The number of novel coronavirus (COVID-19) cases worldwide continues to grow, and the gap between reports from China and statistical estimates of incidence based on cases diagnosed outside China indicates that a substantial number of cases are underdiagnosed (Nishiura et al., 2020a). Estimation of the asymptomatic ratio—the percentage of carriers with no symptoms—will improve understanding of COVID-19 transmission and the spectrum of disease it causes, providing insight into epidemic spread. Although the asymptomatic ratio is conventionally estimated using seroepidemiological data (Carrat et al., 2008, Hsieh et al., 2014), the collection of these data requires significant logistical effort, time, and cost. Instead, we propose a method of estimating the asymptomatic ratio by using information on Japanese nationals who were evacuated from Wuhan, China on charter flights.
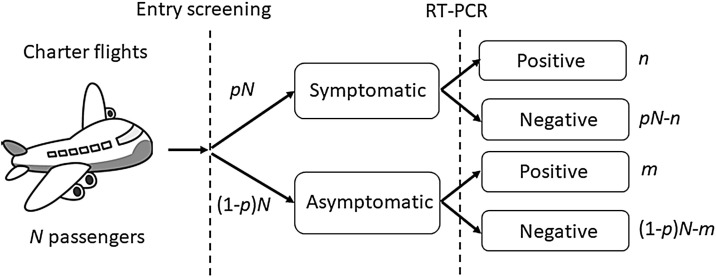
Figure 1 illustrates the flow of the evacuation process. By February 6, 2020 a total of N = 565 citizens had been evacuated. Among them, pN = 63 (11.2%) were considered symptomatic upon arrival based on (1) temperature screening before disembarkation, and (2) face-to-face interviews eliciting information on symptoms including fever, cough, and other non-specific symptoms consistent with COVID-19. Reverse transcription PCR (RT-PCR) testing was performed for all passengers, and m = 4 asymptomatic and n = 9 symptomatic passengers tested positive for COVID-19.
Figure 1.
Flow diagram of symptom screening and viral testing for passengers on chartered evacuation flights from Wuhan, China to Japan. The flow of Japanese residents evacuating from Wuhan and screened in Japan. A total of N passengers were evaluated, of whom a fraction p were symptomatic upon arrival. Among symptomatic and asymptomatic individuals, n and m persons, respectively, tested positive for the virus by RT-PCR.
Employing a Bayes theorem, the asymptomatic ratio is defined as
which can be calculated as m/(n + m), as seen in Figure 1. Using a binomial distribution, the asymptomatic ratio among evacuees was thus estimated to be 30.8% (95% confidence interval 7.7–53.8%). On March 6, 2020, a minimum of 30 days had elapsed since the citizens had departed from Wuhan – a length of observation sufficiently longer than the COVID-19 incubation period (Li et al., 2020, Linton et al., 2020). Thus, there was very little probability that the four virus-positive asymptomatic individuals would develop symptoms.
In general, asymptomatic infections cannot be recognized if they are not confirmed by RT-PCR or other laboratory testing, and symptomatic cases may not be detected if they do not seek medical attention (Nishiura et al., 2020b). Estimates such as this therefore provide important insight by using a targeted population to assess the prevalence of asymptomatic viral shedding (Kupferschmidt and Cohen, 2020). It should be noted that the limited sensitivity of RT-PCR does not affect the estimate of the asymptomatic ratio, because the sensitivity is cancelled out from the right-hand side of the equation. However, a weakness of this study is that age-dependence and other aspects of heterogeneity were ignored, because the samples relied on Japanese evacuees from Wuhan. Despite the small sample size, this estimation indicates that perhaps less than a half of COVID-19-infected individuals are asymptomatic. This ratio is slightly smaller than that for influenza, which has been estimated at 56–80% (Hsieh et al., 2014) using similar definitions for symptomatic individuals.
There is great need for further studies on the prevalence of asymptomatic COVID-19 infections to guide epidemic control efforts.
Ethical approval
Not required.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgements
HN received funding support from the Japan Agency for Medical Research and Development (grant number JP18fk0108050), the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI in Japanese abbreviation; grant numbers 17H04701, 17H05808, 18H04895 and 19H01074), and the Japan Science and Technology Agency (JST) Core Research for Evolutional Science and Technology (CREST) program (grant number JPMJCR1413). NML received a graduate study scholarship from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Carrat F., Vergu E., Ferguson N.M., Lemaitre M., Cauchemez S., Leach S. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008;167(7):775–785. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- Hsieh Y., Tsai C., Lin C. Asymptomatic ratio for seasonal H1N1 influenza infection among schoolchildren in Taiwan. BMC Infect Dis. 2014;14:80. doi: 10.1186/1471-2334-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K., Cohen J. ‘This beast is moving very fast.’ Will the new coronavirus be contained—or go pandemic? Science. 2020;(February) doi: 10.1126/science.abb1701. [DOI] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.-M. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Jung S.M., Linton N.M., Kinoshita R., Yang Y., Hayashi K. The extent of transmission of novel coronavirus in Wuhan, China. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020330. pii: E330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Yang Y., Hayashi K., Miyama T., Kinoshita R. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: estimation using japanese passengers data on evacuation flights. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020419. pii: E419. [DOI] [PMC free article] [PubMed] [Google Scholar]