, 2016, with a 5-year follow-up, including prespecified intermediate analysis at 24-month follow-up. The study compares the effect of RYGB and best medical treatment on kidney outcomes in patients with early-stage CKD, type 2 diabetes, and obesity (BMI of 30-35). Ethical approval for the study was granted by the Institutional Research Ethics Committee of Hospital Alemão Oswaldo Cruz. All participants gave formal written informed consent, and all data were deidentified. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol can be found in Supplement 1.
Participants
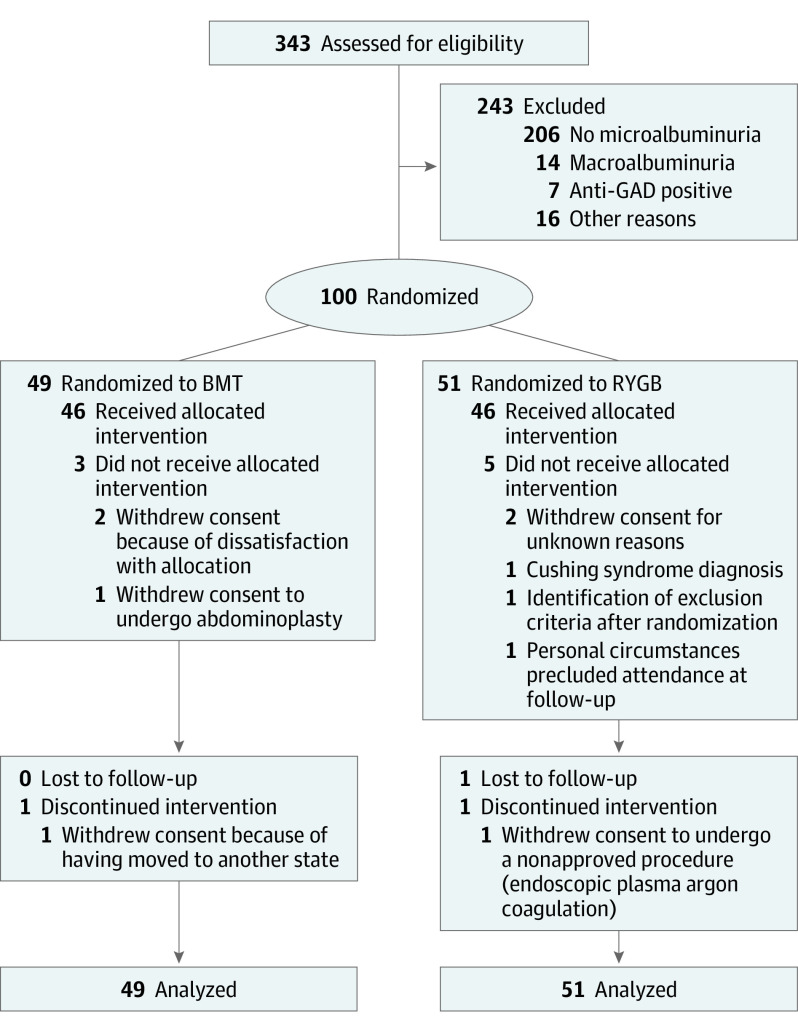
Using a computer-generated, centrally concealed, and sex-stratified 1:1 randomization sequence, we assigned 100 eligible patients to best medical treatment or RYGB (Figure 1). Patients’ eligibility was confirmed at screening. Duration of diabetes (time since diagnosis) was known, but data on the duration of microalbuminuria were not uniformly available. The inclusion criteria were uACR greater than 30 mg/g, type 2 diabetes with glycated hemoglobin (HbA1c) levels less than 12% (to convert to proportion of total hemoglobin, multiply by 0.01), age of 18 to 65 years, and a BMI of 30 to 35. All patients were classifiable as having stage G1 to G3 and A2 to A3 CKD.
Figure 1. CONSORT Diagram of Screening, Enrollment, and 24-Month Follow-up in the Microvascular Outcomes after Metabolic Surgery (MOMS) Trial.
The intention-to-treat population included 49 patients in the best medical treatment (BMT) group and 51 patients in the Roux-en-Y gastric bypass (RYGB) group, whereas the safety population included 46 patients in each group. BMT indicates best medical treatment; GAD, glutamic acid decarboxylase; and RYGB, Roux-en-Y gastric bypass.
Study Treatments
At 24 months of follow-up, medical treatment algorithms in our protocol were consistent with the updated 2019 American Diabetes Association (ADA) and European Association for Study of Diabetes guidelines.20 Drugs with a beneficial effect on microvascular and macrovascular outcomes were administered early after the trial commenced if patients were not already taking these medications. Use of the drugs was continued in the best medical treatment group even in the event of metabolic targets being met and remission of albuminuria occurring. Use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and statins was continued in the RYGB group irrespective of whether albuminuria remitted. Glucose-lowering drugs, including insulin, were dose adjusted. Metformin treatment was maintained for all patients; however, doses were reduced or metformin use stopped when the HbA1c level was less than 5.7% (39 mmol/mol), the fasting plasma glucose level was less than 100 mg/dL (to convert to millimoles per liter, multiply by 0.0555), or metformin-related gastrointestinal adverse effects occurred.
RYGB was performed laparoscopically by a single surgeon (R.V.C.) and consisted of a 30-mL gastric pouch, a 150-cm alimentary limb, and an 80-cm biliopancreatic limb (eFigure 1 in Supplement 2). The RYGB group received standard supplementation and was assessed for nutritional deficiencies at 6, 12, 18, and 24 months.
Data Collection
Patients were assessed at 1 and 4 weeks after randomization and then at each 3-month intervals until the end of the second year. Values of uACR, eGFR (calculated using the Chronic Kidney Disease Epidemiology Collaboration formula21), HbA1c, fasting plasma glucose, blood pressure, lipid profiles, and body weight were assessed. Retinopathy grading was conducted by ophthalmologists in a blinded fashion.17 Neuropathy was assessed by unblinded diabetologists (T.B.Z.P. and L.P.C.d.S.). A detailed description of the study outcomes can be found in eTable 1 in Supplement 2.
Outcomes
The primary outcome was remission of microalbuminuria defined as uACR levels less than 30 mg/g at 24 months after intervention. Albuminuria was quantified in early morning spot urine samples (eTable 1 in Supplement 2).
Prespecified secondary outcomes were change in uACR; metabolic control, including normalization of glycemic control (fasting glucose level <100 mg/dL and HbA1c<6.0%), blood pressure (systolic blood pressure <130 mm Hg and diastolic blood pressure <80 mm Hg), and lipid levels (low-density lipoprotein cholesterol [LDL-C]<100 mg/dL and <70 mg/dL in patients with cardiovascular disease [to convert to millimoles per liter, multiply by 0.0259], high-density lipoprotein cholesterol [HDL-C] level >50 mg/dL [to convert to millimoles per liter, multiply by 0.0259], and triglyceride levels <150 mg/dL [to convert to millimoles per liter, multiply by 0.0113]); discontinuation of pharmacologic therapy for type 2 diabetes; change in quality of life (validated Brazilian-Portuguese language version of the Medical Outcomes Study 36-Item Short-Form Health Survey questionnaire22); rate of retinopathy reversal; development or worsening of peripheral neuropathy; and adverse events and safety profile. Non-prespecified analyses included remission of CKD, defined as remission of albuminuria with an eGFR greater than 60 mL/min. All changes to secondary trial outcomes are listed in eTable 2 in Supplement 2.
Statistical Analysis
Fifty participants per group provided 90% or greater power at the 1.7% significance level (Bonferroni-corrected level for 3 assessments at 12, 24, and 60 months) for the detection of a 5-fold difference in the achievement of the primary outcome between groups (10% in the best medical treatment group vs 50% in the RYGB group) at 60 months, assuming a 20% loss to follow-up. The proportion of patients expected to achieve remission was sourced from previous publications, empirical data from a surgeon (R.V.C.) with extensive experience in bariatric surgery, and a consensus and expert opinion of a panel of specialists (C.W.l.R.) in diabetic kidney disease.
Continuous variables are summarized as means (SDs), means (95% CIs), or medians (interquartile ranges [IQRs]). However, because the distribution of uACR is substantially skewed, this outcome was analyzed with log-transformed uACR values, and results are presented as geometric means (95% CIs). Binary and categorical variables are summarized as numbers (percentages), proportions, risk differences or odds ratios (ORs) with 95% CIs.
For all efficacy outcomes, we performed full intention-to-treat analyses, in which all randomized patients were included in the analyses as randomized and contributed to the analyses. Complete-case (per protocol) analyses are presented for comparison. For the primary analysis, we used multilevel mixed-effects regression models. Exploratory analyses (comparative analyses) were performed with fixed-effects models, ignoring random effects and/or nonindependence in the data. Specifically, for binary outcomes, we used mixed-effects logistic regression models, whereas continuous outcomes were analyzed via mixed-effects linear regression models. Ordered categorical outcomes were analyzed by mixed-effects ordered logistic regression models. All models explicitly accounted for the repeated-measures nature of the data and nonindependence between measurements over time. Fixed effects were time (as a categorical variable) and treatment group. Models were fitted with interactions between time and treatment allocation. To avoid convergence problems or inaccurate estimates, all models were fitted with a random intercept for each participant only. To evaluate safety outcomes, only participants who received the allocated treatment were analyzed (safety population). The statistical approach for missing data is detailed in the eMethods in Supplement 2. Because of sparse data, all adverse events were analyzed using exact logistic regression models.
Comparisons between groups at baseline were performed by 2-tailed, unpaired t tests or Fisher exact tests. A 2-sided α level of 1.7% was considered to indicate statistical significance for the primary outcome. For all the remaining outcomes, 2-sided P < .05 was considered statistically significant. All analyses were performed with Stata software, version 14.0 (StataCorp).
Results
A total of 100 participants (mean [SD] age, 51.4 [7.6] years; 55 [55.0%] male) were randomized to receive best medical treatment (n = 49) or RYGB (n = 51). Both groups were similar with regard to demographic and baseline clinical characteristics (Table 1). Ninety-two patients had a complete assessment at 24 months with no crossovers. Eight patients did not receive the assigned intervention: 3 in the best medical treatment group and 5 in the RYGB arm (Figure 1). Details on missing data for each variable are given in eTable 3 in Supplement 2. The distribution (quartiles) of all continuous variables at baseline by treatment arm is given in eTable 4 in Supplement 2. All the protocol deviations were minor and are reported in eTable 5 in Supplement 2. The efficacy (intention-to-treat) population encompassed all 100 participants, whereas the population in which safety and medication use were assessed was composed of 92 patients (46 in each group).
Table 1. Baseline Characteristics of the Trial Participantsa.
| Characteristic | Best medical treatment (n = 49) | RYGB (n = 51) |
|---|---|---|
| Age, mean (SD), y | 50.2 (7.5) | 52.5 (7.6) |
| Diabetes duration, median (IQR), y | 9 (5-13) | 10 (6-12) |
| Males | 27 (55) | 28 (55) |
| Race/ethnicityb | ||
| White | 34 (69) | 46 (90) |
| Black | 2 (4) | 0 |
| Asian | 3 (6) | 1 (2) |
| Mixed | 8 (16) | 4 (8) |
| Undeclared | 2 (4) | 0 |
| Waist circumference, mean (SD), cm | 111.1 (8.1) | 112.2 (8.01) |
| BMI, mean (SD) | 32.6 (2.1) | 32.5 (1.9) |
| Creatinine level, median (IQR), mg/dL | 0.80 (0.65-0.95) | 0.78 (0.64-0.98) |
| Urinary creatinine level, median (IQR), mg/dL | 0.95 (0.64-1.12) | 0.95 (0.71-1.33) |
| Urinary albumin to creatinine ratio, median (IQR), mg/g | 73 (52-168) | 72 (53-143) |
| eGFR, mean (SD), mL/min/1.73 m2 | 96.18 (21.41) | 94.59 (16.17) |
| Retinopathy status | ||
| Not available or undetermined | 6 (12) | 3 (6) |
| None | 29 (59) | 31 (61) |
| NPDR | 9 (18) | 11 (22) |
| PDR | 5 (10) | 6 (12) |
| Neuropathy status | ||
| Not available | 1 (2) | 3 (6) |
| None | 25 (51) | 25 (49) |
| Any | 23 (47) | 23 (45) |
| Glycemia | ||
| HbA1c level, mean (SD), % | 8.94 (1.96) | 8.80 (1.86) |
| Fasting plasma glucose level, mean (SD), mg/dL | 174 (142-232) | 167 (145-208) |
| Lipid levels, mean (SD), mg/dL | ||
| Total cholesterol | 192.8 (46.6) | 185.2 (38.4) |
| HDL-C | 39.0 (11.4) | 41.1 (12.4) |
| LDL-C | 108.6 (41.1) | 102 (36.5) |
| Proportion of patients with LDL-C level <100 mg/dL | 22 (45) | 24 (47) |
| Triglyceride levels, median (IQR), mg/dL | 214 (150-334) | 195 (145-293) |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 137.3 (15.5) | 141.5 (17.2) |
| Diastolic | 85.7 (8.0) | 88.1 (12.7) |
| Medications | ||
| Biguanides | 45 (92) | 40 (78) |
| Thiazolidinediones | 4 (8) | 2 (4) |
| GLP-1 analog or receptor agonists | 13 (26) | 23 (45) |
| SGLT2 inhibitors | 2 (4) | 2 (4) |
| Secretagogues | 20 (41) | 21 (41) |
| Insulin | 12 (24.) | 20 (39) |
| Lipid-lowering agentsc | 18 (37) | 30 (59) |
| β-blockers | 6 (12) | 8 (16) |
| Calcium channel blockers | 7 (14) | 13 (26) |
| ACE inhibitors or ARBs | 30 (61) | 37 (72) |
| Diuretics | 17 (35) | 15 (29) |
| Anticoagulants | 16 (33) | 17 (33) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; GLP-1, glucagonlike peptide 1; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RYGB, Roux-en-Y gastric bypass; SGLT2, sodium coupled glucose transporter 2.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; to convert total cholesterol, HDL-C, and LDL-C to millimoles per liter, multiply by 0.0259; to convert HbA1c to proportion of total hemoglobin, multiply by 0.01; to convert glucose, to millimoles per liter, multiply by 0.0555; to convert triglycerides to millimoles per liter, multiply by 0.0113.
Data are presented as number (percentage) of participants unless otherwise indicated.
Race/ethnicity was self-reported.
P = .03. All other comparisons were not statistically significant.
Primary Outcome
After 24 months, among participants with complete data, the primary outcome had been achieved in 36 of 43 patients (84%) in the RYGB group compared with 24 of 43 participants (56%) in the best medical treatment group (risk difference, 0.279; 95% CI, 0.094-0.464). In an intention-to-treat analysis, albuminuria remission occurred in 55% of patients (95% CI, 39.0%-70.0%) after best medical treatment and 82% of patients (95% CI, 72%-93%) after RYGB (P = .006) (Table 2 and Figure 2).
Table 2. Primary and Secondary Outcomes at 24 Monthsa.
| Outcome | Best medical treatment (n = 49) | RYGB (n = 51) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| Albumin to creatinine ratio <30 mg/g of creatinine | ||||
| ITT analysis, % | 54.6 (38.8 to 70.3) | 82.3 (72.1 to 92.6) | 27.8 (8.7 to 46.8) | .006 |
| Complete case analysis, No. (%)b | 24 (56) | 36 (84) | 27.9 (9.4 to 46.4) | .003 |
| Secondary outcomes | ||||
| Albuminuria, geometric mean (95% CI), mg/g of creatinine | 23.6 (17.9 to 31.2) | 10.7 (8.1 to 14.1) | 0.45 (0.30 to 0.67)c | <.001 |
| EGFR, mL/min/1.73 m2 | 91.2 (86.1 to 96.3) | 96.6 (91.5 to 101.6) | 5.3 (−1.9 to 12.5) | .15 |
| HbA1c level, % | 6.72 (6.34 to 7.09) | 6.18 (5.80 to 6.56) | −0.54 (−1.07 to −0.004) | .05 |
| HbA1c level ≤7.0%, % | 70.2 (56.9 to 83.6) | 83.0 (72.4 to 93.60) | 12.7 (−4.3 to 29.7) | .16 |
| HbA1c level ≤6.5%, % | 50.5 (36.3 to 64.8) | 70.9 (57.8 to 84.0) | 20.4 (1.03 to 39.7) | .05 |
| HbA1c level ≤6.0%, % | 24.4 (12.3 to 36.7) | 44.5 (29.8 to 59.2) | 20.1 (1.00 to 39.1) | .05 |
| Fasting glucose level, mg/dL | 121.6 (108.0 to 135.2) | 104.1 (90.2 to 118.0) | −17.5 (−37.0 to 1.92) | .08 |
| Glucose level ≤100 mg/dL, % | 30.40 (17.2 to 43.6) | 44.4 (29.6 to 59.3) | 14.0 (−5.80 to 33.9) | .18 |
| BP, mm Hg | ||||
| Systolic | 129.9 (125.1 to 134.6) | 130.8 (125.9 to 135.6) | 0.91 (−5.88 to 7.70) | .79 |
| Diastolic | 82.5 (79.5 to 85.5) | 79.7 (76.6 to 82.8) | −2.80 (−7.12 to 1.53) | .21 |
| Systolic BP <130 mm Hg, % | 37.8 (23.6 to 51.9) | 32.5 (18.6 to 46.5) | −5.2 (−2.5 to 14.7) | .61 |
| Diastolic BP <80 mm Hg, % | 20.1 (8.40 to 31.9) | 28.0 (14.5 to 41.4) | 7.8 (−9.98 to 25.6) | .39 |
| BMI | 31.22 (30.47 to 31.98) | 24.26 (23.51 to 25.01) | −6.96 (−8.02 to −5.89) | <.001 |
| Waist circumference, cm | 107.2 (104.7 to 109.8) | 90.69 (88.1 to 93.29) | −16.51 (−20.15 to −12.87) | <.001 |
| Cholesterol level, mg/dL | ||||
| Total | 173.5 (163.3 to 185.2) | 161.4 (150.6 to 172.5) | −12.73 (−28.2 to 2.8) | .11 |
| LDL-C | 101.6 (92.2 to 110.9) | 85.7 (76.3 to 95.0) | −15.9 (−29.1 to −2.65) | .02 |
| HDL-C | 41.6 (38.1 to 45.4) | 53.6 (51.0 to 58.3) | 12.9 (7.8 to 18.0) | <.001 |
| Triglycerides | 180.7 (157.7 to 207.2) | 107.8 (90.6 to 140.3) | −67 (−102.1 to −31.9) | <.001 |
| LDL-C level <100 mg/dL, % | 51.2 (37.1 to 66.5) | 72.6 (59.4 to 85.2) | 20.5 (0.9 to 40) | .05 |
| HDL-C level >50 mg/dL, % | 18.7 (6.2 to 27.8) | 44.8 (32.9 to 61.2) | 30 (12.2 to 48) | .004 |
| Triglyceride levels <150 mg/dL, % | 41.9 (26.9 to 55.1) | 80.0 (70.2 to 92.6) | 40.4 (22.4 to 58) | <.001 |
| CKD remission, %d | 48.2 (32.2 to 64.1) | 81.9 (71.8 to 92.1) | 33.8 (14.8 to 53) | .002 |
| Metabolic controle | ||||
| ITT analysis, % | 16.6 (8.4 to 24.8) | 30.8 (19.1 to 42.5) | 14.2 (0.6 to 28) | .04 |
| Complete cases analysis, No. (%)b | 10 (22) | 13 (30) | 8.0 (−10.3 to 26.3) | .39 |
| Retinopathy status, % | ||||
| None | 73.3 (71.3 to 75.3) | 74.1 (70.6 to 77.7) | 0.8 (−2.5 to 4.1) | .61 |
| NPDR | 21.2 (17.3 to 25.2) | 21.5 (19.0 to 24.0) | 0.30 (−3.2 to 3.8) | |
| PDR | 5.4 (0.15 to 10.7) | 4.4 (1.3 to 7.5) | −1.0 (−5.5 to 3.4) | |
| Neuropathy, % | 25.3 (12.3 to 38.3) | 20.3 (9 to 31.6) | −5 (−22.1 to 12) | .57 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; CKD, chronic kidney disease; EGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; ITT, intention to treat; LDL-C, low-density lipoprotein cholesterol; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RYGB, Roux-en-Y gastric bypass.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; to convert total cholesterol, HDL-C, and LDL-C to millimoles per liter, multiply by 0.0259; to convert HbA1c to proportion of total hemoglobin, multiply by 0.01; to convert glucose, to millimoles per liter, multiply by 0.0555; to convert triglycerides to millimoles per liter, multiply by 0.0113.
Data are presented as mean (95% CI) unless otherwise indicated.
Fixed-effects analysis ignoring random effects and nonindependence in the data. Only individuals with complete data were included.
Ratio of geometric means.
American Diabetes Association composite criteria, defined as urinary albumin to creatinine ratio less than 30 mg/g of creatinine and eGFR greater than 60 mL/min/1.73 m2.
Defined as HbA1c level less than 7%, LDL-C level greater than 100 mg/dL, systolic BP less than 130 mg Hg, and diastolic BP less than 80 mm Hg.
Figure 2. Albuminuria Remission Rates at 12 and 24 Months of Follow-up and Longitudinal Biochemical Measures of Urinary Albumin-Creatinine Ratio (uACR) and Metabolic Control.
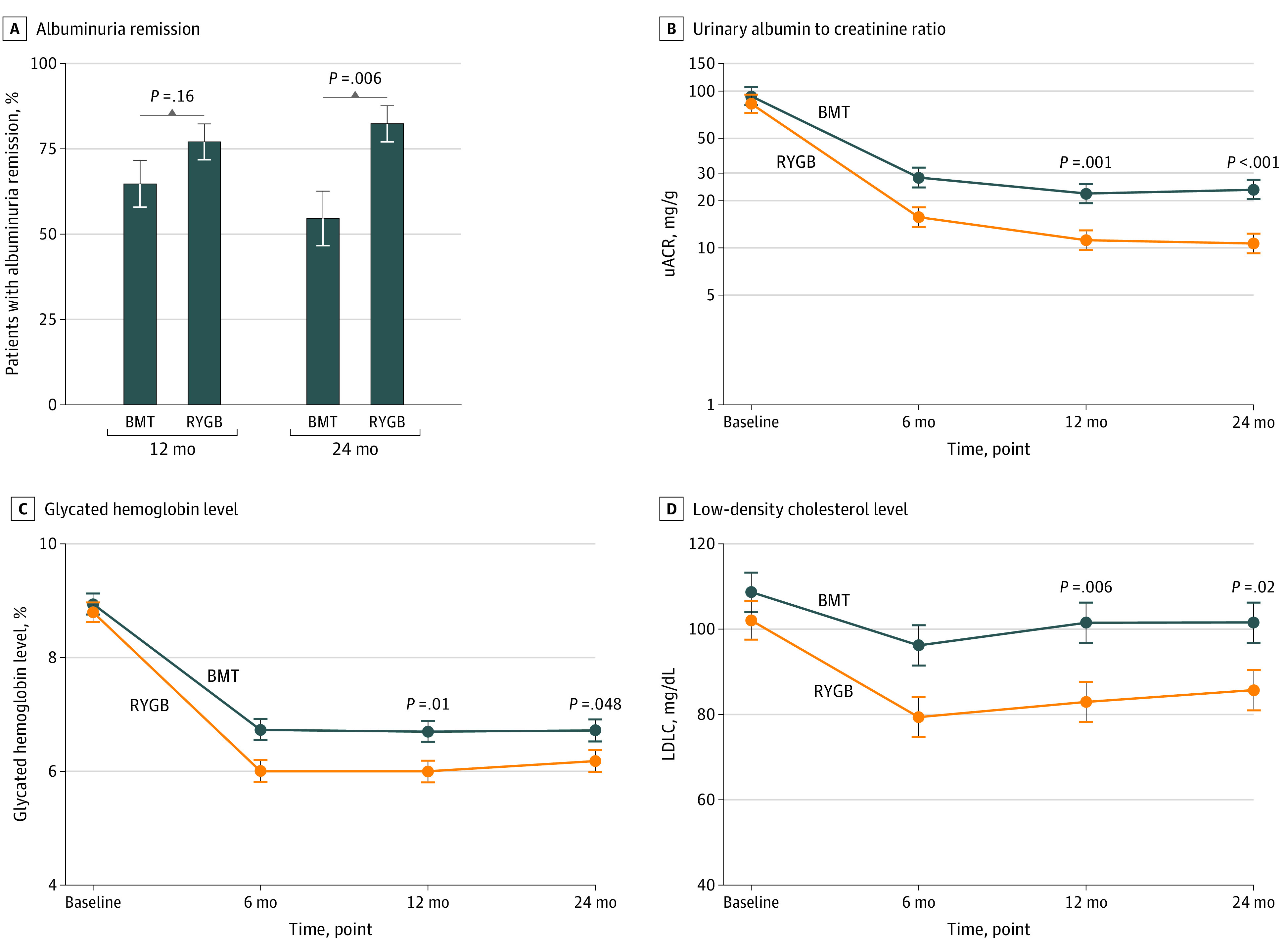
A, Rates of albuminuria remission (uACR <30 mg/g) at 12- and 24-month follow-up. B-D, Longitudinal trajectories of uACR (B), glycated hemoglobin (to convert to proportion of total hemoglobin, multiply by 0.01) (C), and low-density lipoprotein cholesterol (LDL-C) (to convert to millimoles per liter, multiply by 0.0259) (D) from baseline to 24-month follow-up. Error bars indicated SEs. BMT indicates best medical treatment; RYGB, Roux-en-Y gastric bypass.
Exploratory Secondary Outcomes
CKD Staging
Microalbuminuria was modeled on a log scale. Exponentiated model estimates of the RYGB to best medical treatment geometric mean ratio yielded a value of 0.45 (95% CI, 0.30-0.67; P < .001) derived from mean uACR values of 23.6 mg/g (95% CI, 17.9-31.2 mg/g) in the best medical treatment group and 10.7 mg/g (95% CI, 8.1-14.1 mg/g) in the RYGB group. The estimated remission rate of early-stage CKD was 48.2% (95% CI, 32.2%-64.1%) among patients after best medical treatment and 81.9% (95% CI, 71.8%-92.1%) after RYGB (P = .002) (Table 2 and eTable 6 in Supplement 2).
Metabolic Control
The HbA1c level after 24 months was reduced by 2.2% after best medical treatment and by 2.6% after RYGB, with a mean difference of −0.54% (95% CI, −1.07 to −0.004; P = .048) at 24 months (Table 1, Table 2, and Figure 2). At 24 months, the estimated proportion of patients reaching the ADA target for remission of diabetes23 (HbA1c level ≤6.0% [<42 mmol/mol]) was 24.4% after best medical treatment and 44.5% after RYGB (P = .051). No difference was found between study groups in the number of patients reaching the ADA definition of partial remission (HbA1c level <6.5%: risk difference, 0.204; 95% CI, 0.01-0.397; P = .05) or of good control (HbA1c level <7%: risk difference, 0.127; 95% CI, −0.043 to 0.297; P = .16) (Table 2).
At baseline, 78% of patients were treated for hypertension or had blood pressure in the hypertensive range (Table 1). After 24 months, no difference was found in systolic or diastolic blood pressure between the groups or the proportion of patients in either group reaching the ADA targets for systolic or diastolic blood pressure (Table 2).
After 24 months, significantly more participants in the RYGB group reached LDL-C level targets compared with the best medical treatment group (73% vs 51%; P = .048). Analogously, absolute measures of LDL-C level were lower after RYGB compared with best medical treatment (86 vs 102 mg/dL; mean difference, −15.9 mg/dL; 95% CI, −29.1 to −2.65 mg/dL; P = .02) (Table 2 and Figure 2).
The triglyceride levels target of 150 mg/dL was achieved by 41% of patients after best medical treatment and by 81% after RYGB (P < .001). HDL-C levels remained unchanged after best medical treatment (mean change, 2.8 mg/dL; 95% CI, 0.21-5.3 mg/dL) but increased by 13.5 mg/dL (95% CI, 10.97-16.1 mg/dL) after RYGB (P < .001 for posttreatment scores) (Table 2).
At 24 months, the mean percentage change in total body weight was −4.5% (95% CI, −6.1% to −3.1%) in the best medical treatment group and −25.4% (95% CI, −26.9% to −23.8%) in the RYGB group. The estimated mean BMI of patients was 31.2 after best medical treatment and 24.3 after RYGB (mean difference, −6.96; 95% CI, −8.0 to −5.9; P < .001) (Table 2). Less than 5% of patients in the best medical treatment group achieved 15% body weight loss, whereas more than 95% of patients in the RYGB group lost more than 15% body weight (eFigure 2 in Supplement 2). The estimated proportion of patients who achieved a BMI in the normal range was 51% after RYGB and 0% after best medical treatment (P < .001).
Retinopathy and Neuropathy
No differences were found between the best medical treatment and RYGB groups regarding the progression of diabetic retinopathy and neuropathy (Table 2).
Medication Use
Medication profiles are detailed in eTable 7 in Supplement 2. The median number of pharmacologic agents for metabolic control was 6 (IQR, 3-9) in the best medical treatment group and 1 (IQR, 1-3) in the RYGB group (P < .001). Metformin therapy was continued for most patients in both groups (45 [97.8%] vs 35 [76.1%]; P = .004). The metformin dose was reduced or treatment stopped after RYGB when patients reached an HbA1c level less than 5.7% or a fasting plasma glucose level less than 100 mg/dL or when metformin was associated with gastrointestinal adverse effects. After 24 months, patients in the RYGB group were 5 times less likely to use insulin or insulin analogs (11% vs 54%; risk difference, −0.43; 95% CI, −0.60 to −0.26; P < .001). Except for diuretics, which were more frequently prescribed as part of best medical treatment (14 [30.4%] vs 5 [10.9%]; P = .04), patients in the RYGB group were equally likely to receive angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (40 [87.0%] vs 41 [89.1%]; P > .99), β-blockers (10 [21.7%] vs 6 [13.0%]; P = .41), and calcium channel blockers (10 [21.7%] vs 5 [10.9%]; P = .26). No significant differences between groups were observed in the median number of antihypertensive drugs (1.5 [IQR, 1-3] vs 2 [IQR, 1-2]; P = .65).
Quality of Life
Baseline Medical Outcomes Study 36-Item Short-Form Health Survey scores for pain (best medical treatment: mean, 54.54; 95% CI, 47.61-61.47; RYGB: mean, 65.68; 95% CI, 58.68-72.68; P = .03) and social functioning (best medical treatment: mean, 63.78; 95% CI, 56.57-70.98; RYGB: mean, 76.04; 95% CI, 68.77-83.32; P = .02) differed between groups (eTable 8 in Supplement 2). After 24 months, higher scores were observed in both groups for general health. However, patients randomized to receive RYGB had a greater improvement in their general health (mean difference, 17.85; 95% CI, 10.0-25.7; P < .001), emotional well-being (mean difference, 8.93; 95% CI, 0.73-17.15; P = .03), physical health (mean difference, 19.9; 95% CI, 3.46-36.35; P = .02), physical role functioning (mean difference, 14.15; 95% CI, 5.09-23.21; P = .002), and vitality (mean difference, 14.40; 95% CI, 6.07-22.73; P = .001).
Adverse Events and Safety
The safety profile of RYGB was comparable to that of best medical treatment during 24 months (eTable 9 in Supplement 2); adverse events that occurred in 5% or more of the patients are listed in Figure 3. No deaths, episodes of serious hypoglycemia, malnutrition, or excessive weight loss occurred.
Figure 3. Adverse Events.
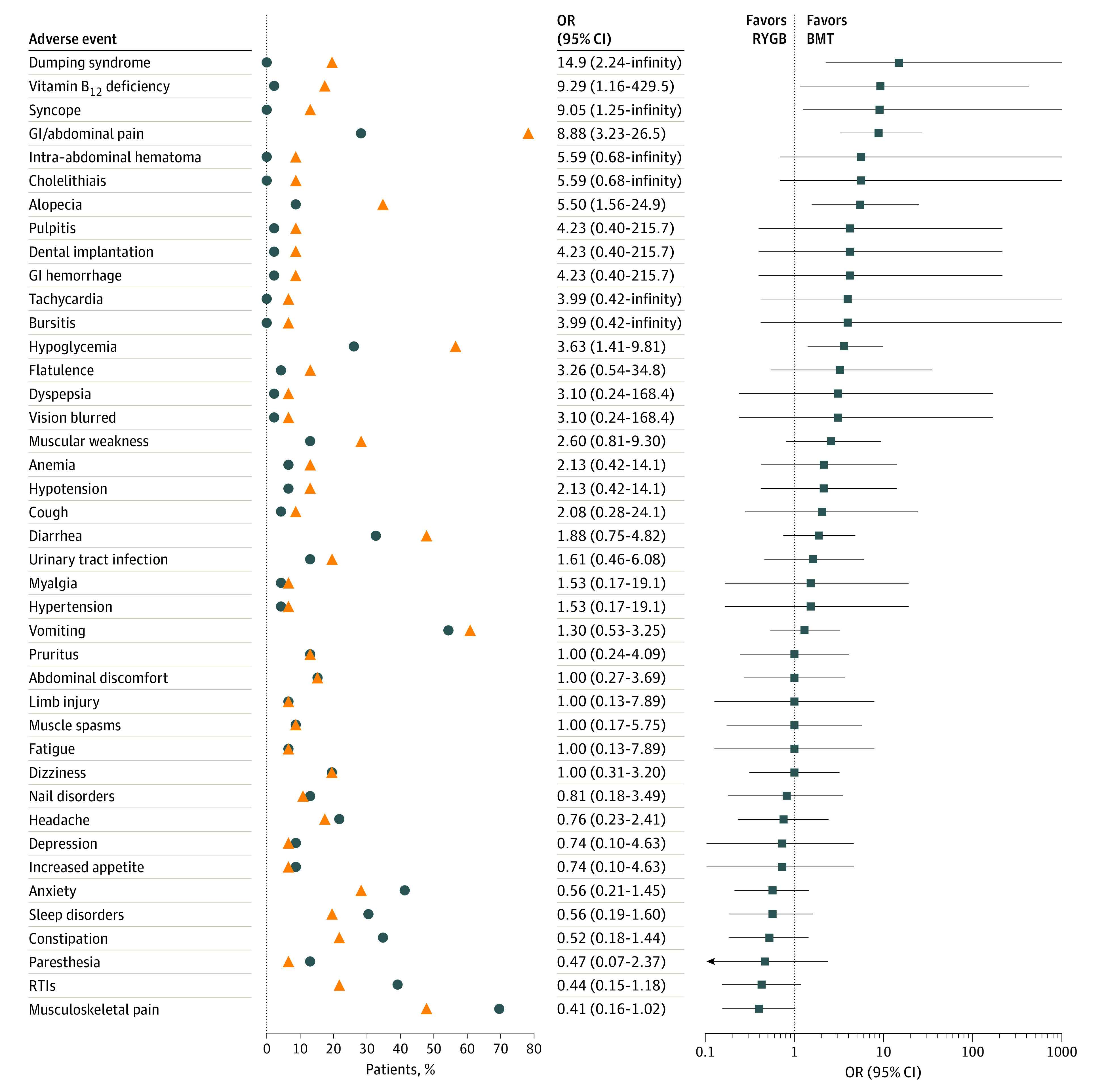
Common adverse events that occurred in 5% or more of patients in the Roux-en-Y gastric bypass (RYGB) arm. Circles and triangles represent the proportion of patients who had adverse events in each arm. Analysis was per protocol, with 46 patients per group. Circles denote proportions for the best medical treatment, and triangles display the corresponding estimates in the RYGB group. Adverse events were ranked by the odds ratio (OR). Squares represent the OR computed by an exact logistic regression model. Horizontal lines depict 95% CIs around the point estimate. Upper limits for the ORs were truncated at 1000 when estimated values exceed the length of the horizontal axis. BMT indicates best medical treatment; GI, gastrointestinal; RTI, respiratory tract infection.
Serious adverse events occurred in 6 of 46 participants (13%) in the best medical treatment group and in 6 of 46 participants (13%) in the RYGB group (P > .99). In the best medical treatment group, there was 1 case each of nephrolithiasis, chest pain, anaphylactic shock, erysipelas, septic shock due to foot infection, and diabetic foot infection. In the RYGB group, there was 1 case of sepsis due to osteomyelitis unrelated to surgery, 1 case of appendicitis, 1 case of cholelithiasis, 1 case of enterorrhagia (day 10; Clavien-Dindo grade II24), and 2 endoscopic interventions (1 to correct an anastomotic stricture [day 28; Clavien-Dido grade IIIb] and 1 to contain a gastric pouch leak [day 2; Clavien-Dido grade IIIa]). No cases of acute kidney injury, nephrolithiasis, or oxalate nephropathy occurred in the RYGB group.
Discussion
The present study revealed that RYGB is more effective for achieving remission of albuminuria and early CKD than is best medical treatment in patients with type 2 diabetes, a uACR greater than 30 mg/g, and a BMI of 30 to 35. Therapeutic interventions in diabetic kidney disease that have a beneficial effect on microalbuminuria in the short to medium term are associated with better preservation of kidney function in the longer term, as best exemplified in a previous study25 that provided the basis for renin-angiotensin-aldosterone system blockade in CKD. Thus, the 24-month outcomes of both the best medical treatment and the RYGB protocols used in the present study can be interpreted in a positive light. Expansion of the range of pharmacologic treatment options in type 2 diabetes has occurred during the past 5 years. In addition, the use of drug classes with proven glycemia- and albuminuria-lowering effects is becoming the standard of care,26 which likely explains the better than anticipated benefit of best medical treatment on albuminuria. The data presented herein confirm observational data that RYGB is significantly associated with reduced urinary albumin excretion in patients with type 2 diabetes27,28 and furthermore demonstrate that the judicious use of RYGB with state-of-the-art medication enhances remission of albuminuria and stage G1 to G3 and A2 to A3 CKD.
In the higher-risk population, no deaths occurred. Serious adverse events in the RYGB group were also easily managed without any sequelae. Most of the adverse event burden in the RYGB group was associated with discrete, self-limiting, or easily resolvable postoperative factors within the expected range for the first 2 postoperative years.
Although RYGB is the procedure of choice in our center, recently, the number of sleeve gastrectomy (SG) operations performed in patients with type 2 diabetes has been increasing internationally.29 However, the randomized clinical trials30 to date have been powered to examine weight loss as the primary outcome. Recently, Hofsø et al31 conducted a trial that suggested that gastric bypass represents the preferred bariatric procedure for patients with obesity and type 2 diabetes. Despite limited evidence on the durability of the metabolic effects of SG or its effect on CKD and other microvascular complications after SG, if any clinical or technical (previous major abdominal or intestinal operations or inflammatory bowel disease) issues contraindicate RYGB, SG may be an option to achieve better metabolic control than best medical treatment alone. Future well-controlled studies are needed to evaluate the effectiveness of SG for treatment of CKD.
Limitations
This study has several limitations, including the inherent open-label design, the short follow-up of 24 months, and minor baseline differences in lipid-lowering medication use and race/ethnicity between groups that were statistically different albeit not deemed to be clinically relevant. Of importance, the primary outcome was based on a single first-morning urine sample. This approach could have led to misclassification, but the randomization procedure was performed centrally via computer-generated random blocks stratified by sex, ensuring a nondifferential outcome misclassification and well-balanced groups at the study start. This type of nondifferential information bias in standard superiority trials is usually associated with a downward bias in the relative risk and risk difference, resulting in more conservative estimates of efficacy.32 Moreover, with the magnitude of the effect size observed in the trial, although increasing the number of measures would result in enhanced precision, it would not markedly enhance the ability to detect the size of the effect observed in the primary outcome.33
Durability and long-term tolerability remain uncertain. With regard to the latter, our inferences from 24-month data in relation to adverse events and relative safety profile are necessarily tempered by recognition that the study, being a superiority randomized clinical trial by design, focuses on relative efficacy on therapeutic primary end points, and prespecification of the type and frequency of anticipated adverse events was not established a priori. Moreover, particularly in trials that compare surgical and medical therapies, there is scope for significant heterogeneity in the type of adverse events that occur; therefore, how best to balance or compare relative severity remains problematic. Initiatives to address these matters are ongoing.34 We acknowledge that an ability to discern such differences is key, given that the prospective harm-benefit balance and therapeutic gap between surgery and medicine may narrow if the efficacy of new classes of glucose-lowering and bariatric medical therapies is sustained at long-term follow-up.35
Modern surgical and medical practices worldwide are increasing in standardization and adoption of the guidelines of the ADA and the International Federation for Surgery of Obesity. Therefore, caution should be used because the results reported in this trial are likely to be replicated only in other centers with experienced multidisciplinary teams that adhere to international guidelines.
The data obtained support a role for future mechanistic studies designed to elucidate the basis for the effects observed. On the basis of preclinical data showing the protective effect of RYGB on diabetic kidney disease in rodents,36,37 these studies may focus on assessing the structural and functional integrity in the renal glomerulus and tubule.
Conclusions
After 24 months, RYGB was more effective than best medical treatment for achieving remission of albuminuria and CKD stage G1 to G-3 and A2 to A-3 in patients with type 2 diabetes and obesity. Our findings highlight the potential of RYGB as a new treatment paradigm that should be considered to slow or arrest CKD progression in patients with type 2 diabetes and obesity.
Trial Protocol
eFigure 1. RYGB Reconstruction
eFigure 2. Patterns of Weight Change
eTable 1. Outcome Definitions
eTable 2. Changes to Trial Nonprimary End Points After the Trial Commenced
eTable 3. Variables With Missing Data
eTable 4. Clinical Variables by Quartile
eTable 5. Minor Protocol Deviations (Eligibility Waivers)
eTable 6. Chronic Kidney Disease Assessment According KDIGO Criteria
eTable 7. Medication Use at Baseline and 24 Months
eTable 8. SF-36 Scores
eTable 9. Adverse Events
eMethods. Statistical Analysis Missing Data Approach
eReferences
Data Sharing Statement
References
- 1.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302-308. doi: 10.1681/ASN.2012070718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahaffey KW, Neal B, Perkovic V, et al. ; CANVAS Program Collaborative Group . Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137(4):323-334. doi: 10.1161/CIRCULATIONAHA.117.032038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073-2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21-28. doi: 10.7326/0003-4819-144-1-200601030-00006 [DOI] [PubMed] [Google Scholar]
- 8.Coleman KJ, Haneuse S, Johnson E, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39(8):1400-1407. doi: 10.2337/dc16-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman AN, Wolfe B. Is bariatric surgery an effective treatment for type II diabetic kidney disease? Clin J Am Soc Nephrol. 2016;11(3):528-535. doi: 10.2215/CJN.07670715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018;169(5):300-310. doi: 10.7326/M17-2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network . Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423-429. doi: 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 12.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069-1077. doi: 10.1681/ASN.2008070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parving HH, Persson F, Rossing P. Microalbuminuria: a parameter that has changed diabetes care. Diabetes Res Clin Pract. 2015;107(1):1-8. doi: 10.1016/j.diabres.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 14.Scirica BM, Mosenzon O, Bhatt DL, et al. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol. 2018;3(2):155-163. doi: 10.1001/jamacardio.2017.4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant. 2004;19(11):2784-2788. doi: 10.1093/ndt/gfh470 [DOI] [PubMed] [Google Scholar]
- 16.Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium . Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26(8):2055-2064. doi: 10.1681/ASN.2014070688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen RV, Pereira TV, Aboud CM, et al. ; MOMS Study Investigators . Microvascular Outcomes after Metabolic Surgery (MOMS) in patients with type 2 diabetes mellitus and class I obesity: rationale and design for a randomised controlled trial. BMJ Open. 2017;7(1):e013574. doi: 10.1136/bmjopen-2016-013574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JP, Pinkney JH. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J. 2006;82(966):280-284. doi: 10.1136/pmj.2005.039032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray N, Picone G, Sloan F, Yashkin A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med J. 2015;108(1):29-36. doi: 10.14423/SMJ.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summary of revisions: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S4-S6. doi: 10.2337/dc19-Srev01 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622-627. doi: 10.1053/j.ajkd.2010.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciconelli R, Ferraz M, Santos W, Meinao IR, Quaresma M. Brazilian-Portuguese version of the SF-36: a reliable and valid quality of life outcome measure. Rev Bras Reumatol. 1999;39(3):143-150. [Google Scholar]
- 23.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133-2135. doi: 10.2337/dc09-9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65(6):2309-2320. doi: 10.1111/j.1523-1755.2004.00653.x [DOI] [PubMed] [Google Scholar]
- 26.Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23(7):579-591. doi: 10.1080/14728222.2019.1624721 [DOI] [PubMed] [Google Scholar]
- 27.Alexander JW, Goodman HR, Hawver LR, Cardi MA. Improvement and stabilization of chronic kidney disease after gastric bypass. Surg Obes Relat Dis. 2009;5(2):237-241. doi: 10.1016/j.soard.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 28.Carlsson LMS, Sjöholm K, Karlsson C, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271-279. doi: 10.1016/S2213-8587(17)30061-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27(9):2279-2289. doi: 10.1007/s11695-017-2666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255-265. doi: 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(12):912-924. doi: 10.1016/S2213-8587(19)30344-4 [DOI] [PubMed] [Google Scholar]
- 32.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488-495. doi: 10.1093/oxfordjournals.aje.a112408 [DOI] [PubMed] [Google Scholar]
- 33.Kröpelin TF, de Zeeuw D, Andress DL, et al. Number and frequency of albuminuria measurements in clinical trials in diabetic nephropathy. Clin J Am Soc Nephrol. 2015;10(3):410-416. doi: 10.2215/CJN.07780814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open. 2019;9(2):e024537. doi: 10.1136/bmjopen-2018-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miras AD, le Roux CW. Metabolic surgery in a pill. Cell Metab. 2017;25(5):985-987. doi: 10.1016/j.cmet.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 36.Canney AL, Cohen RV, Elliott JA, et al. Improvements in diabetic albuminuria and podocyte differentiation following Roux-en-Y gastric bypass surgery. Diab Vasc Dis Res. 2020;17(1):1479164119879039. doi: 10.1177/1479164119879039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neff KJ, Elliott JA, Corteville C, et al. Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis. 2017;13(1):21-27. doi: 10.1016/j.soard.2016.08.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. RYGB Reconstruction
eFigure 2. Patterns of Weight Change
eTable 1. Outcome Definitions
eTable 2. Changes to Trial Nonprimary End Points After the Trial Commenced
eTable 3. Variables With Missing Data
eTable 4. Clinical Variables by Quartile
eTable 5. Minor Protocol Deviations (Eligibility Waivers)
eTable 6. Chronic Kidney Disease Assessment According KDIGO Criteria
eTable 7. Medication Use at Baseline and 24 Months
eTable 8. SF-36 Scores
eTable 9. Adverse Events
eMethods. Statistical Analysis Missing Data Approach
eReferences
Data Sharing Statement