Abstract
Aim:
Glycosphingolipids are conserved lipids displaying a variety of functions in fungal cells, such as determination of cell polarity and virulence. They have been considered as potent targets for new antifungal drugs. The present work aimed to test two inhibitors, myriocin and DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol, in Scedosporium boydii, a pathogenic fungus which causes a wide range of disease.
Materials & methods:
Mass spectrometry, microscopy and cell biology approaches showed that treatment with both inhibitors led to defects in fungal growth and membrane integrity, and caused an increased susceptibility to the current antifungal agents.
Conclusion:
These data demonstrate the antifungal potential of drugs inhibiting sphingolipid biosynthesis, as well as the usefulness of sphingolipids as promising targets for the development of new therapeutic options.
Keywords: : antifungal drugs, antifungal therapy, biosynthetic pathway, glycosphingolipids, new targets, Scedosporium
Scedosporium species are filamentous fungi widely found in nature, especially in human-impacted areas [1,2]. Scedosporium boydii is one of the species associated with fungal infections in immunocompromised and immunocompetent patients. Scedosporiosis is cosmopolitan and has been reported in North and South America, Europe, Asia and Oceania [3]. It causes wide-spectrum diseases, ranging from cutaneous and subcutaneous tissue infections and traumatic inoculation to invasive and disseminated cases as result of conidia inhalation [4].
Glycosphingolipids, a class of lipid molecules present on cell surfaces and in membranes, play crucial roles in fungal growth and virulence. They are composed of a sphingoid backbone linked to a fatty acid chain through an amide bond and the presence of a sugar unit [5]. Disruption of genes, such as gcs1 (glucosylceramide synthase), mts1 (C9-methyltransferase) and sld (Δ8 desaturase), has provided knowledge that highlights the importance of glycosphingolipid synthesis for fungal cells. Mutants of Cryptococcus neoformans, Aspergillus nidulans, Fusarium graminearum, Penicillium digitatum and Candida albicans displayed decreased virulence in animal and plant infection models, deficient fungal growth and hyphal elongation, defects in alkali tolerance, higher susceptibility to membrane stressors and reduced extravesical secretion [6–10]. Molecules that directly bind to glycosphingolipids on fungal surfaces, such as defensins produced by plants (MsDef1 and RsAFP2) and monoclonal antibodies against glucosylceramides, are known to alter cell shape, conidia germination and yeast-to-hyphae transition, as observed for C. albicans, Pichia pastoris, Fonsecaea pedrosoi, Colletotrichum gloeosporioides, Pseudallescheria boydii and Scedosporium apiospermum [11–16].
Inhibitors of sphingolipid biosynthetic pathways have also been already tested in some pathogenic fungi. Myriocin and sphingofungin are inhibitors of serine palmitoyltransferase, important in the first step of sphigolipid biosynthetic pathway, and have been shown to impair Candida and Aspergillus species biofilm formation [17,18]. Fumonisin B1 and australifungin are ceramide synthase inhibitors displaying antifungal activities in Cryptococcus, Candida and Aspergillus species [19]. Inositolphosphoryl ceramide (IPC) synthases are inhibited by aureobasidin A, khakrefungin and galbonolide, which possess antifungal activity against C. albicans and C. neoformans by causing ceramide accumulation to toxic levels [20,21]. D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-threo-PDPM) blocks glucosylceramide synthase which is involved in the last step of glucosylceramide synthesis, resulting in reduced hyphal germination and colony growth in A. nidulans and A. fumigatus [22].
The development of new therapeutic approaches to treat fungal diseases is an urgent need, especially for invasive infections, since the current antifungal options are limited. In this context, fungal sphingolipids have been considered a potential target for new antifungal drugs because their structures and biosynthetic pathway show crucial differences compared with those of mammals [23]. This work aimed to evaluate the effects of sphingolipid inhibition on S. boydii growth, morphology, membrane integrity and susceptibility to other antifungal drugs, by treating cells with two sphingolipid inhibitors, myriocin and DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol (PPMP).
Materials & methods
Microorganism & growth conditions
Scedosporium boydii CBS 120157, a clinical strain isolated from the lung of a patient with leukemia, was kindly provided by Sybren de Hoog from the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. Cells were maintained in modified Sabouraud media (0.5% yeast extract, 1% peptone and 2% glucose). To produce conidia, a 7-day culture on Sabouraud-agar plates at room temperature was scraped off in phosphate-buffered saline (PBS) and the collected conidia were filtered and washed twice with sterile PBS.
Myriocin (Sigma-Aldrich, M1177) and PPMP (Sigma-Aldrich, P4194) were added to RPMI 1640 media (Sigma-Aldrich, R8758, MO, USA).
Sphingolipid profiling of treated cells
Scedosporium boydii conidia (105) were grown in RPMI 1640 medium at 37°C for 12 h in the presence of myriocin (4, 8 and 16 μg/ml) or PPMP (32, 64 and 128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol, the solvent used to dissolve both inhibitors. After incubation, the cultures were centrifuged and lipids were extracted from cell pellets by adding ethanol:H2O:diethylether:pyridine:1 M NH4OH (15:15:5:1:0.018; v/v) for 1 h at 60°C [19]. Partitioning of neutral lipids was performed using methanol:chloroform (2:1; v/v) for 1 h at 37°C [24] followed by base hydrolysis. Electrospray ionization mass spectrometry of the extracted lipids was used to measure sphingolipid content [25].
Inhibitors effect on fungal growth
The effect of myriocin and PPMP in S. boydii growth was evaluated as described previously [26]. Conidia (105) were grown in a 10 ml culture of RPMI 1640 medium at 37°C in the presence of myriocin (4, 8 and 16 μg/ml) or PPMP (32, 64 and 128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol. Aliquots were collected at different time points, diluted and plated onto Sabouraud's agar and incubated at 30°C for 72 h to count colony-forming units (CFUs).
A Cytation 5 Imaging Reader (BioTek, VT, USA) was used to measure optical density (O.D.) at 660 nm and to photograph germinating conidia. Conidia (105) were grown in 24-well plates in the same conditions as described above and O.D. was measured every hour during 48 h. Additionally, each well was photographed every hour to monitor germination.
Biofilm formation & quantification
Scedosporium boydii biofilms were obtained as described previously [27]. Conidia (107) were incubated in 96-well plates at 36°C for 1.5 h (adherence phase). Then, RPMI 1640 media supplemented with 2% glucose and 20% fetal bovine serum (Gibco, MD, USA) was added in the presence of myriocin (4, 8 and 16 μg/ml) or PPMP (32, 64 and 128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol. After 24 h of incubation at 37°C, biofilm formation was evaluated using three parameters: violet crystal to measure overall biomass, safranin to analyze extracellular matrix and XTT for metabolic activity [28].
Filipin staining
Scedosporium boydii conidia were stained with filipin [29]. Cells were firstly incubated in RPMI 1640 media for 12 h at 37°C, in the presence of myriocin (16 μg/ml) or PPMP (128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol. Germinated conidia were further stained with 50 μg/ml of filipin (Sigma-Aldrich, F9765) for 2 h at room temperature protected from light. After washing twice with PBS, cells were visualized using a fluorescence microscope (Axioplan Imager 2, Carl Zeiss).
Transmission electron microscopy
Conidia of S. boydii (105) were treated with myriocin (16 μg/ml) or PPMP (128 μg/ml) for 12 h at 37°C. Control cells were grown in RPMI 1640 supplemented with 2% methanol. After the incubation, cells were washed in 0.01 M PBS (pH 7.2) and fixed overnight in 2.5% glutaraldehyde and 4% formaldehyde in 0.1 M cacodylate buffer. The samples were washed in the same buffer, postfixed in 1% osmium tetroxide and 1.25% potassium ferrocyanide for 40 min, and dehydrated in a series of ethanol solutions with increasing concentrations (30, 50, 70, 90, 100% and ‘ultra-dry' ethanol) for 30 min at each concentration. Then, the cells were embedded in Spurr's resin. At last, ultramicrotomy (LEICA U6) was used to cut ultra-thin sections of 60 nm that were stained with uranyl acetate and lead citrate for observations under transmission electron microscopy (TEM; ZEISS 900; FEI, Eindhoven, The Netherlands).
Susceptibility to antifungal agents
Scedosporium boydii susceptibility to different antifungal agents was performed in the presence of subinhibitory concentrations of myriocin (4 μg/ml) and PPMP (64 μg/ml). Fluconazole, amphotericin B and caspofungin were used as antifungals at concentrations ranging from 1.57 to 100 μg/ml. The checkerboard method to detect synergistic interactions was used according to EUCAST guidelines [30]. Cells were incubated at 37°C for 72 h and minimal inhibitory concentration (MIC) was evaluated at 600 nm using a spectrophotometer (iMark, Bio-Rad, CA, USA). An XTT reduction assay evaluated cell viability. Fractional inhibitory index (FIC) was calculated according to the following formula: (MIC combined/MIC drug A alone) + (MIC combined/MIC drug B alone). Results were classified as: strongly synergistic effect, FIC of <0.5; synergistic effect, FIC of <1; additive effect, FIC of 1; no effect, FIC between 1 and 2; antagonistic effect, FIC of 2 [31].
Susceptibility to membrane stressors
Scedosporium boydii cells were pretreated with subinhibitory concentrations of myriocin (4 μg/ml) or PPMP (64 μg/ml) for 24 h at 37°C in RPMI 1640 media. SDS (120 μg/ml) and NaCl (5%) were added to the media and, after another 24 h-incubation, cell viability was measured by XTT-reduction assay.
Results & discussion
Myriocin & PPMP alter sphingolipid levels in S. boydii
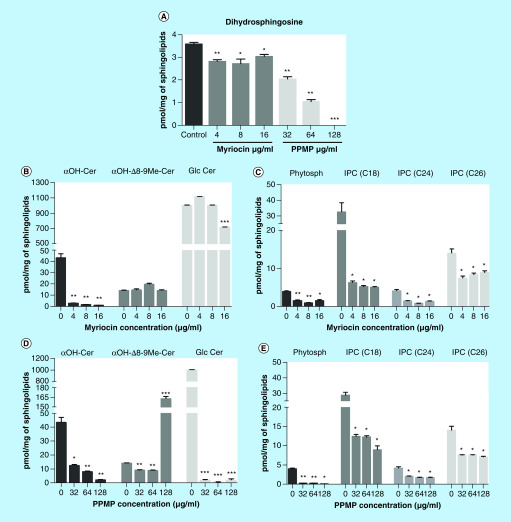
Since myriocin and PPMP are sphingolipid inhibitors, we determined whether the treatment affected lipid profiles in S. boydii cells. The sphingolipid biosynthetic pathway, highlighting the steps in which myriocin and PPMP act, is summarized in Supplementary Figure 1. Dihydrosphingosine, one of the first intermediates of the biosynthetic pathway, was reduced by both myriocin and PPMP (Figure 1A).
Figure 1. . Quantification of sphingolipid intermediates.
Cells were incubated for 12 h in the presence of myriocin (4, 8 and 16 μg/ml) or PPMP (32, 64 and 128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol, the solvent used to dissolve both inhibitors. After lipid extraction, the samples were analyzed by mass spectrometry and the values were normalized to the total amount of extracted sphingolipids. The following intermediates of sphingolipid biosynthetic pathway were analyzed: Dihydrophingosine (A), Glucosylceramide branch intermediates (B & D) and IPC branch intermediates (C & E).
*p < 0.05; **p < 0.03; ***p < 0.001.
αOH-Cer: Dihydroceramide; αOH-Δ8-9Me-Cer: 9-methyl-4,8-sphingadienine; GlcCer: Glucosylceramide; IPC: Inositolphosphorylceramide; Phytosph: Phytosphingosine; PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Regarding the glucosylceramide branch of the pathway, myriocin at 4, 8 and 16 μg/ml strongly decreased dihydroceramide (αOH-Cer) levels, but did not alter 9-methyl-4,8-sphingadienine (αOH-Δ8-9Me-Cer), (Figure 1B). PPMP treatment strongly decreased αOH-Cer at 32, 64 and 128 μg/ml, but αOH-Δ8-9Me-Cer only accumulated in cells treated with 128 μg/ml of PPMP (Figure 1D), indicating that the amount of GlcCer was reduced even in the absence of αOH-Δ8-9Me-Cer accumulation. Interestingly, the final product glucosylceramide is differently affected by both inhibitors. While PPMP reduced GlcCer production at all concentrations, myriocin only led to changes in GlcCer levels at 16 μg/ml, suggesting that higher concentrations are needed for myriocin to decrease GlcCer levels (Figure 1B & D).
Intermediates of IPCs were also analyzed. Both myriocin and PPMP at all concentrations used led to a reduction in phytosphingosine and IPCs (C18, C24 and C26) (Figure 1C & E). All these data indicated that sphingolipid inhibitors modify the pattern of the biosynthetic pathway and alter sphingolipid content in S. boydii.
Singh and colleagues observed in C. neoformans that dhSph, dhCer/phytoCer and GlcCer represent 1.2–2.6, 49–80 and 16–45%, respectively, of the total sphingolipid content [25]. These data corroborate our measurements, in which αOH-Cer, IPCs and GlcCer were the most abundant molecules in S. boydii, and also those most reduced by myriocin and PPMP, suggesting that a high concentration of these intermediates is important for sphingolipid biosynthesis.
Scedosporium boydii growth & biofilm formation is reduced by myriocin & PPMP treatment
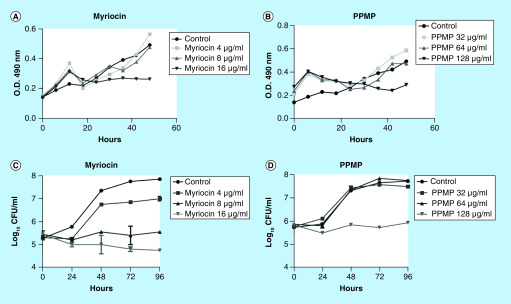
In order to check the effect of sphingolipid inhibitors on S. boydii growth, conidia were grown in RPMI 1640 supplemented with myriocin (4, 8 and 16 μg/ml) and PPMP (32, 64 and 128 μg/ml). O.D. at 600 nm was measured hourly for 48 h. Myriocin at 16 μg/ml and PPMP at 128 μg/ml decreased cell density, whereas lower concentrations of both inhibitors did not affect fungal growth (Figure 2A & B).
Figure 2. . Effect of myriocin and DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol on fungal growth.
Conidia were incubated in RPMI in the presence of myriocin (4, 8 and 16 μg/ml) or PPMP (32, 64 and 128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol. Growth measurement was performed at different time points by optic density (A & B) and CFU counting (C & D).
CFU: Colony-forming unit; PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
CFUs were counted to evaluate fungal viability in the presence of both inhibitors. Myriocin at 8 and 16 μg/ml and PPMP at 128 μg/ml impaired fungal growth compared with the control, since CFU did not increase during the incubation time (up to 96 h) (Figure 2C & D).
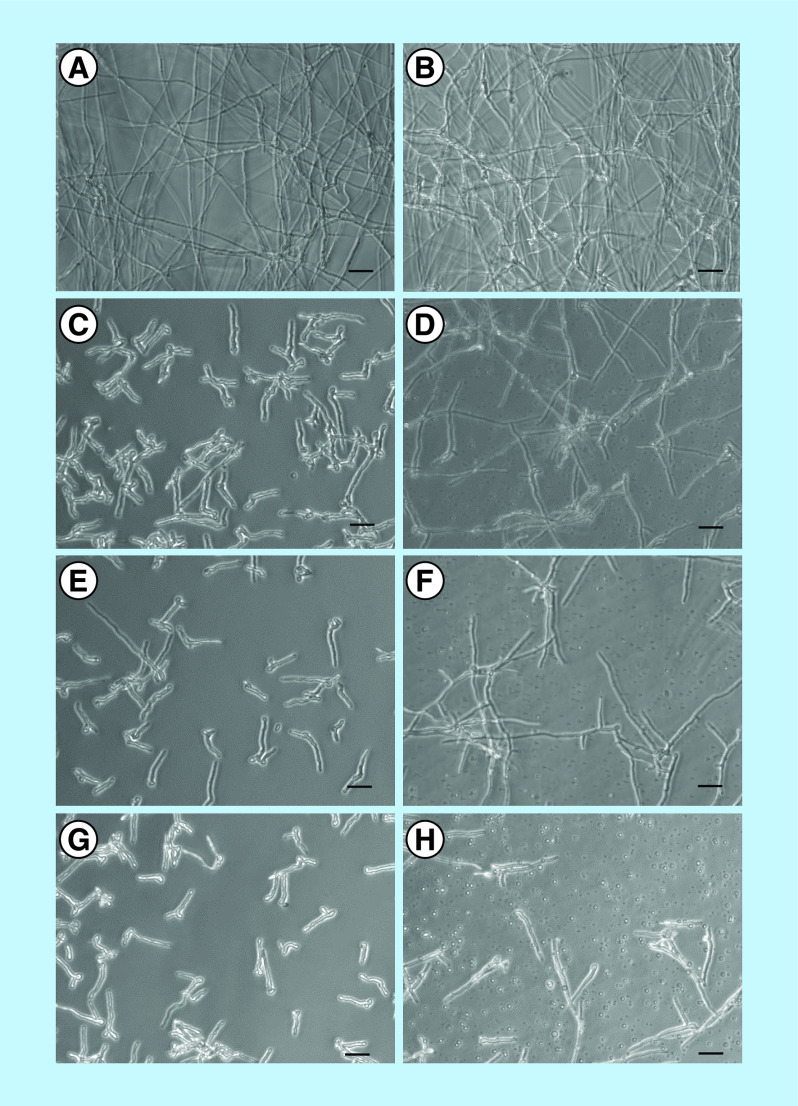
To understand whether these inhibitors alter fungal differentiation, conidial germination was assayed by incubating conidia for 12 h at 37°C in the presence or absence of myriocin and PPMP. Compared with the control in which mature hyphae developed after 12 h (Figure 3A & B), myriocin-treated cells showed only short germ tubes (Figure 3C, E & G), indicating an impairment of the germination process. PPMP treatment resulted in longer germ tubes than myriocin treatment, but hyphae failed to mature as observed in the control (Figure 3D, F & H). These data suggested that sphingolipids are crucial for hyphae elongation and consequently, for fungal growth.
Figure 3. . Germination assay of Scedosporium boydii conidia.
Cells were incubated for 12 h in RPMI in the presence of myriocin or PPMP. Control cells were grown in RPMI 1640 supplemented with 2% methanol (A & B). (C, E & G) S. boydii incubation with myriocin at 4, 8 and 16 μg/ml, respectively. (D, F & H) S. boydii incubation with PPMP at 32, 64 and 128 μg/ml, respectively. After the incubation time, the plates were visualized using an optical microscope.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
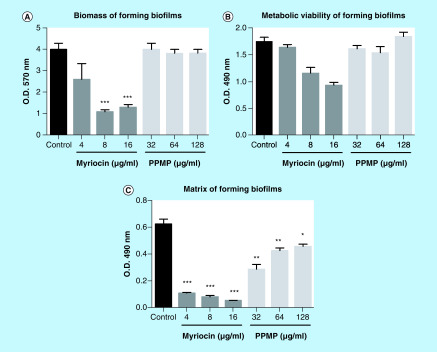
Because biofilm is an important structure for fungal virulence, the influence of sphingolipid inhibitors on S. boydii biofilm formation was assessed. Myriocin at 8 and 16 μg/ml reduced fungal biomass, metabolic activity and matrix production, whereas PPMP (32, 64 and 128 μg/ml) only decreased matrix production, suggesting that sphingolipids are important molecules for biofilm development, especially for the synthesis of a robust extracellular matrix (Figure 4).
Figure 4. . Biofilm formation by Scedosporium boydii in the presence of myriocin (4, 8 and 16 μg/ml) or DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol (32, 64 and 128 μg/ml).
Control cells were grown in RPMI 1640 supplemented with 2% methanol. Cells were incubated for 24 h and then the following parameters were measured: biomass by crystal violet staining (A); cell viability by XTT-reduction assay (B); and matrix quantification by safranin staining (C).
*p < 0.05; **p < 0.03; ***p < 0.001.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Similar effects were observed in A. fumigatus and C. albicans, with reduced CFU, growth and biofilm formation after 24 h of myriocin treatment [18], as well as disruption of lipid raft organization [17], suggesting that this point of inhibition in sphingolipid biosynthesis is conserved in pathogenic fungi. Several PPMP analogs, such as PDMP ((±) – threo – 1 – Phenyl – 2 – decanoylamino – 3 – morpholino – 1 – propanol hydrochloride) and EDO-P4, have been tested in A. fumigatus and A. nidulans [22]. Each analog displayed a differentiated influence in Aspergillus species, indicating that chemical modifications in the structure are a valid approach to increase the effects of these inhibitors.
Similar observations were also reported for A. fumigatus mutants lacking glucosylceramide synthase and Δ8-desaturase genes, in which the absence of glucosylceramide resulted in deficient conidiation and radial growth [32], showing that the inability to synthetize glucosylceramide lead to similar patterns found when inhibitors are used.
We observed that myriocin and PPMP alter cell membrane differently, especially regarding GlcCer production, suggesting that fungal growth can be affected by a variety of changes in membrane lipids. In fact, studies of Aspergillus, Fusarium and Candida mutant strains presenting gene disruptions in other genes than that coding for GlcCer synthase, such as the genes for delta-8 desaturase and C9-methyltransferase, also display membrane alterations and decreased fungal virulence [7,9,10,32]. These findings indicate that an imbalance of sphingolipid biosynthesis could lead to phenotypic alterations in fungal cells. This could be a reason why myriocin reduced S. boydii growth at 16 μg/ml even causing a lesser decrease of GlcCer compared with PPMP.
Myriocin & PPMP affect lipid raft accumulation in hyphal tips
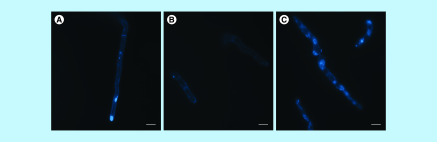
Lipid raft accumulation is crucial for polarized cell growth because it modulates vesicle recruitment and cell wall synthesis [33,34]. For this reason, S. boydii cells were stained with filipin to visualize sterol-enriched microdomains in plasma membrane (Figure 5). Filipin is a polyene macrolide that binds to ergosterol present in lipid rafts and is commonly used in sphingolipid studies [32,35]. Since a defect in S. boydii germination was observed in the presence of sphingolipid inhibitors, cells were treated with filipin to check lipid raft organization in fungal membrane after exposure to myriocin and PPMP. Compared with the control, which displayed the expected lipid raft accumulation in the apical region, cells treated with myriocin were homogeneously stained by filipin, showing a lack of accumulation along the plasma membrane (Figure 5). In PPMP treated cells, lipid rafts randomly accumulated along the membrane, suggesting a disorganization in lipid microdomains (Figure 5).
Figure 5. . Sterol staining using filipin.
Scedosporium boydii conidia were incubated for 12 h in the presence of myriocin (16 μg/ml) or PPMP (128 μg/ml). Control cells were grown in RPMI 1640 supplemented with 2% methanol. After incubation time, cells were fixed and stained with filipin (50 μg/ml) for 2 h. Samples were analyzed using a fluorescent microscope. (A) Control. (B) Myriocin. (C) PPMP.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Similar patterns were observed in A. fumigatus mutants lacking gcs1 and sld genes, in which filipin staining revealed a cell polarity defect [32]. These data suggest that disrupting glucosylceramide synthesis, either by disrupting genes or by using chemical inhibitors, results in deficient lipid raft accumulation in tip zones which could explain the impairment of fungal germination when filamentous fungi are treated with these inhibitors.
Sphingolipid inhibitors affect S. boydii morphology & membrane integrity
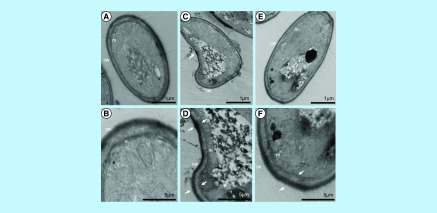
In order to visualize the effect of sphingolipid inhibitors in S. boydii, cells were treated with myriocin (16 μg/ml) and PPMP (128 μg/ml) for 12 h and the samples were processed for TEM. Compared with the control, both treatments resulted in punctual membrane alterations that seemed to be accompanied by a thicker cell wall region, suggesting that the loss of membrane integrity could be followed by a compensation mechanism in the cell wall (Figure 6). Additionally, an intracellular accumulation of electron dense material was observed in both myriocin and PPMP-treated cells.
Figure 6. . Transmission electron microscopy of Scedosporium boydii cells incubated for 12 h in the presence of myriocin (16 μg/ml) or PPMP (128 μg/ml).
Control cells were grown in RPMI 1640 supplemented with 2% methanol. After the incubation time, the samples were processed as described in the methodology section and visualized using a ZEISS 900 microscope. White arrows indicate altered membrane (m) and cell wall (cw). (A & B) Control. (C & D) Myriocin. (E & F) PPMP.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Myriocin-treated A. fumigatus cells displayed similar patterns, as demonstrated by TEM analysis [18]. After 4 h of incubation, plasma membrane became nonhomogeneous, suggesting the loss of bilayer integrity when cells were in contact with myriocin.
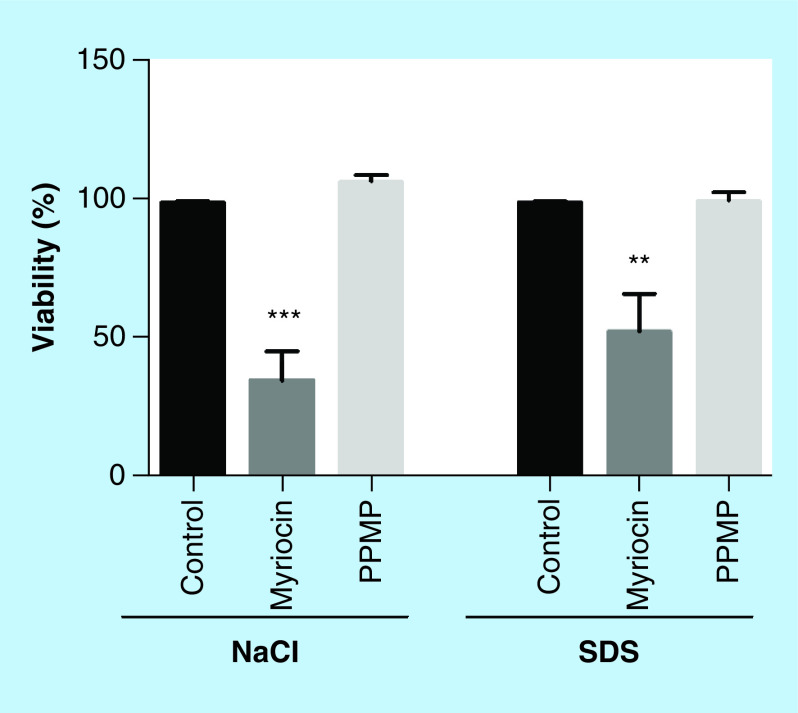
To confirm the hypothesis that plasma membrane is punctually affected by sphingolipid inhibitors, as observed in TEM, S. boydii cells were grown in the presence of membrane stressors, such as SDS and NaCl, and cell viability was measured. When previously treated with myriocin (subinhibitory concentration), cells were more susceptible to SDS and NaCl compared with the control, indicating that the reduction of sphingolipid content leads to a more fragile plasma membrane (Figure 7). PPMP treatment did not alter the susceptibility to membrane stressors comparing to the control, suggesting that each inhibitor acts differently in plasma membrane susceptibility (Figure 7). Interestingly, A. fumigatus mutants lacking gcs1 and sld genes are more resistant to cell wall stressors, such as calcofluor and Congo red [32], which corroborates with the results found in SEM images, in which a thicker S. boydii cell wall was observed in the regions where plasma membrane presented less integrity.
Figure 7. . Scedosporium boydii susceptibility to membrane stressors after incubation with myriocin (16 μg/ml) or DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol (128 μg/ml).
Cells were pre-incubated with the inhibitors for 12 h and then the media were changed for those supplemented with NaCl (5%) or SDS (120 μg/ml). After 24 h in the presence of stressors, cell viability was analyzed by XTT-reduction assay.
**p < 0.03; ***p < 0.001.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Sphingolipid inhibition influences S. boydii susceptibility to antifungal agents
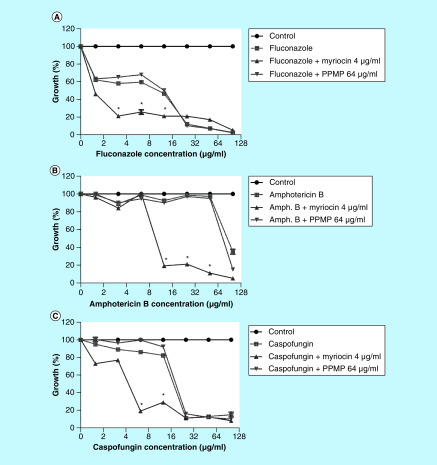
Since sphingolipids are considered a potent target for new antifungal drugs, myriocin and PPMP were used in combination with three different antifungal agents with distinct modes of action: fluconazole, amphotericin B and caspofungin. Myriocin increased S. boydii susceptibility to all antifungals tested (Figure 8). The MIC values of fluconazole, amphotericin B and caspofungin decreased from 25, 100 and 25 μg/ml, respectively, from when tested alone, to 3.125, 12.5 and 6.25 μg/ml, respectively, when combined with myriocin, resulting in a synergistic effect (Supplementary Table 1). In contrast, PPMP did not alter susceptibility of S. boydii to the antifungals (Figure 8). Although we are currently not suggesting myriocin and PPMP as therapeutic options, these data add additional support for exploring sphingolipids as targets for future drug development.
Figure 8. . Scedosporium boydii susceptibility to antifungal agents in the presence of subinhibitory concentrations of myriocin and DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Antifungal drugs (fluconazole, amphotericin B and caspofungin) were tested in serial dilution (100–1.57 μg/ml) in the presence or absence of myriocin (4 μg/ml) and PPMP (64 μg/ml).
*p < 0.05.
PPMP: DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol.
Little is known about synergism between sphingolipid inhibitors and antifungal drugs. Studies in S. apiospermum using monoclonal antibodies against glucosylceramide revealed that these molecules increase the efficacy of itraconazole, but not of amphotericin B [15], suggesting that targeting sphingolipids and sterol simultaneously could enhance the therapeutic approach. Lazzarini and colleagues tested some synthetic acylhydrazone compounds, which target fungal sphingolipid synthesis, and also displayed synergistic effect with azoles [31]. On the other hand, RsAFP2, a plant defensin that directly targets fungal glucosylceramide, presented an antagonistic effect to caspofungin in C. albicans [12]. These data clearly highlight the need for further studies regarding the interaction between sphingolipid inhibitors and antifungal agents.
Myriocin has already been described to be toxic for mammalian cells and to increase host mortality in a C. albicans infection model using Galleria mellonella [36]. However, a myriocin–nanocarrier solution has been shown to be nontoxic to mice and also to be efficient in reducing pathological inflammation and A. fumigatus infection [37], demonstrating that the use of molecules capable to inhibit sphingolipids is a promising field. In this context, our study highlights the effect of two sphingolipid inhibitors, myriocin and PPMP, showing their effect on S. boydii growth, lipid biosynthesis and cell surface integrity. Since the resistance of Scedosporium species to the current antifungal agents continues to emerge, the recognition of new possible drug targets in these fungi is highly relevant to the medical mycology community.
Conclusion
The effect of different inhibitors of sphingolipid biosynthetic pathway was demonstrated in S. boydii cells. When treated with myriocin and PPMP, sphingolipids content was reduced, fungal growth was impaired and membrane integrity was affected. Moreover, susceptibility to current antifungal drugs was potentiated when cells were simultaneously exposed to myriocin.
These data highlight the usefulness of sphingolipids as new targets for fungal treatments, as well as the potential of inhibitor molecules for the development of new drugs in therapeutic approaches.
Future perspective
Treatment of fungal infections has become a challenge because the current antifungal drugs used in the clinics present a variety of limitations, such as high toxicity to patients and low efficacy due to fungal resistance. During the last decades, sphingolipids have been considered attractive targets for the development of new antifungal drugs, opening a promising field for development.
The use of plant defensins, monoclonal antibodies, biosynthetic inhibitors and synthetic chemical compounds has demonstrated that targeting sphingolipids from pathogenic yeast and filamentous fungi lead to growth defect and reduced virulence. In this context, a promising route to development of new antifungal drugs could be modifying inhibitor molecules to broaden pathogen spectrum and reduce toxicity for testing in infection models for different pathogenic fungi.
Summary points.
Sphingolipid inhibitors led to a reduction of lipid content in treated Scedosporium boydii cells.
Myriocin and DL-threo-1-Phenyl-2-palmitoylamino-3-morpholino-1-propanol were shown to be efficient in decreasing fungal growth and hyphal elongation.
Inhibitor mechanism of action was suggested, since lipid raft accumulation and membrane integrity were affected in treated cells.
Myriocin seems to present a synergistic effect with different antifungal drugs, suggesting a promising use for treatment of resistant strains.
All data support the potential use of sphingolipid inhibition molecules to treat fungal infections, highlighting the need of more studies which describe new compounds targeting sphingolipids and test fungal virulence.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants AI136934, AI116420 and AI125770, by Merit Review Grant I01BX002924 from the Veterans Affairs Program to MD Poeta, who is Burroughs Welcome Investigator in Infectious Diseases. Additionally, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do estado do Rio de Janeiro (Faperj). MD Poeta is a Co-Founder and Chief Scientific Officer (CSO) of MicroRid Technologies Incorporated. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank W Oelemann for English revision.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Kaltseis J, Rainer J, De Hoog GS. Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Med. Mycol. 47(4), 398–405 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Rougeron A, Schuliar G, Leto J. et al. Human-impacted areas of France are environmental reservoirs of the Pseudallescheria boydii/Scedosporium apiospermum species complex. Environ. Microbiol. 17(4), 1039–1048 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Luplertlop N. Pseudallescheria/Scedosporium complex species: from saprobic to pathogenic fungus. J. Mycol. Med. 28(2), 249–256 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Cortez KJ, Roilides E, Quiroz-Telles F. et al. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 21(1), 157–197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents a complete review of Scedosporium biology and infections.
- 5.Marques JT, Marinho HS, De Almeida RFM. Sphingolipid hydroxylation in mammals, yeast and plants – an integrated view. Prog. Lipid Res. 71, 18–42 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rittershaus PC, Kechichian TB, Allegood JC. et al. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J. Clin. Invest. 116(6), 1651–1659 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the roles of glucosylceramide synthase on fungal virulence.
- 7.Ramamoorthy V, Cahoon EB, Thokala M, Kaur J, Li J, Shah DM. Sphingolipid C-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot. Cell 8(2), 217–229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu C, Wang M, Wang W. et al. Glucosylceramides are required for mycelial growth and full virulence in Penicillium digitatum. Biochem. Biophys. Res. Commun. 455(3-4), 165–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the glucosylceramide relevance for mycelial growth and fungal virulence.
- 9.Oura T, Kajiwara S. Disruption of the sphingolipid Delta8-desaturase gene causes a delay in morphological changes in Candida albicans. Microbiology 154(Pt 12), 3795–3803 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Oura T, Kajiwara S. Candida albicans sphingolipid C9-methyltransferase is involved in hyphal elongation. Microbiology 156(Pt 4), 1234–1243 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Thevissen K, Warnecke DC, Francois IE. et al. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 279(6), 3900–3905 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Thevissen K, De Mello Tavares P, Xu D. et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 84(1), 166–180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva AFC, Rodrigues ML, Farias SE, Almeida IC, Pinto MR, Barreto-Bergter E. Glucosylceramides in Colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett. 561(1-3), 137–143 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Nimrichter L, Barreto-Bergter E, Mendonca-Filho RR. et al. A monoclonal antibody to glucosylceramide inhibits the growth of Fonsecaea pedrosoi and enhances the antifungal action of mouse macrophages. Microb. Infect. 6(7), 657–665 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Rollin-Pinheiro R, Liporagi-Lopes LC, De Meirelles JV, Souza LM, Barreto-Bergter E. Characterization of Scedosporium apiospermum glucosylceramides and their involvement in fungal development and macrophage functions. PLoS ONE 9(5), e98149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Characterizes the glucosylceramide in a Scedosporium species and evidences its roles in fungi–host interactions.
- 16.Pinto MR, Rodrigues ML, Travassos LR, Haido RM, Wait R, Barreto-Bergter E. Characterization of glucosylceramides in Pseudallescheria boydii and their involvement in fungal differentiation. Glycobiology 12(4), 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Lattif AA, Mukherjee PK, Chandra J. et al. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology 157(Pt 11), 3232–3242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perdoni F, Signorelli P, Cirasola D. et al. Antifungal activity of Myriocin on clinically relevant Aspergillus fumigatus strains producing biofilm. BMC Microbiol. 15, 248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the antifungal activity of myriocin in an important pathogenic filamentous fungus.
- 19.Mandala SM, Thornton RA, Frommer BR. et al. The discovery of australifungin, a novel inhibitor of sphinganine N-acyltransferase from Sporormiella australis. Producing organism, fermentation, isolation, and biological activity. J. Antibiot. (Tokyo) 48(5), 349–356 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Cerantola V, Guillas I, Roubaty C. et al. Aureobasidin A arrests growth of yeast cells through both ceramide intoxication and deprivation of essential inositolphosphorylceramides. Mol. Microbiol. 71(6), 1523–1537 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Tan HW, Tay ST. The inhibitory effects of aureobasidin A on Candida planktonic and biofilm cells. Mycoses 56(2), 150–156 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Levery SB, Momany M, Lindsey R. et al. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 525(1-3), 59–64 (2002). [DOI] [PubMed] [Google Scholar]; •• Shows the antifungal activity of glucosylceramide synthase inhibitors in a model of pathogenic filamentous fungi.
- 23.Rollin-Pinheiro R, Singh A, Barreto-Bergter E, Del Poeta M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 8(12), 1469–1484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights the potential of sphingolipids as a new target for antifungal drugs, as well as reviews the main data regarding sphingolipid functions in fungal pathogens.
- 24.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(8), 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Mackenzie A, Girnun G, Del Poeta M. Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains. J. Lipid Res. 58(10), 2017–2036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mor V, Rella A, Farnoud AM. et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. MBio 6(3), e00647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila TV, Chaturvedi AK, Rozental S, Lopez-Ribot JL. In vitro activity of miltefosine against Candida albicans under planktonic and biofilm growth conditions and in vivo efficacy in a murine model of oral candidiasis. Antimicrob. Agents Chemother. 59(12), 7611–7620 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rollin-Pinheiro R, De Meirelles JV, Vila TVM. et al. Biofilm formation by Pseudallescheria/Scedosporium species: a comparative study. Front. Microbiol. 8, 1568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R, Li J, Zhang T. et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy 10(7), 1241–1255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subcommittee on Antifungal Susceptibility Testing of The EECFaST. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14(10), 982–984 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Lazzarini C, Haranahalli K, Rieger R. et al. Acylhydrazones as antifungal agents targeting the synthesis of fungal sphingolipids. Antimicrob. Agents Chemother. 62(5), e00156–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes CM, De Castro PA, Singh A. et al. Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Delta8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Mol. Microbiol. 102(3), 488–505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Evidences the effects of disrupting sphingolipid biosynthesis on fungal growth and membrane integrity.
- 33.Taheri-Talesh N, Horio T, Araujo-Bazan L. et al. The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19(4), 1439–1449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makushok T, Alves P, Huisman SM, Kijowski AR, Brunner D. Sterol-rich membrane domains define fission yeast cell polarity. Cell 165(5), 1182–1196 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Li S, Du L, Yuen G, Harris SD. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell 17(3), 1218–1227 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Melo NR, Abdrahman A, Greig C. et al. Myriocin significantly increases the mortality of a nonmammalian model host during Candida pathogenesis. PLoS ONE 8(11), e78905 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caretti A, Torelli R, Perdoni F. et al. Inhibition of ceramide de novo synthesis by myriocin produces the double effect of reducing pathological inflammation and exerting antifungal activity against A. fumigatus airways infection. Biochim. Biophys. Acta 1860(6), 1089–1097 (2016). [DOI] [PubMed] [Google Scholar]