Key Points
Question
What are the clinical manifestations of children and adolescents hospitalized with coronavirus disease 2019 (COVID-19)?
Findings
In this case series of 50 children and adolescents hospitalized with COVID-19 infection, respiratory symptoms, while common, were not always present. Children hospitalized with COVID-19 commonly had comorbidities, infants had less severe disease, those with obesity were likely to receive mechanical ventilation, and elevated markers of inflammation at admission and during hospitalization were associated with severe disease.
Meaning
Expanded testing, maintaining a high suspicion for severe acute respiratory syndrome coronavirus 2 infection given the variable presentation of COVID-19, risk stratification, and recognition of findings suggestive of immune dysregulation are crucial to effective COVID-19 management in children.
Abstract
Importance
Descriptions of the coronavirus disease 2019 (COVID-19) experience in pediatrics will help inform clinical practices and infection prevention and control for pediatric facilities.
Objective
To describe the epidemiology, clinical, and laboratory features of patients with COVID-19 hospitalized at a children’s hospital and to compare these parameters between patients hospitalized with and without severe disease.
Design, Setting, and Participants
This retrospective review of electronic medical records from a tertiary care academically affiliated children’s hospital in New York City, New York, included hospitalized children and adolescents (≤21 years) who were tested based on suspicion for COVID-19 between March 1 to April 15, 2020, and had positive results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Exposures
Detection of SARS-CoV-2 from a nasopharyngeal specimen using a reverse transcription–polymerase chain reaction assay.
Main Outcomes and Measures
Severe disease as defined by the requirement for mechanical ventilation.
Results
Among 50 patients, 27 (54%) were boys and 25 (50%) were Hispanic. The median days from onset of symptoms to admission was 2 days (interquartile range, 1-5 days). Most patients (40 [80%]) had fever or respiratory symptoms (32 [64%]), but 3 patients (6%) with only gastrointestinal tract presentations were identified. Obesity (11 [22%]) was the most prevalent comorbidity. Respiratory support was required for 16 patients (32%), including 9 patients (18%) who required mechanical ventilation. One patient (2%) died. None of 14 infants and 1 of 8 immunocompromised patients had severe disease. Obesity was significantly associated with mechanical ventilation in children 2 years or older (6 of 9 [67%] vs 5 of 25 [20%]; P = .03). Lymphopenia was commonly observed at admission (36 [72%]) but did not differ significantly between those with and without severe disease. Those with severe disease had significantly higher C-reactive protein (median, 8.978 mg/dL [to convert to milligrams per liter, multiply by 10] vs 0.64 mg/dL) and procalcitonin levels (median, 0.31 ng/mL vs 0.17 ng/mL) at admission (P < .001), as well as elevated peak interleukin 6, ferritin, and D-dimer levels during hospitalization. Hydroxychloroquine was administered to 15 patients (30%) but could not be completed for 3. Prolonged test positivity (maximum of 27 days) was observed in 4 patients (8%).
Conclusions and Relevance
In this case series study of children and adolescents hospitalized with COVID-19, the disease had diverse manifestations. Infants and immunocompromised patients were not at increased risk of severe disease. Obesity was significantly associated with disease severity. Elevated inflammatory markers were seen in those with severe disease.
This case series study examines the epidemiology, clinical, and laboratory features of children hospitalized with coronavirus disease 2019 (COVID-19) in New York City, New York.
Introduction
Community transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is now widespread in the US, with New York City (NYC), New York, continuing to be the epicenter. Detailed reports of clinical features among hospitalized adults in the US are increasingly available. International pediatric data suggest lower rates of severe coronavirus disease 2019 (COVID-19) in children and higher rates of asymptomatic infection. Risk factors for severe disease in pediatric populations have not been clearly identified and the high prevalence of SARS-CoV-2 in NYC offers an opportunity to describe severe pediatric disease in more detail. Being younger than 1 year has been suggested as a predisposing factor for hospitalization in initial reports, including from the US Centers for Disease Control and Prevention (CDC). Detailed descriptions of the clinical course, laboratory parameters, and findings associated with severe disease in children and adolescents could help inform clinical practice and infection prevention and control (IPC) preparedness at children’s hospitals. In this article, we present the epidemiology, clinical characteristics, treatment, and outcomes of 50 pediatric patients admitted with COVID-19 to our children’s hospital from March 1 to April 15, 2020.
Methods
The study site was NewYork-Presbyterian Morgan Stanley Children’s Hospital, a tertiary care children’s hospital affiliated with Columbia University Irving Medical Center. Electronic medical records for all patients with SARS-CoV-2 detected in nasopharyngeal swabs between March 1 and April 15, 2020, were reviewed. The inclusion criteria for detailed medical record review included (1) inpatient hospitalization, (2) symptoms consistent with COVID-19 as described in the literature, and (3) age 21 years or younger.
Since April 6, 2020, our hospital system has performed universal SARS-CoV-2 testing for all patients being newly hospitalized and for patients undergoing surgical procedures with the potential for aerosol generation. Asymptomatic patients who received positive test results as part of screening were excluded from further analysis.
All testing was conducted at the Columbia University Microbiology Laboratory using a real-time reverse transcriptase–polymerase chain reaction (PCR) assay (Roche Cobas SARS-CoV-2 Test). The Columbia University Irving Medical Center institutional review board approved this study, and a waiver of informed consent was provided because of the minimal risk.
Data Collection and Study Definitions
For patients who met eligibility criteria, the following groups of variables were collected from the electronic medical record: presenting symptoms, duration of symptoms before presentation, comorbid conditions, hospital course, disease severity, laboratory parameters, radiologic findings, administered antiviral therapies, clinical course during hospitalization, and patterns of repeated test positivity when available. Demographic data collected included age, for which infants were defined as patients younger than 12 months and adolescents as patients ages 11 through 21 years. Race and ethnicity were as defined by documentation in the electronic medical record.
All symptoms reported at time of presentation were documented, including COVID-19 symptoms as outlined by the CDC: fever (subjective or temperature ≥38 °C), cough, shortness of breath, chills, muscle pain, new loss of taste or smell, vomiting or diarrhea, and/or sore throat. Respiratory distress was defined by persistent tachypnea, use of accessory muscles documented in physical examination findings, or respiratory distress as noted in treating clinician’s documentation.
The epidemiology of admissions was assessed in association with the introduction of nonpharmaceutical interventions (NPIs) implemented within NYC. Health care–associated cases were defined as detection of SARS-CoV-2 more than 14 days after admission and/or associated with a confirmed exposure within the hospital. The presence of household contacts with illness was stratified based on compatible symptomatology or laboratory confirmation of SARS-CoV-2.
Specific comorbidities ascertained based on published work on COVID-19 included infancy (age younger than 1 year), obesity, asthma, and immunosuppression. Obesity and overweight status were defined based on the CDC’s child and teen body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) calculator for all patients 2 years and older (BMI at or above the 95th percentile for age/sex categorized as obesity; 85%-95% as overweight) and the weight for length percentiles for patients younger than 2 years (overweight above 95th percentile). Immunosuppression was defined as the concurrent use of an immunosuppressive agent either for hematologic or solid malignancy, solid organ transplant, hematopoietic stem cell transplant, or ongoing severe cytopenia.
Severe disease was defined as requirement for mechanical ventilation during hospitalization. Our health care system recommended minimizing the use of noninvasive positive pressure ventilation (NIPPV) to limit aerosol generation. Time to intubation from symptom onset was collected for intubated patients. Other markers of disease severity (eg, length of stay, discharge status) were collected.
Suspected and confirmed bacterial and respiratory viral coinfections were reviewed. Our health care system restricted respiratory PCR testing starting on March 27, 2020, to conserve test supplies. Specific laboratory parameters collected included complete blood cell counts for which lymphopenia was defined as an absolute lymphocyte count (ALC) less than 4500/μL in infants younger than 8 months (to convert to ×109 per liter, multiply by 0.001) or an ALC of less than 1500/μL in patients 8 months or older, kidney/hepatic function, and routine markers of inflammation (C-reactive protein, procalcitonin) for all patients. More comprehensive laboratory testing was performed for select patients, informed by adult protocols at our center, and included additional markers of inflammation: ferritin, D-dimer, and interleukin 6 (IL-6). Severely affected children without additional known comorbidities underwent a workup for primary immune deficiency. Radiology results were reviewed for the presence of bilateral patchy opacities, effusions, pneumothorax, and/or focal consolidation. Finally, we also reviewed readmissions and repeated testing data for included hospitalized patients.
Statistical Analysis
A bivariate analysis was performed comparing comorbidities and laboratory parameters between patients with and without severe disease. This was conducted using nonparametric tests (Wilcoxon rank sum) or parametric tests (t test) for continuous variables as appropriate. Fisher exact tests or χ2 tests were used to compare categorical variables between patients with and without severe disease. An α of .05 was predetermined as the level of significance. All analyses were conducted using R, version 3.53 (R Foundation for Statistical Computing).
Results
Study Population
Between March 1 and April 15, 2020, 387 unique patients (484 tests total) 21 years and younger were tested for SARS-CoV-2 from the emergency department or an inpatient location, with a total of 73 positive results (15%). Among these patients with a positive SARS-CoV-2 result, 54 patients (74.0%) were hospitalized 1 day or longer. Four patients (5.5%) did not have symptoms consistent with COVID-19 and were tested before transfer to an inpatient psychiatry facility (n = 2), or after admission with another diagnosis (axillary abscess and incarcerated inguinal hernia; n = 2). The remaining 50 patients were eligible for the current study.
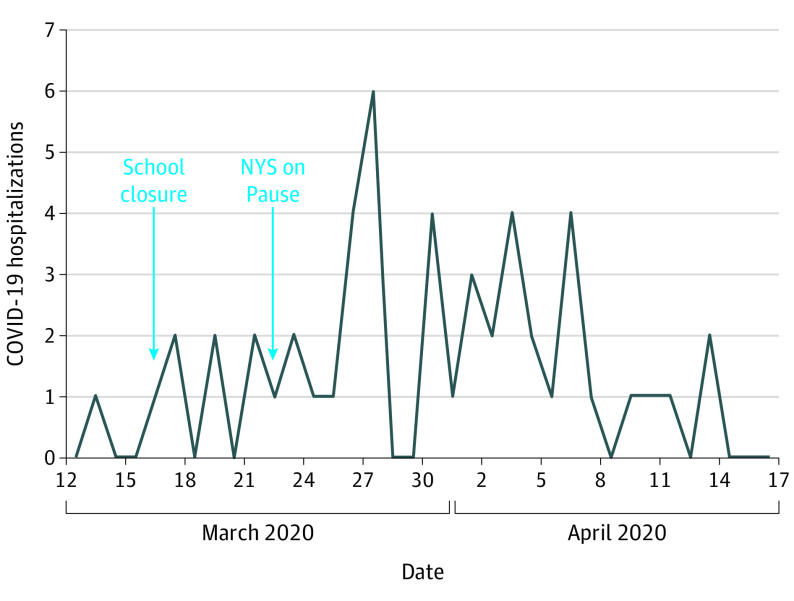
Forty-nine of these 50 patients (98%) acquired SARS-CoV-2 in the community; 1 infection (2%) was possibly health care associated. Close to half (27 [54%]) were male and Hispanic (25 [50%]) (Table 1). The peak number of hospitalizations (6 [12%]) occurred on March 27, 2020. The number of daily hospitalizations in association with citywide school closures (March 16, 2020) and a social distancing measure enforcement called New York State on Pause (March 22, 2020) are shown in Figure 1.
Table 1. Demographics, Clinical Presentation, and Comorbidities in Nonsevere and Severe Disease.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| Nonsevere (n = 41) | Severe (n = 9) | ||
| Male sex | 23 (56) | 4 (44) | .71 |
| White | 22 (54) | 5 (56) | >.99 |
| Hispanic | 21 (51) | 4 (44) | >.99 |
| Age, median (range) | 9 y (6 d-21 y) | 14 y (8 y-19 y) | <.001 |
| Infants younger than 1 y | 14 (34) | 0 | .05 |
| Signs or symptoms at time of admission | |||
| Fever | 31 (76) | 9 (100) | .17 |
| Cough | 17 (41) | 6 (67) | .27 |
| Shortness of breath/dyspnea | 9 (22) | 8 (89) | <.001 |
| Chest pain | 6 (15) | 3 (33) | .33 |
| Gastrointestinal tract symptomsa | 3 (7) | 4 (44) | .01 |
| Sore throat | 4 (10) | 2 (22) | .29 |
| Congestion/runny nose | 3 (7) | 3 (33) | .06 |
| Comorbidities | |||
| Any | 25 (61) | 8 (89) | .14 |
| Obesityb | 5 (20) | 6 (67) | .03 |
| Asthma | 4 (10) | 2 (22) | .29 |
| Immunosuppressionc | 7 (17) | 1 (11) | .66 |
| Neurologicd | 6 (15) | 1 (11) | >.99 |
| Sickle cell disease | 1 (2) | 1 (11) | .33 |
| Cardiac diseasee | 3 (7) | 1 (11) | .56 |
| Diabetes | 1 (2) | 2 (22) | .79 |
| Genetic syndromes | 3 (7) | 2 (22) | .22 |
| Chronic respiratory diseasef | 2 (5) | 0 | >.99 |
Gastrointestinal tract symptoms included abdominal pain (5 [10%]), vomiting (3 [6%]), and diarrhea (2 [4%]).
Denominator for this row only includes patients 2 years and older (25 nonsevere [50%] and 9 severe [18%]).
Immunosuppressed patients included solid organ transplant recipients (2 [4%]), those with hematologic malignancy (2[4%]), those with solid tumors (2 [4%]), hematopoietic stem cell transplant recipients (1 [2%]), and those with aplastic anemia (1 [2%]).
Neurologic conditions included seizure disorder, neurodegenerative disorders, and cerebral palsy.
Cardiac disease includes restrictive cardiomyopathy and congenital heart disease.
Includes bronchiolitis obliterans.
Figure 1. Distribution of Hospitalizations in Relation to the Implementation of Citywide Nonpharmaceutical Interventions.
COVID-19 indicates coronavirus disease 2019; NYS, New York State.
Clinical Presentations
Most patients (26 [52%]) had a documented adult family member or household contact with symptoms compatible with COVID-19 (eg, congestion/rhinorrhea, sore throat, cough, fever, and/or myalgia), of whom 9 (18%) had an exposure to an individual with a confirmed case of COVID-19. No patient had a history of international travel within 14 days before symptom onset. Two parents developed symptoms consistent with COVID-19 while visiting their hospitalized children.
The median time to admission from development of symptoms was 2 days (interquartile range [IQR], 1-5 days) but was longer in adolescents (median, 4 days; IQR, 2-7 days) compared with younger children and infants (median, 1 day; IQR, 1-2 days) (P < .001). Most patients had fever (40 [80%]) or upper/lower respiratory tract symptoms (eg, cough, congestion, sore throat, and/or shortness of breath) (32 [64%]). Infants were significantly less likely to present with respiratory distress compared with older children (1 of 14 [7%] vs 16 of 36 [44%]; P = .02). Atypical presentations included seizures or seizure-like activity (3 [6%]), severe odynophagia (1 [2%]), loss of smell (3 [6%]), recurrent pneumothorax (1 [2%]), and hepatitis in a patient who received a liver transplant for whom the donor was found to be SARS-CoV-2–positive posttransplant (1 [2%]). One infant had seizure-like activity and an abnormal electroencephalogram finding; the cerebrospinal fluid testing result for SARS-CoV-2 using a research PCR assay was negative. Gastrointestinal tract symptoms were observed for 7 patients (14%). Three of these patients only had gastrointestinal tract symptomatology (eg, abdominal pain, vomiting), triggering evaluations for appendicitis. Codetection of other respiratory viruses were found for 4 patients (rhinovirus/enterovirus [2 (4%)], rhinovirus/enterovirus/adenovirus [1 (2%)], and human metapneumovirus [1 (2%)]). Bacterial coinfections during hospitalization included bacteremia (3 [6%]), suspected bacterial pneumonia (9 [18%]), urinary tract infections (5 [10%]), skin and soft tissue infections (3 [6%]), and streptococcus pharyngitis (1 [2%]). Of the patients with bacteremia, 1 was admitted to receive a course of antibiotic therapy for Klebsiella pneumoniae bacteremia diagnosed at another hospital, 1 had methicillin-sensitive Staphylococcus aureus bacteremia on admission, and 1 developed methicillin-resistant S aureus and Enterococcus faecalis bacteremia during hospitalization.
Disease Progression During Hospitalization and Risk Factors for Severe Disease
Most patients were discharged (38 [76%]) with a median length of stay of 3 days (range, 1-30 days). Approximately one-third of the hospitalized patients (16 [32%]) required some form of respiratory support (eg, nasal cannula, NIPPV, or mechanical ventilation). The NIPPV was the maximum respiratory support used for 3 patients (6%) and mechanical ventilation was required for 9 patients (18%). The median time to intubation from symptom onset was 7 days (range, 3-11 days). Two patients had sudden cardiac arrest (pulseless electrical activity) on hospital days 3 and 5, respectively, 1 of whom died. Both cardiac arrests were preceded by a prolonged period of severe hypoxia. Mucus plugging and/or thromboembolism are being considered as potential etiologies.
The most prevalent comorbidity was obesity, seen for 11 patients (22%). An additional 8 patients (16%) had overweight. The distributions of comorbidities in children with severe disease (mechanically ventilated) vs nonsevere disease are summarized in Table 1. Obesity was significantly higher in the severe disease group. Infants were less severely affected. Disease severity was not significantly higher in immunocompromised patients. One previously healthy child who required mechanical ventilation was found on whole-exome sequencing to be heterozygous for a variant of uncertain significance; further workup is ongoing.
Laboratory Markers at Admission and in Severe Illness
On admission, the white blood cell counts were generally normal (median, 7.6 × 103/μL; IQR, 4.6-11.4 × 103/μL), with lymphopenia (median ALC, 1201/μL; IQR, 600-2084/μL) seen in 36 patients (72%). Absolute lymphocyte count at admission did not differ significantly in patients with and without severe disease (median 1201/μL vs 1199.5/μL). Thrombocytopenia was only seen in 7 patients (14%; median, 248 × 103/μL; IQR, 18-733 × 103/μL). Inflammatory markers (C-reactive protein: median, 8.978 mg/dL vs 0.64 mg/dL [to convert to milligrams per liter, multiply by 10]; procalcitonin: median, 0.31 ng/mL vs 0.17 ng/mL) were significantly elevated at admission for patients with severe disease (P < .001 for both). Patients who required mechanical ventilation were characterized by high mean peak inflammatory markers (ferritin, C-reactive protein, procalcitonin, D-dimer, and IL-6) (Table 2).
Table 2. Laboratory Values for Patients With and Without Severe Disease.
| Laboratory studies (normal values) | Peak values, mean (range) | P value | |
|---|---|---|---|
| Nonsevere (n = 41) | Severe (n = 9) | ||
| C-reactive protein (≤10 mg/dL) | 4.45 (0.027-25.7) | 18.825 (12.69-25.78) | <.001 |
| Procalcitonin (≤0.08 ng/mL) | 0.74 (0.04-7.44) | 5.3 (0.13-29.89) | .03 |
| Ferritin (≤150 ng/mL) | NAa | 432.55 (178-1374) | NA |
| Interleukin 6 (≤5 pg/mL) | 139.52 (11.2-315.0) | ||
| D-dimer (≤0.5 μg/mL) | 4.87 (0.95-18.775) | ||
| Partial thromboplastin time (23.9-34.7 s) | 47.77 (32.4-108.5) | ||
| Prothrombin time (11.9-14.4 s) | 17.32 (13.7-20.7) | ||
Abbreviation: NA, not applicable.
SI conversion factors: To convert C-reactive protein to mg/L, multiply by 10; D-dimer to nmol/L, multiply by 5.476; ferritin to μg/L, multiply by 1.
Ferritin, interleukin 6, D-dimer, partial thromboplastin time, and prothrombin time values were not consistently obtained in patients with nonsevere disease.
Radiographic Findings
Chest radiographs were performed for 36 patients (72%). Findings included bilateral patchy or ground glass opacities (25 [69%]), pleural effusion (9 [25%]), focal consolidation (8 [22%]), and pneumothorax (2 [5%]). Five patients without respiratory symptoms had bilateral patchy infiltrates on imaging. Three immunocompromised patients received chest computerized tomography; 1 patient had unilateral multilobar pneumonia but did not develop lower respiratory tract symptoms or a supplemental oxygen requirement.
Administered Therapy and Adverse Drug Events
Hydroxychloroquine was administered to 15 patients (30%), typically at a dose of 6.5 mg/kg, twice daily, for 2 doses, then 6.5 mg/kg, once daily, for 4 doses. Therapy was reserved for patients with escalating oxygen requirements and respiratory distress. In 3 patients, hydroxychloroquine was used as a bridge to remdesivir therapy. Of the 15 patients prescribed hydroxychloroquine, 2 patients required drug discontinuation because of adverse events (blurry vision [1] and severe abdominal pain [1]); both resolved their symptoms with drug discontinuation. One patient had the drug discontinued because of a new diagnosis of Brugada syndrome associated with prolonged QTc of more than 500 milliseconds. One patient who received hydroxychloroquine died on hospital day 5. Remdesivir was administered to 4 patients (3 obtained through a compassionate use mechanism and 1 enrolled through a clinical trial). No adverse events were observed, with 1 patient discharged and 3 still hospitalized. Tocilizumab was administered to 1 patient with elevated IL-6 levels receiving extracorporeal membrane oxygenation who developed methicillin-resistant S aureus and E faecalis bacteremia 10 days after administration. Two patients receiving mechanical ventilation with elevated inflammatory markers received methylprednisolone, 1 mg/kg, once daily, for 5 to 7 days.
Repeated Test Positivity for SARS-CoV-2 and Potential False Negatives
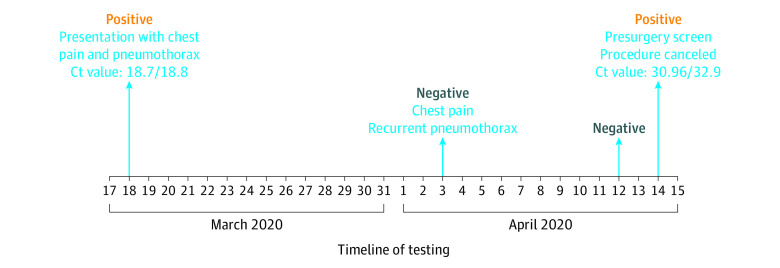
On readmission 15 and 27 days after their first SARS-CoV-2 tests, 2 immunocompetent patients were retested and had positive results. A test positivity sequence demonstrating potential false-negative results for 1 of these patients is illustrated in Figure 2. Two immunocompromised patients were retested 14 and 23 days after their first detection and still had positive results. One patient with fever and fatigue at the time of admission initially had a negative test result but was retested 7 days later and had positive results for SARS-CoV-2. Nasopharyngeal viral load values as represented as the PCR cycle threshold values were available for 1 immunocompromised infant, and these values remained high and unchanged with sequential testing 7 days apart, although the patient had minimal lower respiratory tract disease.
Figure 2. Pattern of Positive and Negative Test Results for a Specific Patient.
Cycle threshold (Ct) values for polymerase chain reaction testing. A lower value corresponds to a higher nasopharyngeal viral load, with 40 being the internal threshold for negativity.
Discussion
In this article, we report the epidemiology, clinical manifestations, and outcomes of pediatric patients hospitalized with COVID-19 at a children’s hospital in New York City. Thus far, the small number of cases are consistent with data suggesting that children have less severe disease requiring hospitalization compared with adults.
Data from China suggested that children were infected early during the community transmission phase of the pandemic. Thus, having a low threshold of suspicion for SARS-CoV-2 testing and adequate test supplies are crucial for mitigation. Consequently, our testing strategy, which is similar to many other centers, has rapidly evolved from using strict CDC person-under-investigation criteria to universal screening of admissions. Most of the patients were clustered within an incubation period starting from citywide NPI implementation, and decreasing numbers of cases could indicate efficacy of these measures. Many patients had household contacts with illness at the time of admission, suggesting household transmission. However, only a few of these contacts had laboratory confirmation of SARS-CoV-2. Expansion of ambulatory testing and easier access must be implemented before decreasing NPIs in communities with high rates of COVID-19. Two parents developed symptoms during their child’s admission, so vigilance for the emergence of symptoms in visitors remains crucial to minimize staff exposures.
Lower respiratory tract symptoms and fever were common manifestations in the study patients. However, we noted diverse COVID-19 presentations. Gastrointestinal symptoms were a feature in many patients, as described in adults. The constellation of abdominal pain and vomiting was seen in 3 patients (6%), and whether gastrointestinal tract inflammation can trigger a clinical picture consistent with appendicitis in children should be further defined. Seizures or seizure-like activity also occurred. Neurologic manifestations have been documented in adults with COVID-19. Whether these atypical presentations result from viral replication vs an immune phenomenon remains to be elucidated. The presence of viral and bacterial coinfections can complicate decisions for pediatric and infection control practitioners when labeling a detection of SARS-CoV-2 as COVID-19.
The significance of obesity as an independent risk factor for severity is now being increasingly described in adult studies of COVID-19, so it was interesting that many of the hospitalized patients in this study had obesity and/or overweight. Obesity was the most significant factor associated with mechanical ventilation in children 2 years and older. Contrary to some previous reports, infants seemed largely spared severe manifestations. Most patients had 1 or more comorbidities. An associated observation is the lack of disproportionate effect on immunosuppressed patients, most of whom remained stable throughout their illness.
As described previously, lymphopenia was the most consistent laboratory finding, but it did not have prognostic value. However, significantly elevated inflammatory markers suggestive of a hyperinflammatory state were seen in patients with severe disease, as described in adults. Radiographic findings were consistent with previous reports, but in some cases, findings were observed without significant symptoms.
Therapy for COVID-19 remains experimental. We reserved antiviral therapy for patients who required escalating respiratory support, usually with NIPPV or higher. The safety of hydroxychloroquine is debated, and in our limited experience, we encountered issues that halted course completion for 20% of patients receiving hydroxychloroquine. Remdesivir administration was not associated with any adverse events. However, the small sample size does not allow inference regarding efficacy. The role of immune modulators and steroids remains controversial and should be addressed through clinical trials.
The dynamics of viral shedding complicate IPC for SARS-CoV-2. The few retested patients showed prolonged positivity even when immunocompetent. Prolonged SARS-CoV-2 detection after symptom resolution has been described, which complicates decision-making around discontinuing isolation or home quarantine. Even more concerning is repeated positivity after multiple interval negative results, as seen in 1 patient. Encouragingly, as rates of community transmission have decreased, our admission screening positivity rates remain low. Only 1 health care–associated case has been identified to date at our center, hopefully attributable to effective hospital IPC practices, including universal masking, universal symptom checks of staff and visitors, and expanded personal protective equipment access.
Limitations
This study has limitations. Our hospital predominantly serves a Hispanic community, so findings may not be generalizable to other populations. The small sample size of this descriptive study may additionally limit generalizability. We may not have captured all comorbidities or signs and symptoms in this retrospective study. In the setting of widespread community transmission, SARS-CoV-2 detection could also be coincidental with other diagnoses and detection could represent previous illness with prolonged shedding, mild symptoms, and/or asymptomatic infection creating misclassification.
Conclusions
As community transmission of SARS-CoV-2 continues, hospitals must be alert to variable presentations of COVID-19, test liberally, attempt early risk stratification of patient populations, and have well-established clinical and IPC protocols. Therapeutic considerations need to consider the risk of toxicity, control of antiviral replication, and early recognition and management of immune dysregulation.
References
- 1.NYC Department of Health . Coronavirus disease 2019 (COVID-19). Accessed March 26, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-main.page
- 2.Harcourt J, Tamin A, Lu X, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis. 2020;26(6):1266-1273. doi: 10.3201/eid2606.200516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. Published online March 19, 2020. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. Published online April 08, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020:e20200702. doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 6.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663-1665. doi: 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344 [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . COVID-19 situation summary. Accessed March 25, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 9.World Health Organization . Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Accessed May 27, 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 10.Centers for Disease Control and Prevention . Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Accessed May 8, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html
- 11.Centers for Disease Control and Prevention . Defining childhood obesity. Accessed April 18, 2020. https://www.cdc.gov/obesity/childhood/defining.html
- 12.Régent A, Kluger N, Bérezné A, Lassoued K, Mouthon L. Lymphocytopenia: aetiology and diagnosis, when to think about idiopathic CD4(+) lymphocytopenia?. Article in French. Rev Med Interne. 2012;33(11):628-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;S0016-5085(20)30448-0. Published online April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. Published online April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. Published online April 9, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169-1174. doi: 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. JAMA Network Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. medRxiv. Preprint. Posted online April 23, 2020. https://www.medrxiv.org/content/10.1101/2020.04.16.20065920v2.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]